Abstract
Background/Objectives: Evidence regarding the associations between coffee and tea consumption and bone mineral density (BMD) in postmenopausal women remains inconclusive. Prior studies have not examined these relationships using repeated measures of both beverage intake and BMD over an extended follow-up. This study aimed to evaluate the longitudinal associations of coffee and tea consumption with BMD in older women. Methods: Data were drawn from the Study of Osteoporotic Fractures (SOF), a prospective cohort of 9704 women aged ≥65 years. Coffee and tea intake were repeatedly assessed via self-administered questionnaires at visits 2, 4, 5, and 6, spanning approximately 10 years. Femoral neck and total hip BMD were repeatedly measured by dual-energy X-ray absorptiometry. Linear mixed-effects models with random intercepts were used to estimate associations, adjusting for demographic, physical activity, comorbidities, and medication use. Nonlinear relationships were assessed using natural splines, and subgroup analyses were conducted using exposure-by-covariate interaction terms. Results: During the 10-year follow-up, tea consumption was positively associated with total hip BMD (least squares mean: 0.718 vs. 0.715 g/cm2; mean difference: 0.003; 95% CI: 0.000–0.005; p = 0.026). No significant overall association was observed on coffee consumption with femoral neck or total hip BMD. However, spline analyses suggested that consuming more than five cups of coffee per day may be associated with lower BMD. Interaction analyses indicated significant interactions between coffee and alcohol intake (p = 0.0147) and between tea consumption and BMI (p = 0.0175). Conclusions: Tea consumption was associated with higher total hip BMD in postmenopausal women, whereas excessive coffee intake (>5 cups/day) may adversely affect BMD. Coffee consumption was negatively associated with femoral neck BMD in women with higher alcohol intake, while tea consumption appeared particularly beneficial for those with obesity.
1. Introduction
Osteoporosis, characterized by low bone mineral density (BMD), is a major global public health concern affecting individuals across all ages, genders, and ethnicities [1,2]. Worldwide, approximately one in three women and one in five men aged 50 years or older experience osteoporotic fractures [3]. According to the Global Burden of Disease Study 2021, low BMD accounted for 8.32 million years lived with disability (YLDs), 17.2 million disability-adjusted life years (DALYs), and 477,000 deaths, with 71% of these deaths directly attributable to low BMD [4]. In the United States, the number of adults aged 50 years and older with low BMD at the femoral neck or lumbar spine is projected to rise by 17.2 million by 2030 [5]. Fractures related to osteoporosis impose substantial morbidity, health care costs, and long-term disability burdens [6]. Women are particularly vulnerable due to smaller bone mass and the accelerated bone loss that occurs after menopause [7].
Dietary factors play a crucial role in the prevention of noncommunicable diseases and may influence bone health [8]. Coffee and tea are among the most widely consumed beverages globally. The International Coffee Organization reported the consumption of 177 million bags of coffee (1 bag = 60 kg) in 2023–2024 [9], while global tea consumption has reached approximately 6.5 million metric tons annually [10]. Caffeine, a major bioactive compound in both beverages, has been the focus of numerous studies investigating its potential effects on health outcomes, including bone metabolism [11,12]. However, evidence regarding the relationship between coffee and tea consumption and BMD remains inconsistent and none has employed repeated measurements of both exposures and outcomes over an extended follow-up period.
This study aimed to examine the longitudinal associations between coffee and tea consumption and BMD among postmenopausal women, using repeated-measures data from the Study of Osteoporotic Fractures (SOF).
2. Materials and Methods
2.1. Data Source
The study data were drawn from the SOF, a multicenter prospective cohort study that followed 9704 primarily White women aged ≥65 years for approximately 20 years, with clinical assessments conducted every two years (nine visits in total). The detailed study design has been described elsewhere [13]. All participants provided written informed consent, and institutional review board approval was obtained. This specific analysis was exempted from ethical review by the Flinders University Human Research Ethics Committee (project number: 9307).
2.2. Study Population
The study population included SOF participants who attended visits 2, 4, 5, and 6, spanning approximately 10 years, during which BMD and coffee and tea consumption were both assessed. Data from visits 1, 3, and 7 were excluded due to the unavailability of either BMD or beverage intake data. Visits 8 and 9 were excluded from the primary analysis because of extensive missing data but were included in the sensitivity analyses.
2.3. Exposure
Exposures were assessed using self-administered questionnaires. The exposure-related questions were as follows: for coffee consumption, “Do you currently drink regular coffee? (yes/no); if yes, on average, how many cups of regular coffee do you usually consume per day?”; for tea consumption, “Do you currently drink tea or iced tea (excluding herbal or decaffeinated varieties)? (yes/no); if yes, on average, how many cups of tea do you usually consume per day?”.
2.4. Outcome
The outcome variables were femoral neck BMD (g/cm2) and total hip BMD (g/cm2), measured at visits 2, 4, 5, and 6 using a Hologic QDR 1000 densitometer, where visit 6 was treated as the primary endpoint. These anatomical sites were selected because of their strong correlation with fracture risk [14,15].
2.5. Covariates
Time-dependent covariates included: visit, age, body mass index (BMI, kg/m2), current cigarette smoking status (yes/no), and the Charlson comorbidity index (CCI), derived from participants’ medical histories. The detailed algorithm for CCI calculation has been described previously [16].
Time-independent covariates were primarily collected at visit 1 and included age at menopause, lifetime alcohol intake (calculated as drinks per week multiplied by years of drinking), lifetime physical activity (a weighted measure of frequency across four life stages: teen, 30s, 50s, and current), oral estrogen use, and oral steroid pill use (both categorized as current, past, or never). Average weekly calcium and protein intake were both assessed at visit 1 using a 20-item dietary scale. Finally, baseline BMD measured at visit 2 was included as a time-independent covariate specific to each corresponding outcome site.
2.6. Statistical Analysis
Coffee and tea consumption, along with covariates measured at each visit, were treated as categorical or continuous variables and are presented as frequencies (percentages) or means (standard deviations), respectively.
The associations between coffee or tea consumption and BMD were assessed using linear mixed-effects models with random intercepts [17]. Two models were specified. Model 1 (minimally adjusted): included the outcome variable (femoral neck or total hip BMD), the primary exposure (coffee or tea consumption), the study visit, the visit × exposure interaction, and baseline BMD corresponding to the outcome site. Model 2 (extensively adjusted): additionally adjusted for time-dependent covariates (age, BMI, current smoking status, Charlson comorbidity index), the other beverage exposure (e.g., tea intake adjusted for coffee intake) and time-independent covariates (age at menopause, lifetime alcohol use, average weekly calcium and protein intake, weighted lifetime physical activity, and oral estrogen and steroid pill use).
Subgroup analyses were performed to evaluate if the associations between coffee or tea intake and BMD varied across participant characteristics. This was achieved by including two-way interaction terms between each exposure and the covariates in the mixed-effects models [18,19,20]. For continuous covariates, the variables were dichotomized at their respective medians for the interaction assessment. Least squares [LS] means and pairwise comparisons were estimated using the LSMEANS and SLICE statements in SAS.
To assess potential non-linear relationships, natural spline regression models were applied for continuous exposures (cups of coffee or tea per day) [21]. Based on previous research [22,23], two knots were placed at 2 and 4 cups per day to define points of interest. Non-linearity was assessed by a likelihood ratio test comparing the non-linear and linear models. Additionally, to ensure stable predictions, the following variables were square root transformed: lifetime alcohol use, average weekly calcium intake, average weekly protein intake, and weighted lifetime physical activity.
For the main analyses, missing data were assumed to be Missing at Random (MAR). No specific imputation procedures were applied, as linear mixed models can appropriately handle missing data under the MAR assumption [24,25].
Sensitivity analyses were conducted by extending the follow-up period to include visits 8 and 9. Natural spline regression models were also re-run using this extended dataset.
All statistical analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC) and R software, version 4.4.0 (R Core Team, Vienna, Austria)
3. Results
The study flow diagram detailing participant inclusion is presented in Figure 1. A total of 9704 women were initially enrolled in the SOF at Visit 1; however, BMD was not measured at this time point. At visit 2 (baseline), 8074 participants had total hip BMD data available, and 7963 had femoral neck BMD data. Subsequent follow-up visits (visits 4, 5, and 6) included 6211, 5656 and 4697 participants, respectively, with both BMD and beverage consumption data collected at each visit. Participants who died or had missing BMD data were excluded from each subsequent wave. After all exclusions across the four included visits (visits 2, 4, 5, and 6), the final total analytic sample comprised 24,638 observations.
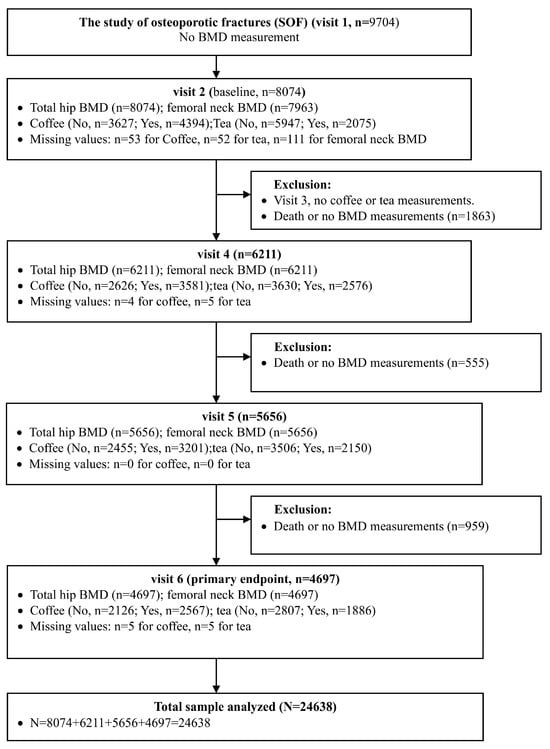
Figure 1.
Study flow diagram.
The mean follow-up period for the analytic sample was 8.1 ± 0.6 years, with a median of 8.0 years (interquartile range [IQR], 0.7 years). Table 1 summarizes the outcome variables and covariates by coffee and tea consumption across the four study visits (2, 4, 5, and 6).

Table 1.
Distribution of outcome variables and covariates by coffee and tea consumption across study visits.
Over the approximately 10-year follow-up period, femoral neck BMD declined from a mean of approximately 0.65 g/cm2 at visit 2 to 0.62 g/cm2 at visit 6. For total hip BMD, the mean value decreased from approximately 0.76 g/cm2 to about 0.73 g/cm2.
The mean age of participants increased from approximately 73 years at visit 2 to 80 years by visit 6. Mean BMI remained relatively stable at around 26 kg/m2 throughout follow-up. The CCI increased, rising from approximately 1.3 at visit 2 to 2.9 at visit 6.
Smoking prevalence decreased across all groups. Among non-coffee drinkers, it decreased from 5.4% to 2.4%, and among coffee drinkers, it decreased from 10.0% to 5.4%. Similarly, smoking prevalence declined from 8.3% to 4.5% among non-tea drinkers and from 6.7% to 3.4% among tea drinkers.
The mean age at menopause across all groups was approximately 48 years. Non-coffee drinkers and non-tea drinkers generally reported higher average weekly calcium consumption than their respective beverage-drinking counterparts, with the exception of the non-tea drinker group at visit 6. Coffee drinkers consumed slightly less protein than non-coffee drinkers, while tea drinkers consumed slightly more protein than non-tea drinkers. Coffee drinkers reported higher alcohol consumption than non-drinkers, whereas tea drinkers reported lower alcohol consumption than non-drinkers, except at visit 2. No clear pattern was observed in lifetime physical activity levels between coffee/tea drinkers and non-drinkers. At baseline, about 15% of women were current oral estrogen users, and less than 3% were current oral steroid pill users.
Table 2 presents the associations between coffee consumption and femoral neck and total hip BMD as estimated by the linear mixed-effects models. Across all four study visits, coffee consumption was not significantly associated with either femoral neck or total hip BMD in the minimally adjusted Model 1 or the extensively adjusted Model 2.

Table 2.
Associations of coffee consumption with femoral neck and total hip BMD.
Table 3 details the associations between tea consumption and femoral neck and total hip BMD. Tea consumption showed no significant association with femoral neck BMD in either the minimally adjusted Model 1 or the extensively adjusted Model 2.

Table 3.
Associations of tea consumption with femoral neck and total hip BMD.
However, tea consumption was significantly associated with higher total hip BMD at Visit 6 in both models. In model 1, the association was observed with a LS mean difference of 0.002 (LS mean: 0.717 vs. 0.715; 95% CI: 0.000–0.004; p = 0.0354). This finding persisted in the extensively adjusted Model 2, which showed a slightly stronger association with an LS mean difference of 0.003 (LS mean: 0.718 vs. 0.715; 95% CI: 0.000–0.005; p = 0.0260).
Figure 2 presents the results from natural spline regression analyses. The likelihood ratio tests indicated no significant non-linear associations between the continuous daily consumption of coffee or tea and either femoral neck or total hip BMD (p for nonlinearity all >0.05).
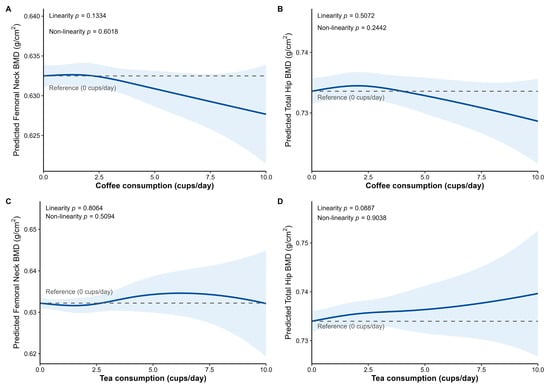
Figure 2.
Association between coffee and tea consumption and femoral neck BMD and total hip BMD. (A) Coffee consumption and femoral neck BMD. (B) Coffee consumption and total hip BMD. (C) Tea consumption and femoral neck BMD. (D) Tea consumption and total hip BMD. Predicted values and their 95% confidence intervals for BMD were obtained from the extensively adjusted linear mixed-effects model utilizing natural splines for coffee or tea consumption (cups/day). The model included adjustment for time-dependent covariates (visit, age, BMI, smoking status, and CCI) and time-independent covariates (age at menopause, lifetime alcohol use, average weekly calcium and protein intake, weighted lifetime physical activity, oral estrogen use, oral steroid pill use, and baseline BMD corresponding to the outcome site). Two knots were placed at 2 and 4 cups per day for both coffee and tea consumption. The dashed line indicates the reference value for the predicted BMD at 0 cups/day, and the shaded blue area denotes the 95% confidence interval. The p-value for linearity was derived from the model treating coffee or tea consumption as a linear term, and the p-value for non-linearity was obtained by comparing the spline and linear models using a likelihood ratio test.
However, the trends suggested by Figure 2A,B indicate that consuming approximately 2 to 3 cups of coffee per day may not affect BMD. Conversely, consumption exceeding 5 cups per day was visually suggested to be potentially associated with lower BMD.
For tea consumption, a weak linear relationship may exist with total hip BMD (β = 0.0006, p for linearity = 0.0887; Figure 2D).
Subgroup analyses revealed significant interaction effects. The association between coffee consumption and femoral neck BMD was significantly modified by lifetime alcohol intake (p for interaction = 0.0147). Specifically, coffee consumption showed a more beneficial effect on femoral neck BMD among participants categorized as consuming less alcohol (LS mean difference = 0.002; 95% CI, 0.000 to 0.004), as illustrated in Figure 3.
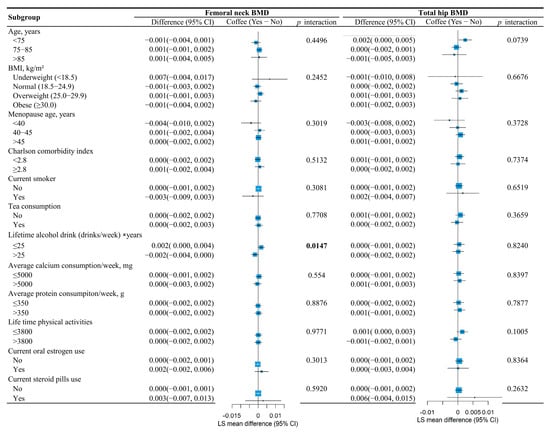
Figure 3.
Subgroup analysis evaluating the association between coffee consumption and femoral neck BMD and total hip BMD among different subgroups through coffee × covariate interactions in the mixed models.
Furthermore, the association between tea consumption and femoral neck BMD was significantly modified by BMI (p for interaction = 0.0175). Tea consumption was associated with higher femoral neck BMD among women classified as having obesity (LS mean difference = 0.001; 95% CI, 0.001 to 0.006), as shown in Figure 4.
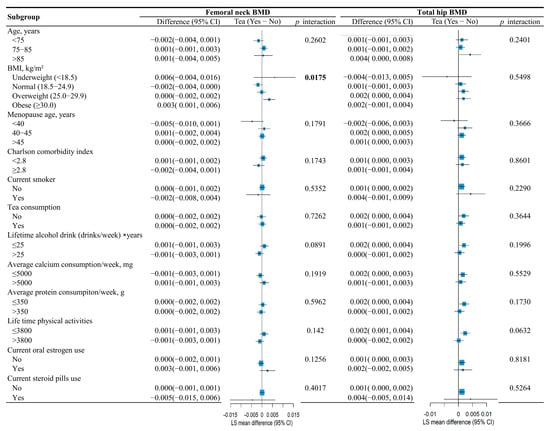
Figure 4.
Subgroup analysis evaluating the association between tea consumption and femoral neck BMD and total hip BMD among different subgroups through tea × covariate interactions in the mixed models.
Sensitivity analyses, extending the follow-up period to include visits 8 and 9, provided additional insights. At visit 9, coffee consumption was associated with lower femoral neck BMD but showed no significant association with total hip BMD. Conversely, tea consumption was associated with higher total hip BMD at visits 6 and 8, but this association was not observed at visit 9 (Appendix A Table A1 and Table A2).
For non-linear relationships, the natural spline regression conducted on the extended dataset visually confirmed the suggestion that consuming more than 5 cups of coffee per day might be associated with lower BMD. For tea consumption and total hip BMD, we observed a significant overall linear association (β = 0.001, p = 0.0221), alongside evidence that the association departs from linearity (likelihood ratio test p = 0.0084). This departure indicates additional curvature beyond the simple linear trend (Appendix A Figure A1).
4. Discussion
In this 10-year repeated-measures study of women aged ≥65 years, we found that tea consumption was positively associated with total hip BMD. While the statistical test for non-linearity was non-significant, our natural spline regression analyses visually suggested that consuming approximately two to three cups of coffee per day might not affect BMD, whereas intake exceeding five cups per day could be linked to lower BMD. Tea consumption showed a linear association with total hip BMD; however, the dose-response relationship was modest (β = 0.0006, p for linearity = 0.0887). Subgroup analyses suggested that women with higher lifetime alcohol intake may benefit from limiting coffee consumption, while women with obesity might derive greater benefits from tea consumption. However, these subgroup findings should be interpreted with caution as they are likely underpowered and may be susceptible to type I error; they are primarily hypothesis-generating and warrant validation in future, larger studies. The sensitivity analyses were largely consistent with our main results, although the finding that coffee consumption was associated with lower femoral neck BMD at visit 9 should be interpreted cautiously due to the substantial missing data at that late time point.
The relationship between coffee consumption and BMD remains inconsistent across the literature. Several studies have reported that higher coffee intake is associated with lower BMD [26,27,28,29], whereas others have found no association [30,31,32], and some have suggested a protective effect [33,34,35,36]. Caffeine, a key component of coffee, is believed to play a significant role in these mixed findings [37]. Mechanistically, caffeine acts as a non-specific antagonist of adenosine receptors, which may inhibit bone formation and enhance bone resorption [28]. In vitro studies further suggest that caffeine may downregulate vitamin D receptor expression and impair the osteogenic actions of 1,25(OH)2D3 in osteoblasts [38]. However human experimental studies indicate that in individuals with inadequate calcium intake, caffeine’s impact is minimal, causing only a slight negative calcium balance due to reduced absorption efficiency. This effect is often negligible that it could be offset by adding one to two tablespoons of milk to a cup of coffee [39]. Furthermore, genetic factors may modify the association between caffeine and osteoporosis risk. For example, a study using Taiwan Biobank data found that individuals carrying the ESR1 rs2982573 genotype who consumed more than three cups of coffee per week had a lower risk of osteoporosis [40].
Findings regarding tea consumption and BMD have also been inconsistent. Some studies, such as those conducted among Korean postmenopausal women and in China [41,42], found that habitual tea drinking was associated with higher BMD at various skeletal sites. Research in the UK among women aged 65 to 76 years [43], as well as studies in Australian women aged 70 to 85 years [44], reported similar positive associations. Two meta-analyses have also supported the potential benefits of tea consumption on BMD [45,46]. However, other studies, including a clinical trial of green tea extract, found no significant associations [47], and another meta-analysis concluded that tea consumption was not associated with higher BMD [31]. The potential mechanisms underlying tea’s effects may involve catechins, particularly epigallocatechin (EGC), which can promote osteoblast activity and inhibit osteoclast differentiation [48]. Animal studies also suggest that EGC may prevent both bone and muscle loss [49].
Our study offers several strengths, notably its basis in a large, well-characterized cohort with repeated measures of both beverage intake and BMD, which helps minimize measurement error. Furthermore, the use of linear mixed-effects models provides a robust analytic framework that effectively accounts for time-varying exposures and covariates, better reflecting real-world changes over time.
However, limitations must be considered. First, the self-reported nature of coffee and tea consumption may introduce measurement bias. In addition, treating intrinsically time-varying variables, such as physical activity and alcohol consumption, as time-independent, by only assessing lifetime consumption at baseline, may also introduce recall bias. Furthermore, more detailed information, such as cup size, beverage strength, and beverage types, was not collected, which may further contribute to measurement error. Second, the long follow-up period resulted in high rates of mortality and missing data. While mixed models can handle missingness under the assumption of MAR, this assumption may not fully hold, potentially introducing bias. Third, despite extensive covariate adjustment, residual confounding cannot be entirely ruled out. Fourth, although the difference in BMD between tea drinkers and non-drinkers (≈0.003 g/cm2) is statistically significant, it is below the threshold typically considered clinically meaningful for individual patient management [50], However, such a small difference may still be relevant from an epidemiological and public health perspective, as even modest shifts in the population distribution of BMD could translate into reductions in fracture risk at the group level. Future longitudinal studies with fracture as a primary endpoint are necessary to confirm whether the small BMD differences associated with tea consumption have a meaningful impact on reducing osteoporotic fractures. Finally, the study population consisted almost entirely of White older women in the United States. This lack of racial and ethnic diversity severely restricts the applicability of our findings to broader populations. Because BMD, fracture risk, and dietary patterns differ meaningfully across racial/ethnic groups, our results should be interpreted with considerable caution when applied outside this demographic.
5. Conclusions
Our findings suggest tea consumption is associated with higher total hip BMD in older postmenopausal women. While no overall association between coffee consumption and BMD was identified during the 10-year follow-up, natural spline analyses suggest that consuming more than five cups of coffee per day might be detrimental to BMD. The observed interaction effects highlight the importance of individual factors: coffee consumption was negatively associated with femoral neck BMD in women with higher alcohol intake, while tea consumption appeared particularly beneficial for those with obesity. These findings underscore the importance of personalized dietary recommendations in promoting optimal bone health among aging women.
Author Contributions
Conceptualization, E.L. and R.Y.L.; methodology, E.L.; software, E.L. and R.Y.L.; validation, R.Y.L.; formal analysis, E.L.; data curation, E.L.; writing—original draft preparation, R.Y.L. and E.L.; writing—review and editing, R.Y.L. and E.L.; visualization, E.L. and R.Y.L.; supervision, E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
All participants provided written informed consent, and institutional review board approval was obtained for the main study. This specific analysis was granted an exemption from ethical review by the Flinders University Human Research Ethics Committee (project number: 9307).
Data Availability Statement
The original data presented in the study are openly available in SOF online, at https://sofonline.ucsf.edu (accessed on 9 October 2025). To access data, you must create an account and electronically sign a Data Use Agreement.
Acknowledgments
The funding for the SOF study comes from the National Institute on Aging (NIA) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), supported by grants (AG05407, AR35582, AG05394, AR35584, and AR35583).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMD | Bone mineral density |
| CCI | Charlson comorbidity index |
| DALY | Disability-adjusted life years |
| MAR | Missing at random |
| LS | Least squares |
| SOF | Study of osteoporotic fractures |
| YLD | Years lived with disability |
Appendix A
Appendix A.1
Sensitivity analyses extending follow up visits to visit 8 and visit 9. Associations of coffee consumption with femoral and total hip BMD.

Table A1.
Associations of coffee consumption with femoral neck and total hip BMD.
Table A1.
Associations of coffee consumption with femoral neck and total hip BMD.
| Outcome | Visit | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Difference (95% CI) | p Value | No | Yes | Difference (95% CI) | p Value | ||
| LS means of femoral neck BMD (g/cm2) | 2 | 0.656 | 0.655 | 0.000 (−0.002, 0.002) | 0.6859 | 0.651 | 0.650 | 0.000 (−0.003, 0.002) | 0.7187 |
| 4 | 0.634 | 0.634 | 0.000 (−0.002, 0.002) | 0.9345 | 0.631 | 0.631 | −0.001 (−0.003, 0.002) | 0.6725 | |
| 5 | 0.626 | 0.627 | 0.001 (−0.001, 0.003) | 0.4246 | 0.623 | 0.624 | 0.001 (−0.002, 0.004) | 0.4480 | |
| 6 | 0.619 | 0.619 | 0.000 (−0.002, 0.003) | 0.7388 | 0.617 | 0.618 | 0.001 (−0.002, 0.004) | 0.5827 | |
| 8 | 0.611 | 0.615 | 0.003 (0.000, 0.006) | 0.0565 | 0.613 | 0.616 | 0.003 (−0.001, 0.007) | 0.0921 | |
| 9 | 0.612 | 0.600 | −0.012 (−0.018, −0.005) | 0.0010 | 0.620 | 0.609 | −0.011 (−0.019, −0.003) | 0.0071 | |
| LS means of total hip BMD (g/cm2) | 2 | 0.766 | 0.766 | 0.000 (−0.001, 0.002) | 0.6758 | 0.756 | 0.757 | 0.001 (−0.001, 0.003) | 0.5623 |
| 4 | 0.738 | 0.740 | 0.001 (−0.001, 0.003) | 0.2275 | 0.733 | 0.734 | 0.001 (−0.001, 0.003) | 0.3717 | |
| 5 | 0.727 | 0.728 | 0.001 (−0.001, 0.003) | 0.2608 | 0.725 | 0.726 | 0.001 (−0.001, 0.004) | 0.2916 | |
| 6 | 0.718 | 0.717 | −0.001 (−0.003, 0.001) | 0.3545 | 0.717 | 0.717 | 0.000 (−0.002, 0.003) | 0.8971 | |
| 8 | 0.703 | 0.706 | 0.003 (0.000, 0.006) | 0.0283 | 0.709 | 0.712 | 0.003 (0.000, 0.006) | 0.0697 | |
| 9 | 0.706 | 0.704 | −0.002 (−0.008, 0.005) | 0.6005 | 0.723 | 0.721 | −0.002 (−0.009, 0.005) | 0.5435 | |
Model 1: Adjusted for coffee consumption, visit, visit × coffee interaction, and baseline BMD (measured at visit 2 for each outcome site). Model 2: additionally adjusted for time dependent covariates, age, BMI, current smoking status, Charlson comorbidity index, as well as the other beverage exposure (e.g., tea consumption adjusted for coffee consumption) and time independent covariates age at menopause, lifetime alcohol use, average calcium consumed per week, average protein consumed per week, weighted lifetime physical activity, lifetime oral estrogen used was also included.
Appendix A.2
Sensitivity analyses extending follow up visits to visit 8 and visit 9. Associations of tea consumption with femoral and total hip BMD.

Table A2.
Associations of tea consumption with femoral neck and total hip BMD.
Table A2.
Associations of tea consumption with femoral neck and total hip BMD.
| Outcome | Visit | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Difference (95% CI) | p Value | No | Yes | Difference (95% CI) | p Value | ||
| LS means of femoral neck BMD (g/cm2) | 2 | 0.655 | 0.656 | 0.000 (−0.002, 0.003) | 0.7318 | 0.650 | 0.651 | 0.001 (−0.002, 0.003) | 0.4955 |
| 4 | 0.634 | 0.635 | 0.002 (0.000, 0.004) | 0.1240 | 0.630 | 0.632 | 0.002 (−0.001, 0.004) | 0.1855 | |
| 5 | 0.626 | 0.626 | 0.000 (−0.002, 0.003) | 0.8256 | 0.624 | 0.624 | 0.000 (−0.003, 0.003) | 0.9554 | |
| 6 | 0.620 | 0.619 | −0.001 (−0.004, 0.002) | 0.4261 | 0.619 | 0.617 | −0.002 (−0.005, 0.001) | 0.2360 | |
| 8 | 0.612 | 0.615 | 0.004 (0.000, 0.007) | 0.0286 | 0.613 | 0.616 | 0.003 (−0.001, 0.007) | 0.1139 | |
| 9 | 0.604 | 0.605 | 0.001 (−0.006, 0.008) | 0.7669 | 0.613 | 0.614 | 0.001 (−0.007, 0.009) | 0.8361 | |
| LS means of total hip BMD (g/cm2) | 2 | 0.766 | 0.767 | 0.000 (−0.002, 0.002) | 0.7088 | 0.756 | 0.757 | 0.001 (−0.001, 0.003) | 0.3118 |
| 4 | 0.738 | 0.740 | 0.002 (0.000, 0.004) | 0.1383 | 0.733 | 0.735 | 0.002 ( 0.000, 0.004) | 0.0683 | |
| 5 | 0.728 | 0.728 | 0.001 (−0.002, 0.003) | 0.6265 | 0.725 | 0.726 | 0.001 (−0.002, 0.003) | 0.6044 | |
| 6 | 0.717 | 0.719 | 0.003 (0.000, 0.005) | 0.0329 | 0.716 | 0.719 | 0.003 (0.001, 0.006) | 0.0159 | |
| 8 | 0.703 | 0.707 | 0.005 (0.001, 0.008) | 0.0040 | 0.709 | 0.714 | 0.004 (0.001, 0.007) | 0.0187 | |
| 9 | 0.704 | 0.706 | 0.002 (−0.005, 0.008) | 0.5952 | 0.722 | 0.720 | −0.001 (−0.009, 0.006) | 0.7022 | |
Model 1: Adjusted for tea consumption, visit, visit × tea interaction, and baseline BMD (measured at visit 2 for each outcome site). Model 2: additionally adjusted for time dependent covariates, age, BMI, current smoking status, Charlson comorbidity index, as well as the other beverage exposure (e.g., tea consumption adjusted for coffee consumption) and time independent covariates age at menopause, lifetime alcohol use, average calcium consumed per week, average protein consumed per week, weighted lifetime physical activity, lifetime oral estrogen used was also included.
Appendix A.3
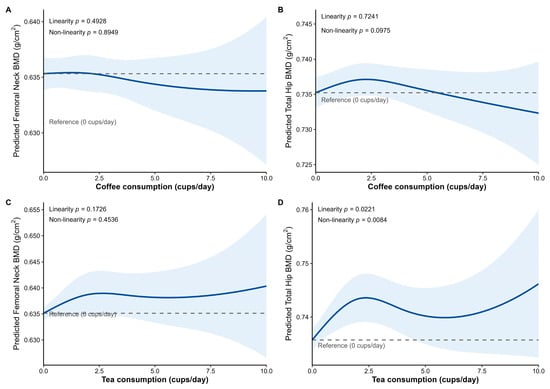
Figure A1.
Association between coffee, tea consumption and femoral neck BMD and total hip BMD. (A) Coffee consumption and femoral neck BMD. (B) Coffee consumption and total hip BMD. (C) Tea consumption and femoral neck BMD. (D) Tea consumption and total hip BMD. Predicted values and 95% confidence intervals for BMD were obtained from a linear mixed-effects model with natural splines for coffee or tea consumption (cups/day), adjusted visit, age, BMI, menopausal age, smoking status, Charlson comorbidity index, and the other beverage, and time independent covariates age at menopause, lifetime alcohol use, average calcium consumed per week, average protein consumed per week, weighted lifetime physical activity, oral estrogen used, steroid pills use and baseline BMD. Two knots were placed at 2 and 4 cups per day for both coffee and tea consumption. The dashed line indicates the reference value at 0 cups/day, and the shaded blue area denotes the 95% confidence interval. The p value for linearity was obtained from the model including coffee or tea consumption as a linear term, and the p value for nonlinearity was obtained by comparing the spline and linear models using a likelihood ratio test.
References
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Kanis, J.A.; Harvey, N.C.; Lorentzon, M.; Liu, E.; Schini, M.; Abrahamsen, B.; Adachi, J.D.; Alokail, M.; Borgstrom, F.; Bruyère, O.; et al. Race-specific FRAX models are evidence-based and support equitable care: A response to the ASBMR Task Force report on Clinical Algorithms for Fracture Risk. Osteoporos. Int. 2024, 35, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Johansson, H.; Harvey, N.; Liu, E.; Vandenput, L.; McCloskey, E.; Kanis, J. Osteoporosis and fractures in women: The burden of disease. Climacteric 2022, 25, 4–10. [Google Scholar] [CrossRef]
- GBD 2021 Low Bone Mineral Density Collaborators. The global, regional, and national burden attributable to low bone mineral density, 1990-2020: An analysis of a modifiable risk factor from the Global Burden of Disease Study 2021. Lancet Rheumatol. 2025, 7, e873–e894. [Google Scholar] [CrossRef]
- Fang, P.; She, Y.; Han, L.; Wan, S.; Shang, W.; Zhang, Z.; Min, W. A promising biomarker of elevated galanin level in hypothalamus for osteoporosis risk in type 2 diabetes mellitus. Mech. Ageing Dev. 2021, 194, 111427. [Google Scholar] [CrossRef]
- Liu, E. Hip fractures: Mortality, economic burden, and organisational factors for improved patient outcomes. Lancet Healthy Longev. 2023, 4, e360–e361. [Google Scholar] [CrossRef]
- McPhee, C.; Aninye, I.O.; Horan, L. Recommendations for Improving Women’s Bone Health Throughout the Lifespan. J. Women’s Health 2022, 31, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. Coffee Report and Outlook. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 9 October 2025).
- Ibrahim, A.A.; Rahman, M.S.; Noman, M.R.A.F.; Nur, A.H. Tea and Tea Industry Scenario: A Review of World and Bangladesh Perspective. Int. J. Tea Sci. 2024, 18, 6–17. [Google Scholar] [CrossRef]
- Walter, K. Caffeine and health. JAMA 2022, 327, 693. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, X.; Li, G.; Wu, L.; Shao, L.; Fan, Y.; Pan, C.-W.; Wu, Y.; Borné, Y.; Ke, C. Habitual Coffee, Tea, and Caffeine Consumption, Circulating Metabolites, and the Risk of Cardiometabolic Multimorbidity. J. Clin. Endocrinol. Metab. 2025, 110, e1845–e1855. [Google Scholar] [CrossRef]
- San Francisco Coordinating Center. About the SOF Study. Available online: https://sofonline.ucsf.edu/Home/About (accessed on 9 October 2025).
- Kanis, J.A.; Johansson, H.; McCloskey, E.V.; Liu, E.; Åkesson, K.E.; Anderson, F.A.; Azagra, R.; Bager, C.L.; Beaudart, C.; Bischoff-Ferrari, H.A.; et al. Previous fracture and subsequent fracture risk: A meta-analysis to update FRAX. Osteoporos. Int. 2023, 34, 2027–2045. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Binkley, N.; Leslie, W.D. The association of hip bone mineral density (BMD) with incident major osteoporotic and hip fractures varies by body mass index. J. Clin. Densitom. 2025, 28, 101577. [Google Scholar] [CrossRef]
- Liu, E.; Liu, R.Y.; Moraros, J.; McCloskey, E.V.; Harvey, N.C.; Lorentzon, M.; Johansson, H.; Kanis, J.A. Association between walking and hip fracture in women aged 65 and older: 20-year follow-up from the study of osteoporotic fractures. Osteoporos. Int. 2025, 36, 1155–1164. [Google Scholar] [CrossRef]
- West, B.T.; Welch, K.B.; Galecki, A.T. Linear Mixed Models: A Practical Guide Using Statistical Software, 3rd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2022. [Google Scholar]
- Schandelmaier, S.; Guyatt, G. Same Old Challenges in Subgroup Analysis—Should We Do More About Methods Implementation? JAMA Netw. Open 2024, 7, e243339. [Google Scholar] [CrossRef]
- Wang, X.; Piantadosi, S.; Le-Rademacher, J.; Mandrekar, S.J. Statistical Considerations for Subgroup Analyses. J. Thorac. Oncol. 2021, 16, 375–380. [Google Scholar] [CrossRef]
- Liu, E.; Liu, R.Y. Imputing Covariance for Meta-Analysis in the Presence of Interaction. Appl. Sci. 2025, 15, 141. [Google Scholar] [CrossRef]
- Gauthier, J.; Wu, Q.V.; Gooley, T.A. Cubic splines to model relationships between continuous variables and outcomes: A guide for clinicians. Bone Marrow Transplant. 2020, 55, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, M.; Hua, Y.; Hui, Z.; Zhang, Y.; Lin, Y.; Du, J.; Ni, C.; Wang, X. Soft drinks, tea and coffee consumption in relation to risk of fracture: Evidence from china health and nutrition survey. J. Bone Miner. Metab. 2023, 41, 621–630. [Google Scholar] [CrossRef]
- Zeng, X.; Su, Y.; Tan, A.; Zou, L.; Zha, W.; Yi, S.; Lv, Y.; Kwok, T. The association of coffee consumption with the risk of osteoporosis and fractures: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 1871–1893. [Google Scholar] [CrossRef] [PubMed]
- Gabrio, A.; Plumpton, C.; Banerjee, S.; Leurent, B. Linear mixed models to handle missing at random data in trial-based economic evaluations. Health Econ. 2022, 31, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Twisk, J.; de Boer, M.; de Vente, W.; Heymans, M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J. Clin. Epidemiol. 2013, 66, 1022–1028. [Google Scholar] [CrossRef]
- Rame, A. Coffee consumption, bone mineral density and incidence hip fracture in Icelandic community dwelling adults. Proc. Nutr. Soc. 2021, 80, E35. [Google Scholar] [CrossRef]
- Adıgüzel, K.T.; Köroğlu, Ö. Caffeine intake and bone mineral density in postmenopausal women. Gulhane Med. J. 2022, 64, 262–267. [Google Scholar] [CrossRef]
- Berman, N.K.; Honig, S.; Cronstein, B.N.; Pillinger, M.H. The effects of caffeine on bone mineral density and fracture risk. Osteoporos. Int. 2022, 33, 1235–1241. [Google Scholar] [CrossRef]
- Jahng, S.Y.; Kim, H.W.; Lee, S.H.; Jeong, J.Y.; Son, H.R. Association between Coffee Consumption and Bone Mineral Density in Korean Men Aged 50 Years and Older: A Cross Sectional Analysis of Korea National Health and Nutrition Examination Survey 2011. Korean J. Fam. Pract. 2020, 10, 15–22. [Google Scholar] [CrossRef]
- Cui, A.; Xiao, P.; He, J.; Fan, Z.; Xie, M.; Chen, L.; Zhuang, Y.; Wang, H. Association between caffeine consumption and bone mineral density in children and adolescent: Observational and Mendelian randomization study. PLoS ONE 2023, 18, e0287756. [Google Scholar] [CrossRef]
- Chen, C.-C.; Shen, Y.-M.; Li, S.-B.; Huang, S.-W.; Kuo, Y.-J.; Chen, Y.-P. Association of coffee and tea intake with bone mineral density and hip fracture: A meta-analysis. Medicina 2023, 59, 1177. [Google Scholar] [CrossRef]
- da Costa, M.S.; da Silva Pontes, K.S.; Guedes, M.R.; Silva, M.I.B.; Klein, M.R.S.T. Association of habitual coffee consumption with obesity, sarcopenia, bone mineral density and cardiovascular risk factors: A two-year follow-up study in kidney transplant recipients. Clin. Nutr. 2023, 42, 1889–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, S.; Xia, B.; He, Q.; Mi, N.; Zhao, J.; Hu, L.; Wang, D.; Zheng, L.; Sheng, P. Association of coffee and tea consumption with osteoporosis risk: A prospective study from the UK biobank. Bone 2024, 186, 117135. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhai, T. Coffee Drinking and the Odds of Osteopenia and Osteoporosis in Middle-Aged and Older Americans: A Cross-Sectional Study in NHANES 2005–2014. Calcif. Tissue Int. 2024, 114, 348–359. [Google Scholar] [CrossRef]
- Li, W.; Xie, Y.; Jiang, L. Coffee and tea consumption on the risk of osteoporosis: A meta-analysis. Front. Nutr. 2025, 12, 1559835. [Google Scholar] [CrossRef]
- Ye, Y.; Zhong, R.; Xiong, X.M.; Wang, C.E. Association of coffee intake with bone mineral density: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1328748. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, T.; Hu, L.; Li, J.; Gan, C.; Xu, J.; Chen, F.; Xiang, Z.; Wang, X.; Sheng, J. Effect of caffeine on ovariectomy-induced osteoporosis in rats. Biomed. Pharmacother. 2019, 112, 108650. [Google Scholar] [CrossRef] [PubMed]
- Rapuri, P.B.; Gallagher, J.C.; Nawaz, Z. Caffeine decreases vitamin D receptor protein expression and 1, 25 (OH) 2D3 stimulated alkaline phosphatase activity in human osteoblast cells. J. Steroid Biochem. Mol. Biol. 2007, 103, 368–371. [Google Scholar] [CrossRef]
- Heaney, R.P. Effects of caffeine on bone and the calcium economy. Food Chem. Toxicol. 2002, 40, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Nfor, O.; Lu, W.-Y.; Tantoh, D.M.; Liaw, Y.-P. Relationship between coffee consumption and osteoporosis risk determined by the ESR1 polymorphism rs2982573. J. Nutr. Health Aging 2022, 26, 558–563. [Google Scholar] [CrossRef]
- Ni, S.; Wang, L.; Wang, G.; Lin, J.; Ma, Y.; Zhao, X.; Ru, Y.; Zheng, W.; Zhang, X.; Zhu, S. Drinking tea before menopause is associated with higher bone mineral density in postmenopausal women. Eur. J. Clin. Nutr. 2021, 75, 1454–1464. [Google Scholar] [CrossRef]
- Lee, D.B.; Song, H.J.; Paek, Y.-J.; Park, K.H.; Seo, Y.-G.; Noh, H.-M. Relationship between Regular Green Tea Intake and Osteoporosis in Korean Postmenopausal Women: A Nationwide Study. Nutrients 2022, 14, 87. [Google Scholar] [CrossRef]
- Hegarty, V.M.; May, H.M.; Khaw, K.-T. Tea drinking and bone mineral density in older women. Am. J. Clin. Nutr. 2000, 71, 1003–1007. [Google Scholar] [CrossRef]
- Devine, A.; Hodgson, J.M.; Dick, I.M.; Prince, R.L. Tea drinking is associated with benefits on bone density in older women. Am. J. Clin. Nutr. 2007, 86, 1243–1247. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Yang, J.-L.; Jiang, H.-C.; Lai, Z.; Wu, F.; Liu, Z.-X. Updated association of tea consumption and bone mineral density: A meta-analysis. Medicine 2017, 96, e6437. [Google Scholar] [CrossRef]
- Sun, K.; Wang, L.; Ma, Q.; Cui, Q.; Lv, Q.; Zhang, W.; Li, X. Association between tea consumption and osteoporosis: A meta-analysis. Medicine 2017, 96, e9034. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Kurzer, M.S. Long-Term Supplementation of Green Tea Extract Does Not Modify Adiposity or Bone Mineral Density in a Randomized Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.H.; Lau, K.M.; Choy, W.Y.; Leung, P.C. Effects of tea catechins, epigallocatechin, gallocatechin, and gallocatechin gallate, on bone metabolism. J. Agric. Food Chem. 2009, 57, 7293–7297. [Google Scholar] [CrossRef] [PubMed]
- Dvoretskiy, S.; Cole, T.; Skelding, M.-B.; Reaves, L.A.; Edens, N.K.; Pereira, S.L. The Green Tea Catechin, EGCg, Preserves Both Muscle and Bone in Aging Sarcopenic Rats. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Pocock, N.; Eisman, J.A. Interpretation of bone mineral density measurement and its change. J. Clin. Densitom. 2000, 3, 107–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).