Obesity and Diabetes in Mexico: An Approach to the Intestinal Microbiota
Abstract
1. Introduction
2. Prevalence of Obesity and Diabetes and Risk Factors
2.1. Obesity in Mexico
2.2. Diabetes in Mexico
3. Relationships of the Intestinal Microbiota with Obesity and Diabetes
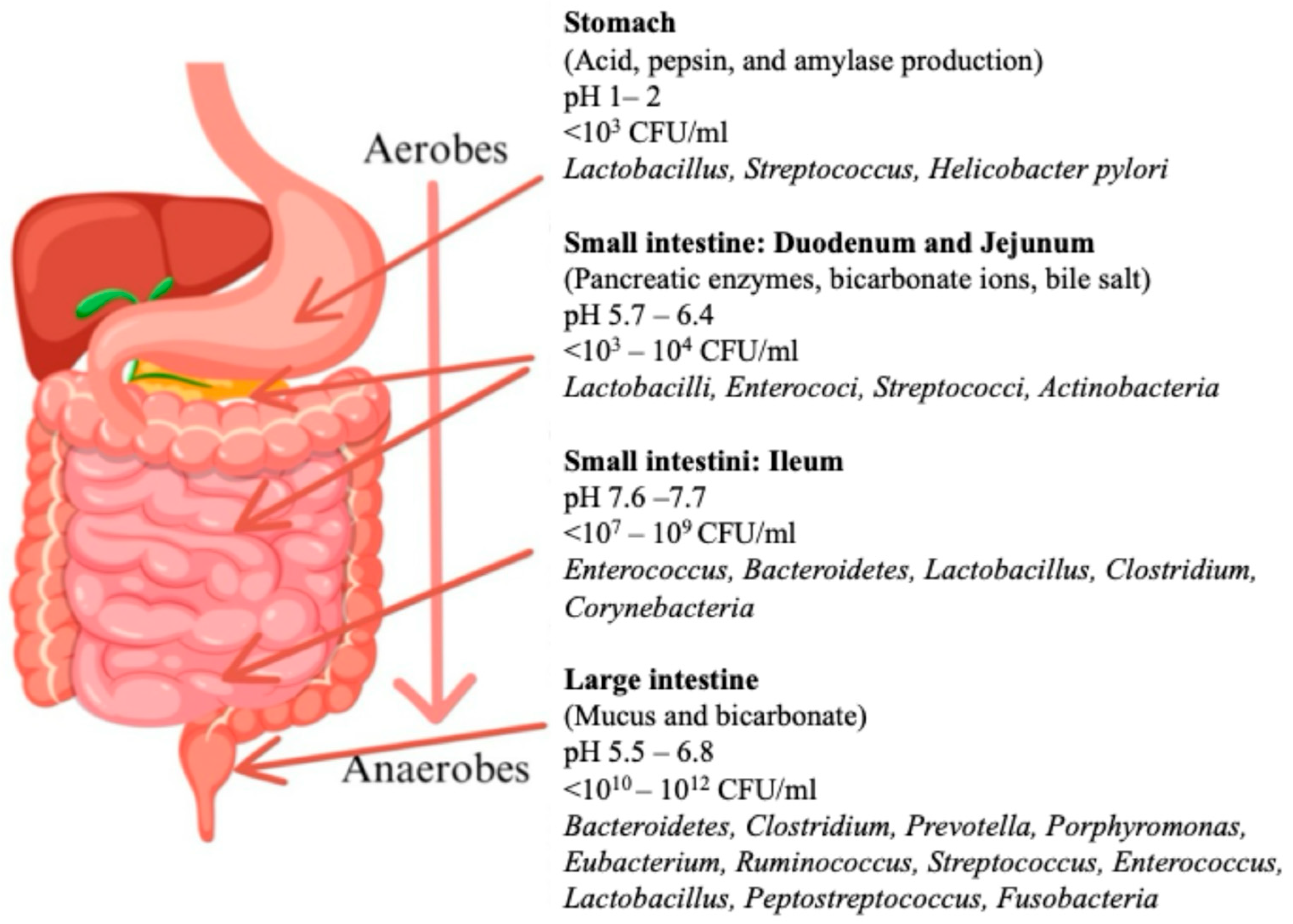
3.1. Intestinal Microbiota
3.2. Diabetes and the Microbiota in Mexico
3.3. Obesity and the Microbiota in Mexico
4. Interventions Aimed at Restoring the Microbiota
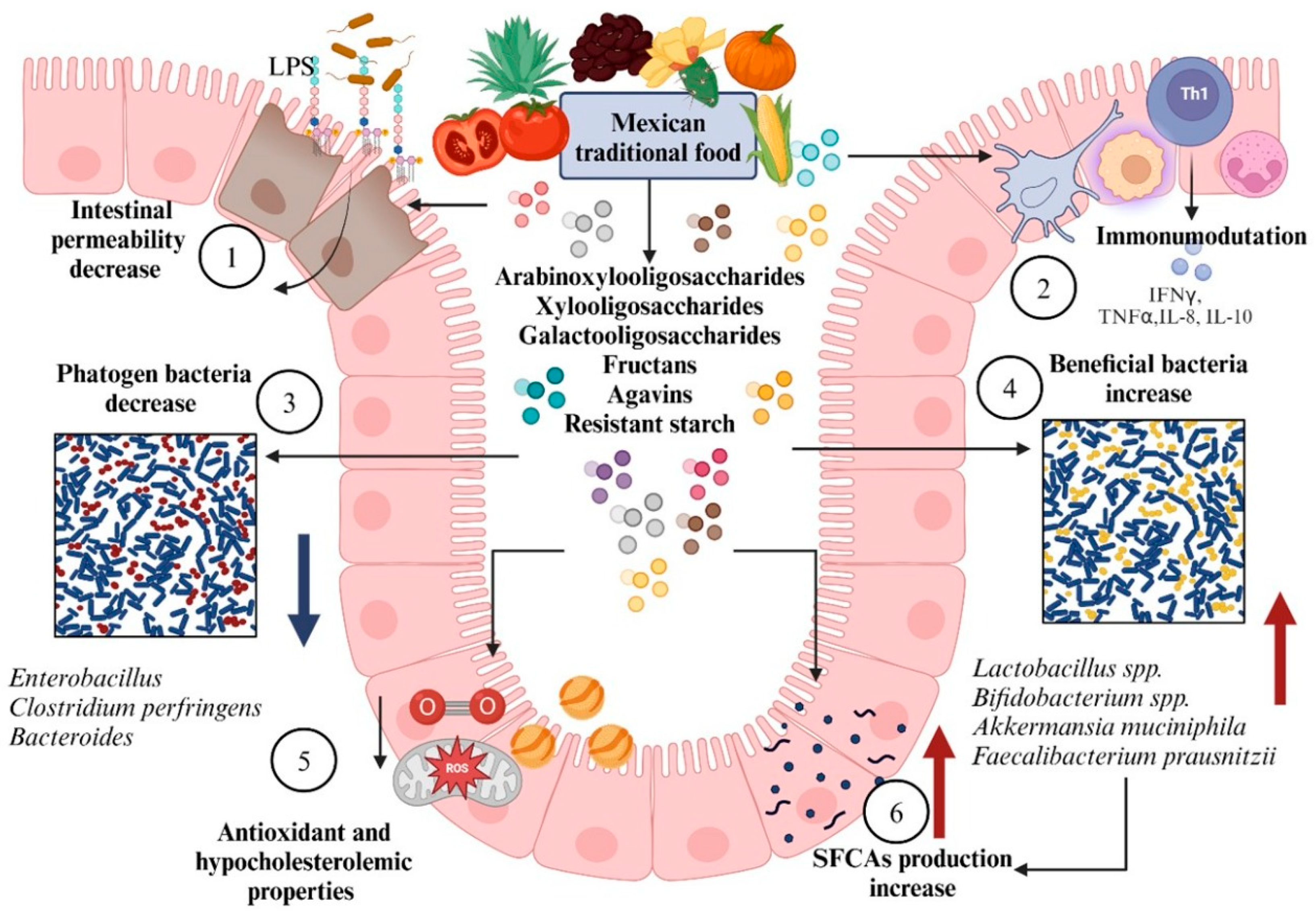
4.1. Diet Modification
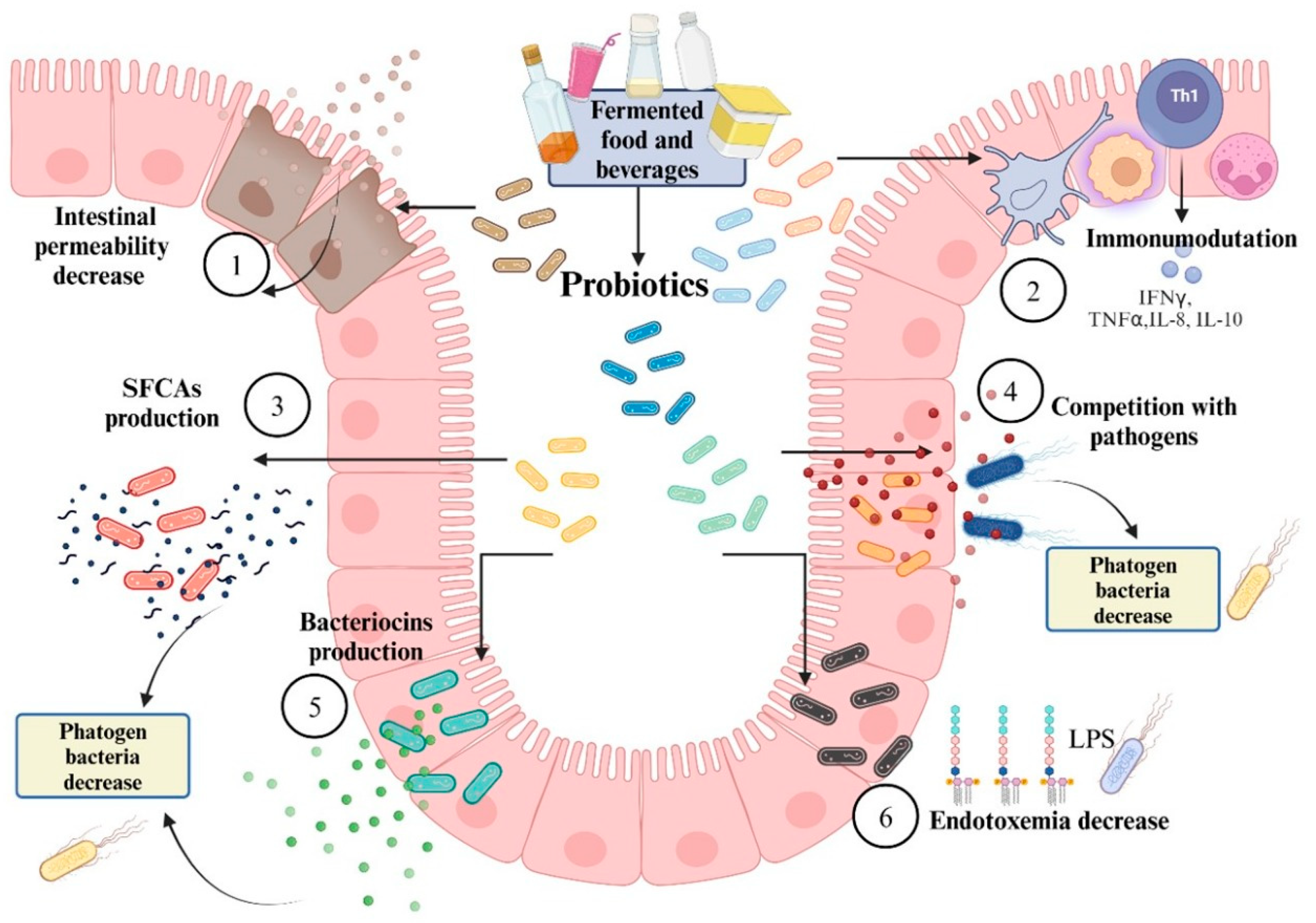
4.2. Incorporation of Probiotics
5. Challenges and Future Directions
- Genomic and microbiota research: More detailed studies on how human genes interact with the gut microbiota are essential. Advances in identifying genetic biomarkers that can predict susceptibility to obesity and diabetes are needed, as well as understanding how these biomarkers are related to the composition of the gut microbiota.
- Personalized interventions: The future of probiotic and prebiotic therapies is rooted in personalization. Advances in precision medicine will enable interventions to be tailored specifically to each individual’s microbiological and genetic traits. This research will also involve the development of data-driven treatment strategies, where gut microbiota studies are integrated with genetic and health data to customize the therapeutic approach.
- Dietary and synbiotic treatments: Diet and synbiotic supplements are anticipated to play crucial roles in managing obesity and diabetes, although optimal combinations for each individual need to be identified. Long-term intervention studies are necessary to evaluate the impact and sustained effectiveness of these treatments.
- Integrative approach: In addition to the microbiota, further research is necessary to understand how additional factors, including socioeconomic status, lifestyle, and patient psychology, influence the gut microbiota and, in turn, obesity and diabetes. An integrative approach that takes all these factors into account will be vital for developing more comprehensive and effective prevention and treatment strategies.
- Advancing the development of new animal and clinical models is essential. Studies involving animal and human models must progress to more accurately replicate the conditions of human patients. Innovative experimental models may provide more detailed data on how the microbiota interacts with metabolism and diseases associated with metabolic syndrome.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| BMI | Body Mass Index |
| CNS | Central Nervous System |
| CFU | Colony Forming Units |
| ENSANUT | National Health and Nutrition Survey |
| FMT | Fecal Microbiota Transplantation |
| GDM | Gestational Diabetes |
| GLUT4 | Glucose Transporters Group 4 |
| HRQoL | Health-Related Quality of Life |
| IDF | International Diabetes Federation |
| LPS | Lipopolysaccharide |
| NGS | Next-Generation Sequencing |
| PAHO | PanAmerican Health Organization |
| SCFAs | Short Chain Fatty Acids |
| T1D | Type 1 Diabetes |
| T2D | Type 2 Diabetes |
| WHO | Worl Health Organization |
References
- Noyola, D.E.; Hermosillo-Arredondo, N.; Ramírez-Juárez, C.; Werge-Sánchez, A. Association between obesity and diabetes prevalence and COVID-19 mortality in Mexico: An ecological study. J. Infect. Dev. Ctries. 2021, 15, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Medina, M.S. Overview of the overweight and obesity epidemic in Mexico. Mex. J. Med. Res. 2020, 8, 65–71. [Google Scholar] [CrossRef]
- Basto-Abreu, A.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; Moreno-Banda, G.L.; Carnalla, M.; Rivera, J.A.; Romero-Martinez, S.B.; Barrientos-Gutierrez, T. Prevalencia de prediabetes y diabetes en México: Ensanut 2022. Salud Publica Mex. 2023, 65, 163–168. [Google Scholar] [CrossRef]
- Huang, A.A.; Huang, S.Y. Diabetes is associated with increased risk of death in COVID-19 hospitalizations in Mexico 2020: A retrospective cohort study. Health Sci. Rep. 2023, 6, e1416. [Google Scholar] [CrossRef]
- Campos-Nonato, I.; Ramírez-Villalobos, M.; Flores-Coria, A.; Valdez, A.; Monterrubio-Flores, E. Prevalence of previously diagnosed diabetes and glycemic control strategies in Mexican adults: ENSANUT-2016. PLoS ONE 2020, 15, e0230752. [Google Scholar] [CrossRef]
- Calcaterra, V.; Vandoni, M.; Marin, L.; Carnevale Pellino, V.; Rossi, V.; Gatti, A.; Patané, P.; Cavallo, C.; Albanese, F.R.; Silvestri, D.; et al. Exergames to limit weight gain and to fight sedentarism in children and adolescents with Obesity. Children 2023, 10, 928. [Google Scholar] [CrossRef]
- Guevara-Ramírez, P.; Cadena-Ullauri, S.; Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Paz-Cruz, E.; Simancas-Racines, D.; Zambrano, A.K. Genetics, genomics, and diet interactions in obesity in the Latin American environment. Front. Nutr. 2022, 9, 1063286. [Google Scholar] [CrossRef]
- Denova-Gutiérrez, E.; Vargas-Chanes, D.; Hernández, S.; Muñoz-Aguirre, P.; Napier, D.; Barquera, S. Linking socioeconomic inequalities and type 2 diabetes through obesity and lifestyle factors among Mexican adults: A structural equations modeling approach. Salud Publica Mex. 2020, 62, 192. [Google Scholar] [CrossRef]
- Pitocco, D.; Di Leo, M.; Tartaglione, L.; De Leva, F.; Petruzziello, C.; Saviano, A.; Pontecovi, A.; Ojetti, V. The role of gut microbiota in mediating obesity and diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1548–1562. [Google Scholar] [PubMed]
- Napolitano, M.; Covasa, M. Microbiota transplant in the treatment of obesity and diabetes: Current and future perspectives. Front. Microbiol. 2020, 11, 590370. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Stirling, K.; Yang, J.J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut microbiome composition in obese and non-obese persons: A systematic review and meta-analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Umirah, F.; Neoh, C.F.; Ramasamy, K.; Lim, S.M. Differential gut microbiota composition between type 2 diabetes mellitus patients and healthy controls: A systematic review. Diabetes Res. Clin. Pract. 2021, 173, 108689. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the Firmicutes/Bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Arfan Ikram, M.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of insulin resistance and type 2 diabetes with gut microbial diversity. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Jess, T.; Jensen, B.W.; Andersson, M.; Villumsen, M.; Allin, K.H. Inflammatory bowel diseases increase risk of type 2 diabetes in a nationwide cohort study. Clin. Gastroenterol. Hepatol. 2020, 18, 881–888.e1. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.B.; Cruz-Guerrero, A.E.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; Calderón-Ramos, Z.G.; Arias-Rico, J.; et al. Impact of the gut microbiota balance on the health–disease relationship: The importance of consuming probiotics and prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Le Roux, C.W.; Cohen, R.V. There is no magic bullet for obesity. Lancet Diabetes Endocrinol. 2023, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. The definition of obesity. Korean J. Fam. Med. 2016, 37, 309. [Google Scholar] [CrossRef][Green Version]
- de Salud, S. NORMA Oficial Mexicana NOM-043-SSA2-2012, Servicios básicos de salud. Promoción y educación para la salud en materia alimentaria. Criterios para brindar orientación. 2013. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5285372&fecha=22/01/2013#gsc.tab=0[M1] (accessed on 12 August 2025).
- Meneses-Sierra, E.; Ochoa-Martínez, C.; Burciaga-Jimenéz, E.; Gómez-Mendoza, R.; Salgado-Loza, L.; Peña-Arriaga, T.; Diaz-Aguilera, M.; Burguete-Garcia, A.I.; Márquez-González, S.M.; Zavala-Cruz, G.G.; et al. Abordaje multidisciplinario del sobrepeso y la obesidad en adultos. Med. Int. Mex. 2023, 39, 329–366. [Google Scholar]
- Reyes-Sánchez, F.; Basto-Abreu, A.; Torres-Álvarez, R.; Carnalla-Cortés, M.; Reyes-García, A.; Swinburn, B.; Meza, R.; Rivera, J.A.; Popkin, B.; Barrientos-Guitiérrez, T. Caloric reductions needed to achieve obesity goals in Mexico for 2030 and 2040: A modeling study. PLoS Med. 2023, 20, e1004248. [Google Scholar] [CrossRef]
- Magallón-Zertuche, V.; Garrido-Dzib, A.G.; Salazar-Gonzalez, E.; González-Castro, D.G.; Chávez-Loría, G.; Avila-Nava, A.; Gutierrez-Solis, A.L. A systematic review and meta-analysis on the prevalence of mild cognitive impairment and dementia in Mexico. Dement. Geriatr. Cogn. Disord. 2024, 53, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, M.D.; Meincke, H.; Le Lay, A.; Fournier, J.; Bakker, E.; Ehrenreich, A. Obesity in Mexico: Prevalence, comorbidities, associations with patient outcomes, and treatment experiences. Diabetes Metab. Syndr. Obes. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Med. Hosp. Gen. Mex. 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Flores, Y.N.; Contreras, Z.A.; Ramírez-Palacios, P.; Morales, L.S.; Edwards, T.C.; Gallegos-Carrillo, K.; Salmerón, J.; Lang, C.M.; Sportiche, N.; Patrick, D.L. Increased prevalence of psychosocial, behavioral, and socio-environmental risk factors among overweight and obese youths in Mexico and the United States. Int. J. Environ. Res. Public Health 2019, 16, 1534. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.A.; Barquera, S.; Campirano, F.; Campos, I.; Safdie, M.; Tovar, V. Epidemiological and nutritional transition in Mexico: Rapid increase of non-communicable chronic diseases and obesity. Public Health Nutr. 2002, 5, 113–122. [Google Scholar] [CrossRef]
- Ojeda-Granados, C.; Panduro, A.; Rivera-Iñiguez, I.; Sepúlveda-Villegas, M.; Roman, S. A regionalized genome-based mexican diet improves anthropometric and metabolic parameters in subjects at risk for obesity-related chronic diseases. Nutrients 2020, 12, 645. [Google Scholar] [CrossRef]
- Trogdon, J.G.; Finkelstein, E.A.; Hylands, T.; Dellea, P.S.; Kamal-Bahl, S.J. Indirect costs of obesity: A review of the current literature. Obes. Rev. 2008, 9, 489–500. [Google Scholar] [CrossRef]
- Cazares-Manríquez, M.A.; Wilson, C.C.; Vardasca, R.; García-Alcaraz, J.L.; Olguín-Tiznado, J.E.; López-Barreras, J.A.; García-Rivera, B.R. A review of carpal tunnel syndrome and its association with age, body mass index, cardiovascular risk factors, hand dominance, and sex. Appl. Sci. 2020, 10, 3488. [Google Scholar] [CrossRef]
- Mateos-Valenzuela, A.G.; González-Macías, M.E.; Ahumada-Valdez, S.; Villa-Angulo, C.; Villa-Angulo, R. Risk factors and association of body composition components for lumbar disc herniation in Northwest, Mexico. Sci. Rep. 2020, 10, 18479. [Google Scholar] [CrossRef]
- Badial, L.J.P.; Badial, A.A. Corelation between severity of obstructive sleep apnea syndrome and body mass index and tonsil size. An. Otorrinolaringol. Mex. 2011, 56, 174–179. [Google Scholar]
- Sánchez-Román, S.; López-Alvarenga, J.C.; Vargas-Martínez, A.; Téllez-Zenteno, J.F.; Vázquez-Velázquez, V.; Arcila-Martínez, D.; González-Blanco, J.; Herrera-Hernández, M.F.; Salín-Pascual, R.J. Prevalence of psychiatric disorders in patients with severe obesity waiting for bariatric surgery. Rev. Investig. Clin. 2003, 55, 400–406. [Google Scholar]
- Opel, N.; Thalamuthu, A.; Milaneschi, Y.; Grotegerd, D.; Flint, C.; Leenings, R.; Goltermann, J.; Ritcher, M.; Hahn, T.; Woditsch, G.; et al. Brain structural abnormalities in obesity: Relation to age, genetic risk, and common psychiatric disorders. Mol. Psychiatry 2021, 26, 4839–4852. [Google Scholar] [CrossRef] [PubMed]
- Aceves-Martins, M.; López-Cruz, L.; García-Botello, M.; Gutierrez-Gómez, Y.Y.; Moreno-García, C.F. Interventions to treat obesity in Mexican children and adolescents: Systematic review and meta-analysis. Nutr. Rev. 2022, 80, 544–560. [Google Scholar] [CrossRef]
- James, E.; Lajous, M.; Reich, M.R. The politics of taxes for health: An analysis of the passage of the sugar-sweetened beverage tax in Mexico. Health Syst. Reform 2020, 6, e1669122. [Google Scholar] [CrossRef] [PubMed]
- Lazarevich, I.; Irigoyen-Camacho, M.E.; Velázquez-Alva, M.C. Obesity, eating behaviour and mental health among university students in Mexico City. Nutr Hosp. 2013, 28, 1892–1899. [Google Scholar]
- Fernández Carrasco, M.P. Relation between eating habits and risk of developing diabetes in Mexican university students. Nutr. Clín. Diet. Hosp. 2020, 4, 32–40. [Google Scholar] [CrossRef]
- Asociación latinoamericana de Diabetes (ALAD). Guías ALAD sobre el Diagnóstico, Control y Tratamiento de la Diabetes Mellitus Tipo 2 con Medicina Basada en Evidencia Edición 2019. 2019, pp. 11–22. Available online: https://www.revistaalad.com/guias/5600AX191_guias_alad_2019.pdf (accessed on 17 July 2025).
- International Diabetes Federation (IDF). IDF Diabetes Atlas 10th edition. 2021, pp. 14–41. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf (accessed on 23 January 2025).
- Maedler, K. Beta cells in type 2 diabetes—A crucial contribution to pathogenesis. Diabetes Obes. Metab. 2008, 10, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global etiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Global Burden of Diseases Study (GBD) 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation (IDF). IDF Diabetes Atlas 10th edition. 2021, pp. 14–18. Available online: https://fmdiabetes.org/wp-content/uploads/2022/01/IDF_Atlas_10th_Edition_2021-comprimido.pdf (accessed on 10 February 2025).
- Basto-Abreu, A.C.; López-Olmedo, N.; Rojas-Martínez, R.; Aguilar-Salinas, C.A.; De la Cruz-Góngora, V.V.; Rivera-Dommarco, J.; Shamah-Levy, T.; Romero-Martínez, M.; Barquera, S.; Vallapando, S.; et al. Prevalence of diabetes and glycemic control in Mexico: National results from 2018 and 2020. Salud Publica Mex. 2021, 63, 725–733. [Google Scholar] [CrossRef]
- Seiglie, J.A.; Franco, R.R.; Wirtz, V.J.; Meigs, J.B.; Mendoza, M.A.; Miranda, J.J.; Gómez-Dantés, H.; Lozano, R.; Wexler, D.J.; Serván-Mori, E. Regional and state-level patterns of type 2 diabetes prevalence in Mexico over the last three decades. Diabetes Res. Clin. Pract. 2021, 177, 108927. [Google Scholar] [CrossRef]
- Barbiellini Amidei, C.; Fayosse, A.; Dumurgier, J.; Machado-Fragua, M.D.; Tabak, A.G.; van Sloten, T.; Kivimäki, M.; Dugravot, A.; sabia, S.; Singh-Manoux, A. Association between age at diabetes onset and subsequent risk of dementia. JAMA 2021, 325, 1640. [Google Scholar] [CrossRef]
- Bellary, S.; Kyrou, I.; Brown, J.E.; Bailey, C.J. Type 2 diabetes mellitus in older adults: Clinical considerations and management. Nat. Rev. Endocrinol. 2021, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Lora, A.L.; Vilchis-Gil, J.; Juárez-Comboni, D.B.; Cruz, M.; Klünder-Klünder, M. A genetic risk score improves the prediction of type [2] diabetes mellitus in Mexican youths but has lower predictive utility compared with non-genetic factors. Front. Endocrinol. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Quiroz, L.E.; Roman, S. Influence of genetic and environmental risk factors in the development of hepatocellular carcinoma in Mexico. Ann Hepatol. 2022, 27, 100649. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, M.A.; Bautista-Ortiz, L.F.; Villa, A.R.; Antúnez-Bautista, P.K.; Aldaz-Rodríguez, M.V.; Estrada-Luna, D.; Denova-Gutierrez, E.; Camacho-Diaz, B.H.; Martinez-Salazar, M.F. Prevalence of metabolic syndrome among Mexican adults. Metab. Syndr. Relat. Disord. 2022, 20, 264–272. [Google Scholar]
- Ko, J.; Skudder-Hill, L.; Cho, J.; Bharmal, S.H.; Petrov, M.S. The relationship between abdominal fat phenotypes and insulin resistance in non-obese individuals after acute pancreatitis. Nutrients 2020, 12, 2883. [Google Scholar] [CrossRef]
- Fowler, J.R.; Tucker, L.A.; Bailey, B.W.; Le Cheminant, J.D. Physical activity and insulin resistance in 6,500 NHANES adults: The role of abdominal obesity. J. Obes. 2020, 2020, 3848256. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; Bielemann, R.M.; da Silva, B.G.C.; dos Santos, F.S.; Mintem, G.C.; Flores, T.R.; Arcencio, R.A.; Nunes, B.P. Ultra-processed food and risk of type 2 diabetes: A systematic review and meta-analysis of longitudinal studies. Int. J. Epidemiol. 2022, 51, 1120–1141. [Google Scholar] [CrossRef]
- Li, D.D.; Yang, Y.; Gao, Z.Y.; Zhao, L.H.; Yang, X.; Xu, F.; Yu, C.; Zhang, X.L.; Wang, X.Q.; Wang, L.H.; et al. Sedentary lifestyle and body composition in type 2 diabetes. Diabetol. Metab. Syndr. 2022, 14, 8. [Google Scholar] [CrossRef]
- Howland, C.; Wakefield, B. Assessing telehealth interventions for physical activity and sedentary behavior self-management in adults with type 2 diabetes mellitus: An integrative review. Res. Nurs. Health 2021, 44, 92–110. [Google Scholar] [CrossRef]
- Castro-Porras, L.V.; Rojas-Martínez, R.; Romero-Martínez, M.; Aguilar-Salinas, C.A.; Escamilla-Nuñez, C. The trend in the prevalence of diabetes mellitus in the Mexican indigenous population from 2000 to 2018. AJPM Focus. 2023, 2, 100087. [Google Scholar] [CrossRef]
- Parra-Rodríguez, L.; González-Meljem, J.M.; Gómez-Dantés, H.; Gutiérrez-Robledo, L.M.; López-Ortega, M.; García-Peña, C.; Medina-campos, R.H. The burden of disease in Mexican older adults: Premature mortality challenging a limited-resource health system. J. Aging Health 2020, 32, 543–553. [Google Scholar] [CrossRef]
- Salinas, M.A. Cost of diabetes treatment in Mexico. Mex. J. Med. Res. 2021, 9, 16–21. [Google Scholar] [CrossRef]
- Sagaceta-Mejía, J.; Tolentino-Mayo, L.; Cruz-Casarrubias, C.; Nieto, C.; Barquera, S. Understanding of front of package nutrition labels: Guideline daily amount and warning labels in Mexicans with non-communicable diseases. PLoS ONE 2022, 17, e0269892. [Google Scholar] [CrossRef]
- White, M.; Barquera, S. Mexico adopts food warning labels, why now? Health Syst. Reform. 2020, 6, e1752063. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. 2022, 26, 13. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 2020, 24, 10. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Yoo, J.; Groer, M.; Dutra, S.; Sarkar, A.; McSkimming, D. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Sankarasubramanian, J.; Ahmad, R.; Avuthu, N.; Singh, A.B.; Guda, C. Gut microbiota and metabolic specificity in ulcerative colitis and crohn’s disease. Front. Med. 2020, 7, 606298. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal microbiota transplantation and other gut microbiota manipulation strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef]
- Kim, C.Y.; Ma, J.; Lee, I. HiFi metagenomic sequencing enables assembly of accurate and complete genomes from human gut microbiota. Nat. Commun. 2022, 13, 6367. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, K.; Li, S.; Zhang, X.; Zhao, Q.; Zhao, X.; Liu, Z.; Chang, H.; Liu, Z.X.; Li, X. GutMEGA: A database of the human gut MEtaGenome Atlas. Brief Bioinform. 2021, 22, bbaa082. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chi, L.; Zhu, Y.; Shi, X.; Tu, P.; Li, B.; Yin, J.; Gao, N.; Shen, W.; Schnabl, B. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules 2021, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Schnabl, B.; Knight, R.; Loomba, R. Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab. 2021, 33, 21–32. [Google Scholar] [CrossRef]
- Potter, K.; Gayle, E.J.; Deb, S. Effect of gut microbiome on serotonin metabolism: A personalized treatment approach. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2589–2602. [Google Scholar] [CrossRef]
- Carrizales-Sánchez, A.K.; Tamez-Rivera, O.; Rodríguez-Gutiérrez, N.A.; Elizondo-Montemayor, L.; Gradilla-Hernández, M.S.; García-Rivas, G.; Pacheco, A.; Senés-Guerrero, C. Characterization of gut microbiota associated with metabolic syndrome and type-2 diabetes mellitus in Mexican pediatric subjects. BMC Pediatr. 2023, 23, 210. [Google Scholar] [CrossRef]
- Mejía-León, M.; Barca, A. Diet, microbiota and immune system in type 1 diabetes development and evolution. Nutrients 2015, 7, 9171–9184. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Lopez-Contreras, B.E.; Villarruel-Vazquez, R.; Ocampo-Medina, E.; Macias-Kauffer, L.; Martinez-Medina, J.N.; Villamil-Ramirez, H.; León-Mimila, P.; Del Rio-Navarro, B.; Ibarra-Gonzalez, I. Environmental and intrinsic factors shaping gut microbiota composition and diversity and its relation to metabolic health in children and early adolescents: A population-based study. Gut Microb. 2020, 11, 900–917. [Google Scholar] [CrossRef]
- García-Mena, J.; Corona-Cervantes, K.; Cuervo-Zanatta, D.; Benitez-Guerrero, T.; Vélez-Ixta, J.M.; Zavala-Torres, N.G.; Villalobos-Flores, L.E.; Hernández-Quiroz, F.; Perez-Cruz, C.; Murugesan, S.; et al. Gut microbiota in a population highly affected by obesity and type 2 diabetes and susceptibility to COVID-19. World J. Gastroenterol. 2021, 27, 7065–7079. [Google Scholar] [CrossRef]
- Yamamoto, S.; Saito, M.; Tamura, A.; Prawisuda, D.; Mizutani, T.; Yotsuyanagi, H. The human microbiome and COVID-19: A systematic review. PLoS ONE 2021, 16, e0253293. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Bahena-López, J.P.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; González-Díaz, A.; Márquez-Salinas, A.; Fermín-Martínez, C.A.; Naveja, J.J.; Aguilar-Salinas, C.A. Predicting mortality due to SARS-CoV-2: A mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J. Clin. Endocrinol. Metab. 2020, 105, 2752–2761. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Pizano-Zárate, M.L.; Hernández-Quiroz, F.; Ortiz-Luna, G.F.; Morales-Hernández, R.M.; De Sales-Millán, A.; Hernández-Trejo, M.; García-Vite, A.; Beltrán-Lagunes, L.; Hoyo-Vadillo, C.; et al. Characterization of the gut microbiota of individuals at different T2D stages reveals a complex relationship with the Host. Microorganisms 2020, 8, 94. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; Diaz-Perdigones, C.; Tinahones, F.J. Microbiota y diabetes mellitus tipo 2. Endocrinol. Nutr. 2016, 63, 560–568. [Google Scholar] [CrossRef]
- Diener, C.; Reyes-Escogido, M.L.; Jimenez-Ceja, L.M.; Matus, M.; Gomez-Navarro, C.M.; Chu, N.D.; Zhong, V.; Tejero, M.E.; Alm, E.; Resendis-Antonio, O.; et al. Progressive shifts in the gut microbiome reflect prediabetes and diabetes development in a treatment-naive Mexican cohort. Front. Endocrinol. 2021, 11, 602326. [Google Scholar] [CrossRef]
- Lizárraga, D.; García-Gasca, A.; García-Gasca, T.; Lund, G.; Guerrero, A.; Peraza-Manjarrez, E.; Gómez-Gil, B. A pilot study on the fecal microbiota in Mexican women with gestational diabetes mellitus and their newborns. Diabetology 2024, 5, 464–475. [Google Scholar] [CrossRef]
- Gámez-Valdez, J.S.; García-Mazcorro, J.F.; Montoya-Rincón, A.H.; Rodríguez-Reyes, D.L.; Jiménez-Blanco, G.; Rodríguez, M.T.A.; Pérez-Cabeza de Vaca, R.; Alcorta-García, M.R.; Brunk, M.; Lara-Diaz, V.J.; et al. Differential analysis of the bacterial community in colostrum samples from women with gestational diabetes mellitus and obesity. Sci. Rep. 2021, 11, 24373. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Guerrero, T.; Vélez-Ixta, J.; Juárez-Castelán, C.; Corona-Cervantes, K.; Piña-Escobedo, A.; Martínez-Corona, H.; De Sales-Millán, A.; Cruz-Narvaez, Y.; Gómez-Cruz, C.Y.; Ramírez-Lozada, T.; et al. Gut microbiota associated with gestational health conditions in a sample of Mexican women. Nutrients 2022, 14, 4818. [Google Scholar] [CrossRef]
- Fetissov, S.O.; Lucas, N.; Legrand, R. Ghrelin-reactive immunoglobulins in conditions of altered appetite and energy balance. Front. Endocrinol. 2017, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, H.; Dinan, T.G.; Cryan, J.F. Lean mean fat reducing “ghrelin” machine: Hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology 2010, 58, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. Obesity wars. Cell 2004, 116, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtif, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-Del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut microbiota and predicted metabolic pathways in a sample of Mexican women affected by obesity and obesity plus metabolic syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- Riggen-Bueno, V.; Del Toro-Arreola, S.; Baltazar-Díaz, T.A.; Vega-Magaña, A.N.; Peña-Rodríguez, M.; Castaño-Jiménez, P.A.; Sánchez-Orozco, L.V.; Vera-Cruz, J.M.; Bueno-Topete, M.R. Intestinal dysbiosis in subjects with obesity from western Mexico and its association with a proinflammatory profile and disturbances of folate (B9) and carbohydrate metabolism. Metabolites 2024, 14, 121. [Google Scholar] [CrossRef]
- Radilla-Vázquez, R.B.; Parra-Rojas, I.; Martínez-Hernández, N.E.; Márquez-Sandoval, Y.F.; Illades-Aguiar, B.; Castro-Alarcón, N. Gut microbiota and metabolic endotoxemia in young obese Mexican subjects. Obes. Facts 2016, 9, 1–11. [Google Scholar] [CrossRef]
- Valsecchi, C.; Carlotta Tagliacarne, S.; Castellazzi, A. Gut microbiota and obesity. J. Clin. Gastroenterol. 2016, 50 (Suppl. 2), 157–158. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. A review on gut microbiota: A central factor in the pathophysiology of obesity. Lipids Health Dis. 2021, 20, 65. [Google Scholar] [CrossRef]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.M.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A. Gut microbiota: A new path to treat obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Lankelma, J.M.; Nieuwdorp, M.; de Vos, W.M.; Wiersinga, W.J. The gut microbiota in internal medicine: Implications for health and disease. Neth. J. Med. 2015, 73, 61–68. [Google Scholar]
- Guevara-Cruz, M.; Flores-López, A.G.; Aguilar-López, M.; Sánchez-Tapia, M.; Medina-Vera, I.; Díaz, D.; Tovar, A.R.; Torres, N. Improvement of lipoprotein profile and metabolic endotoxemia by a lifestyle intervention that modifies the gut microbiota in subjects with metabolic syndrome. J. Am. Heart Assoc. 2019, 8, e012401. [Google Scholar] [CrossRef]
- Abreu y Abreu, A.T.; Milke-García, M.P.; Argüello-Arévalo, G.A.; Calderón-de la Barca, A.M.; Carmona-Sánchez, R.I.; Consuelo-Sánchez, A.; Coss-Adame, E.; García-Cedillo, M.F.; Hernández-Rosiles, V.; Icaza-Chávez, M.E.; et al. Fibra dietaria y microbiota, revisión narrativa de un grupo de expertos de la Asociación Mexicana de Gastroenterología. Rev. Gastroenterol. Mex. 2021, 86, 287–304. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Méndez-Trujillo, V.; Hernández-Delgado, N.C.; Bermúdez-Humarán, L.G.; Reyes-Pavón, D. Looking inside Mexican traditional food as sources of synbiotics for developing novel functional products. Fermentation 2022, 8, 123. [Google Scholar] [CrossRef]
- Rodríguez-Lara, A.; Plaza-Díaz, J.; López-Uriarte, P.; Vázquez-Aguilar, A.; Reyes-Castillo, Z.; Álvarez-Mercado, A.I. Fiber consumption mediates differences in several gut microbes in a subpopulation of young Mexican adults. Nutrients 2022, 14, 1214. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A systematic review of its phytochemicals and pharmacological activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef]
- Blando, F.; Albano, C.; Jiménez-Martínez, C.; Cardador-Martínez, A. Opuntia, ficus-indica L. Mill. and other species. In Molecular Mechanisms of Functional Food; John Wiley & Sons Ltd.: Chichester, UK, 2022; pp. 193–237. [Google Scholar]
- Sánchez-Tapia, M.; Aguilar-López, M.; Pérez-Cruz, C.; Pichardo-Ontiveros, E.; Wang, M.; Donovan, S.M.; Tovar, S.M.; Torres, N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017, 7, 4716. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Avila-Nava, A.; Noriega, L.G.; Tovar, A.R.; Granados, O.; Perez-Cruz, C.; Pedraza-Chaverri, J.; Torres, N. Food combination based on a pre-hispanic Mexican diet decreases metabolic and cognitive abnormalities and gut microbiota dysbiosis caused by a sucrose-enriched high-fat diet in rats. Mol. Nutr. Food Res. 2017, 61, 1501023. [Google Scholar] [CrossRef]
- Velázquez-Martínez, J.; González-Cervantes, R.; Hernández-Gallegos, M.; Mendiola, R.; Aparicio, A.; Ocampo, M. Prebiotic potential of Agave angustifolia haw fructans with different degrees of polymerization. Molecules 2014, 19, 12660–12675. [Google Scholar] [CrossRef]
- Catry, E.; Bindels, L.B.; Tailleux, A.; Lestavel, S.; Neyrinck, A.M.; Goossens, J.F.; Lobysheva, I.; Plovier, H.; Essaghir, A.; demoulin, J.B.; et al. Targeting the gut microbiota with inulin-type fructans: Preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 2018, 67, 271–283. [Google Scholar] [CrossRef]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, V.K.; Morrow, L.E.; Gregory, P.J.; Malesker, M.A. Probiotics: History and evolution. J. Anc. Dis. Prev. Rem. 2013, 1, 107. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotech. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Campbell-Platt, G. Fermented foods—A world perspective. Food Res. Int. 1994, 27, 253–257. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhou, D.D.; Gan, R.Y.; Huang, S.Y.; Zhao, C.N.; Shang, A.; Xu, X.Y.; Li, H.B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Ojeda-Linares, C.; Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Casas, A. Traditional fermented beverages of Mexico: A biocultural unseen foodscape. Foods 2021, 10, 2390. [Google Scholar] [CrossRef] [PubMed]
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a Traditional Mexican Alcoholic Fermented Beverage: Historical, Microbiological, and Technical Aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef]
- Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Casas, A. Physical, chemical, and microbiological characteristics of pulque: Management of a fermented beverage in Michoacán, Mexico. Foods 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, M.U.; Vázquez-Maldonado, D.; Ratering, S.; Godínez-Hernández, C.; Ortiz-Basurto, R.I.; Soria-Guerra, R.E.; Schenider, B.; Juárez-Flores, B.I.; Portales-Pérez, D.P.; Schnell, S.; et al. Fructans from Agave enhance probiotic yoghurt by modulating gut microbiota on children with overweight or obesity. Food Biosci. 2022, 46, 101516. [Google Scholar] [CrossRef]
- Cruz-Mora, J.; Martínez-Hernández, N.E.; Martín del Campo-López, F.; Viramontes-Hörner, D.; Vizmanos-Lamotte, B.; Muñoz-Valle, J.F.; García-García, G.; Parra-Rojas, I.; Castro-Alarcón, N. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J. Ren. Nutr. 2014, 24, 330–335. [Google Scholar] [CrossRef] [PubMed]
| Underweight | Normal | Overweight | Obesity | ||
|---|---|---|---|---|---|
| Grade I | Grade II | Grade III | |||
| <18.5 | 18.5–24.9 | 25.0–29.9 | 30.0–34.9 | 35.0–39.9 | >40.0 |
| Abdominal Obesity According to the Official Mexican Standard NOM-043-SSA2-2012 | |||||
| Male | Female | ||||
| <90 cm | <80 cm | ||||
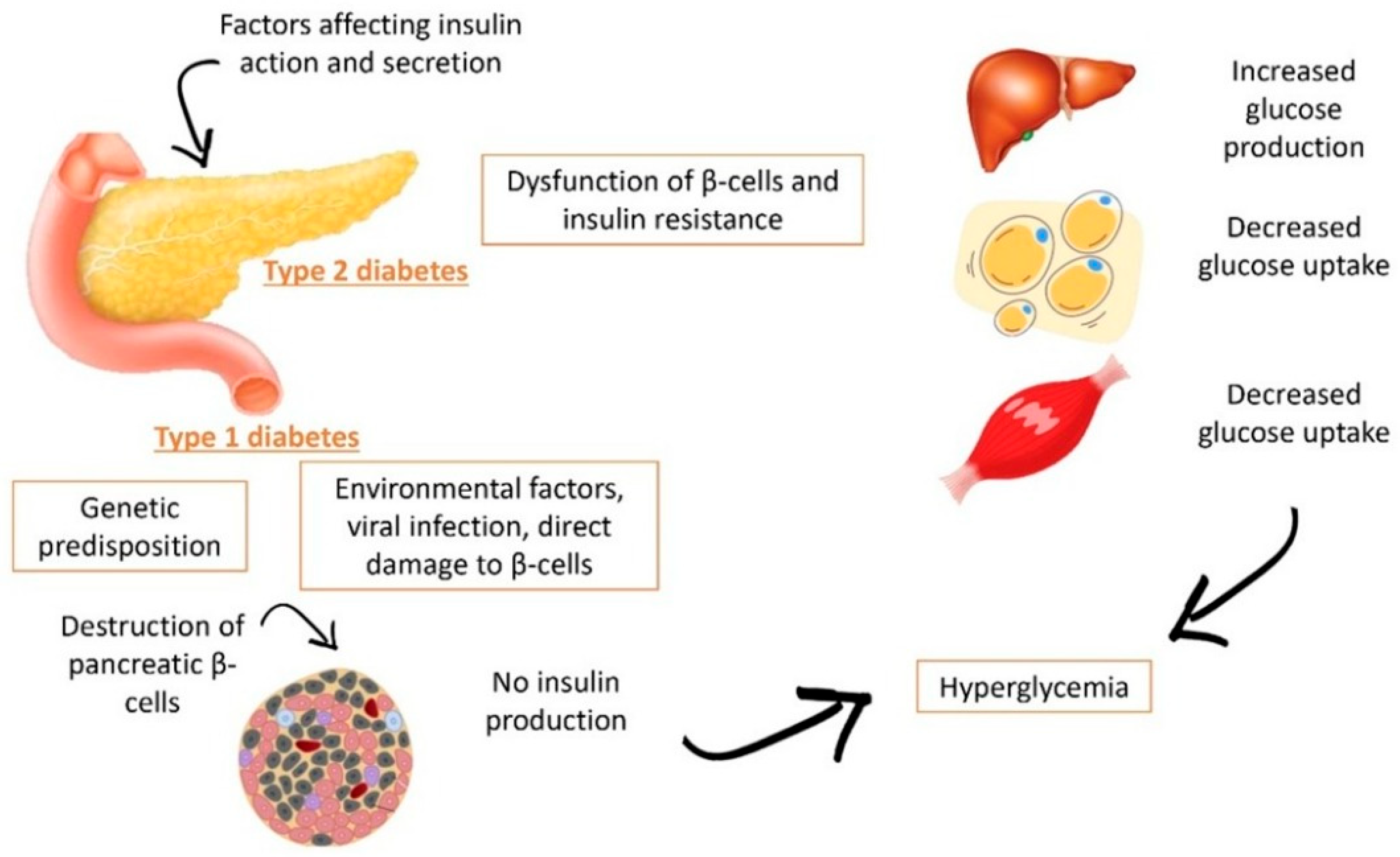
| Type | Definition | Signs and Symptoms |
|---|---|---|
| Type 1 Diabetes | There is an autoimmune destruction of the pancreatic β-cells, resulting in an absolute insulin deficiency | Polyuria, Polydipsia, Polyphagia, Excessive fatigue, Blurry vision, Cuts/bruises that do not heal easily, Weight loss (type 1), Tingling, pain, or numbness in the hands and feet (type 2) (American Diabetes Association) |
| Type 2 Diabetes | Caused by a progressive deficit in insulin secretion from the pancreatic β-cells initiated by an insulin resistance state | |
| Gestational Diabetes | Diagnosed in the second or third month of pregnancy and was not present before gestation | Increased thirst and the sensation of urinating |
| Specific Types of Diabetes | Such as monogenic diabetes syndromes, exocrine pancreatic diseases, and drug or chemical-induced diabetes | Not applied |
| Study | Population | Conditions Investigated | Microbiota Characteristics in Obesity | Other Relevant Findings | Reference |
|---|---|---|---|---|---|
| Cross-sectional analytical study | 67 Mexican women aged 18 to 59 years, without antibiotic treatment | Obesity and obesity plus Mets | The Firmicutes phylum was more abundant in women with obesity. Additionally, a higher number of Faecalibacterium spp. taxa from the Ruminococcaceae family were observed in this group. | Predicted functional pathways associated with MetS: altered carbohydrate metabolism, endotoxemia, and inflammatory pathways | [99] |
| Comparative cross-sectional study | 65 male and female Mexican volunteers aged 18–59 years with BMI > 29.9 kg/m2 | Obesity and intestinal dysbiosis | The Proteobacteria/Firmicutes ratio significantly increased in the obesity group, with a predominance of aerobic and Gram-negative bacteria. The class Negativicutes and the genus Lachnoclostridium were associated with the obesity group, along with the Streptococcaceae family (order Lactobacillales) and Enterobacteriaceae. | Associations between dysbiosis, pro-inflammatory profile, folate (B9) disturbances, and altered carbohydrate metabolism. | [100] |
| Comparative analytical study | 64 young Mexican volunteers, without prior medical treatment with antibiotics, probiotic or prebiotic supplementation, or chronic diseases | Obesity and metabolic inflammation | Young individuals with obesity had a lower total bacterial count compared to the normal BMI group. Specifically, those with obesity showed higher amounts of Clostridium leptum and Lactobacillus, and lower amounts of Prevotella and Escherichia coli. | Endotoxemia correlated with IL-6 and other inflammatory markers. Early obesity linked to systemic low-grade inflammation. | [101] |
| Case–control studies | 66 subjects aged 7 to 17 years with T2D and metabolic syndrome, divided into three groups: (a) T2D (21), (b) metabolic syndrome (25), and (c) controls (20) | Metabolic syndrome and type 2 diabetes in children | The T2D group showed a peculiar presence of Succinibrionaceae. Another abundant genus in both T2D and metabolic syndrome was Prevotella. Specifically, for the T2D group, two genera were found in lower abundances: Lactobacillus and Succinivibrio. | Metabolic alterations associated with early inflammatory profiles. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Meneses, R.M.; Ramírez-Moreno, E.; Olvera-Rosales, L.B.; Cardelle-Cobas, A.; Calderón-Ramos, Z.G.; Rodríguez-Serrano, G.M.; Franco-Abuín, C.M.; Cepeda-Saéz, A.; González-Olivares, L.G.; Mondragón-Portocarrero, A.d.C. Obesity and Diabetes in Mexico: An Approach to the Intestinal Microbiota. Nutrients 2025, 17, 3661. https://doi.org/10.3390/nu17233661
Acosta-Meneses RM, Ramírez-Moreno E, Olvera-Rosales LB, Cardelle-Cobas A, Calderón-Ramos ZG, Rodríguez-Serrano GM, Franco-Abuín CM, Cepeda-Saéz A, González-Olivares LG, Mondragón-Portocarrero AdC. Obesity and Diabetes in Mexico: An Approach to the Intestinal Microbiota. Nutrients. 2025; 17(23):3661. https://doi.org/10.3390/nu17233661
Chicago/Turabian StyleAcosta-Meneses, Ruth Michelle, Esther Ramírez-Moreno, Laura Berenice Olvera-Rosales, Alejandra Cardelle-Cobas, Zuli Guadalupe Calderón-Ramos, Gabriela Mariana Rodríguez-Serrano, Carlos Manuel Franco-Abuín, Alberto Cepeda-Saéz, Luis Guillermo González-Olivares, and Alicia del Carmen Mondragón-Portocarrero. 2025. "Obesity and Diabetes in Mexico: An Approach to the Intestinal Microbiota" Nutrients 17, no. 23: 3661. https://doi.org/10.3390/nu17233661
APA StyleAcosta-Meneses, R. M., Ramírez-Moreno, E., Olvera-Rosales, L. B., Cardelle-Cobas, A., Calderón-Ramos, Z. G., Rodríguez-Serrano, G. M., Franco-Abuín, C. M., Cepeda-Saéz, A., González-Olivares, L. G., & Mondragón-Portocarrero, A. d. C. (2025). Obesity and Diabetes in Mexico: An Approach to the Intestinal Microbiota. Nutrients, 17(23), 3661. https://doi.org/10.3390/nu17233661










