Can Myokines Serve as Supporters of Muscle–Brain Connectivity in Obesity and Type 2 Diabetes? Potential of Exercise and Nutrition Interventions
Abstract
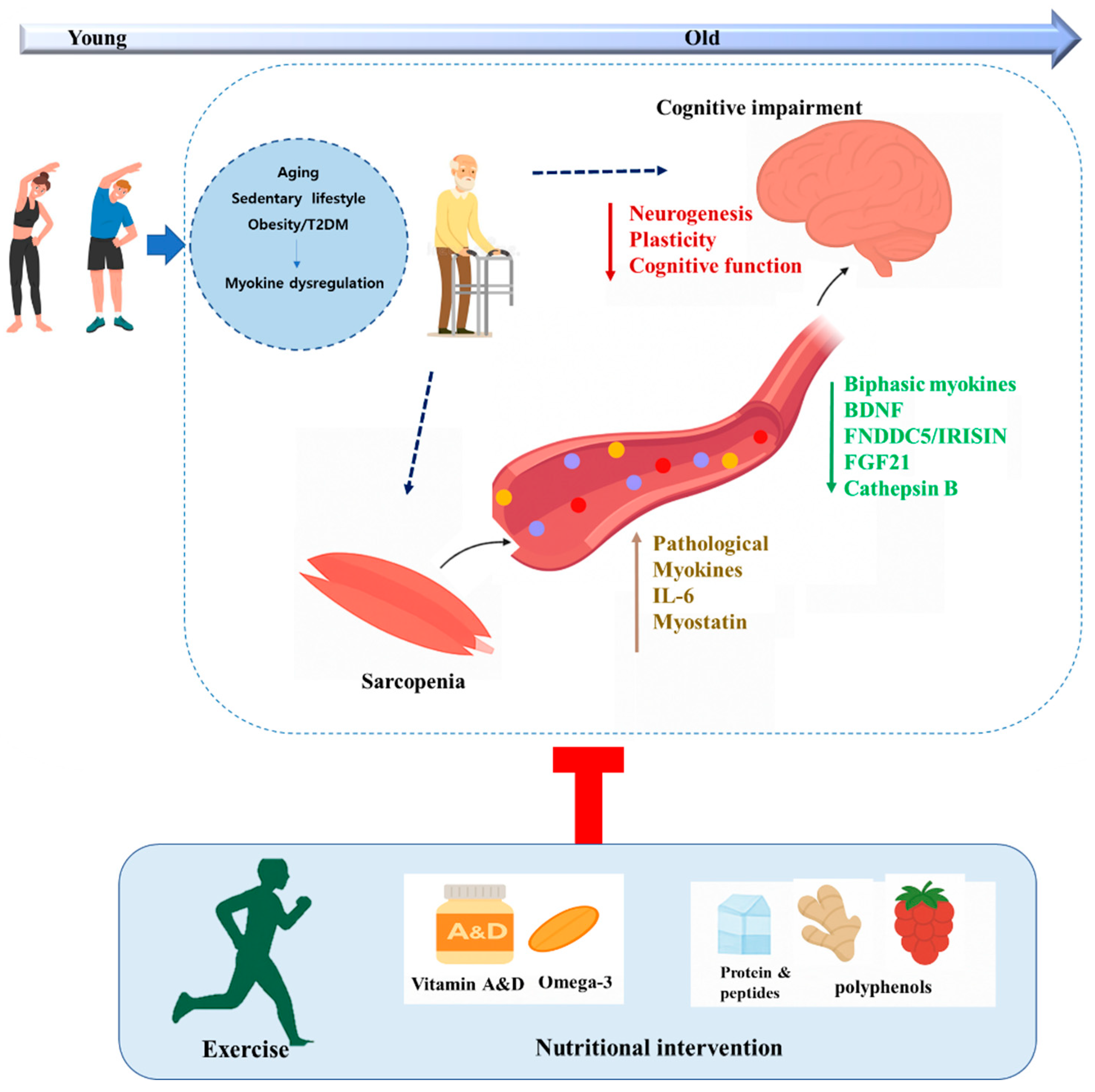
1. Introduction
2. Muscle and Brain Alterations in OB and T2DM
3. Potential Role of Myokines in Muscle–Brain Connectivity in OB and T2DM
3.1. Biphasic Regulation of Myokines Across Disease Progression
3.1.1. BDNF
3.1.2. Irisin
3.1.3. FGF21
3.2. Persistent Dysregulation of Myokines in Metabolic Disease
3.2.1. Cathepsin-B (CTSB)
3.2.2. IL-6
3.2.3. Myostatin
4. Therapeutic Strategies to Enhance Myokine Signaling in the Muscle–Brain Axis During Metabolic Disease
4.1. Exercise
4.2. Dietary Intervention
4.2.1. Vitamin A
4.2.2. Vitamin D
4.2.3. Polyunsaturated Fatty Acids (PUFAs)
4.2.4. Protein and Peptides
4.2.5. Polyphenols
5. Future Directions and Limitations
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Pillon, N.J.; Loos, R.J.F.; Marshall, S.M.; Zierath, J.R. Metabolic Consequences of Obesity and Type 2 Diabetes: Balancing Genes and Environment for Personalized Care. Cell 2021, 184, 1530. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash Parul Singla, A.A.; Kluge, M. Metabolic Effects of Obesity: A Review. World J. Diabetes 2010, 1, 76. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Rai, M.; Demontis, F. Muscle-to-Brain Signaling Via Myokines and Myometabolites. Brain Plast. 2022, 8, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ashraf, M.; Tipparaju, S.M.; Xuan, W. Muscle-Brain Crosstalk in Cognitive Impairment. Front. Aging Neurosci. 2023, 15, 1221653. [Google Scholar] [CrossRef]
- Michailidis, M.; Moraitou, D.; Tata, D.A.; Kalinderi, K.; Papamitsou, T.; Papaliagkas, V. Alzheimer’s Disease as Type 3 Diabetes: Common Pathophysiological Mechanisms between Alzheimer’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 2687. [Google Scholar] [CrossRef]
- Dove, A.; Wang, J.; Huang, H.; Dunk, M.M.; Sakakibara, S.; Guitart-Masip, M.; Papenberg, G.; Xu, W. Diabetes, Prediabetes, and Brain Aging: The Role of Healthy Lifestyle. Diabetes Care 2024, 47, 1794–1802. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–628. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, Y. The Potential Role of Myokines/Hepatokines in the Progression of Neuronal Damage in Streptozotocin and High-Fat Diet-Induced Type 2 Diabetes Mellitus Mice. Biomedicines 2022, 10, 1521. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Zhou, N.; Shen, Y.; Li, B.; Chen, B.E.; Li, X. Association Between Omega-3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose–Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2023, 12, 29512. [Google Scholar] [CrossRef]
- Kou, J.; Wang, M.; Shi, J.; Zhang, H.; Pu, X.; Song, S.; Yang, C.; Yan, Y.; Döring, Y.; Xie, X.; et al. Curcumin Reduces Cognitive Deficits by Inhibiting Neuroinflammation through the Endoplasmic Reticulum Stress Pathway in Apolipoprotein E4 Transgenic Mice. ACS Omega 2021, 6, 6654–6662. [Google Scholar] [CrossRef]
- Chan, A.; Remington, R.; Kotyla, E.; Lepore, A.; Zemianek, J.; Shea, T.B. A Vitamin/Nutriceutical Formulation Improves Memory and Cognitive Performance in Community-Dwelling Adults without Dementia. J. Nutr. Health Aging 2010, 14, 224–230. [Google Scholar] [CrossRef]
- Park, J.E.; Pichiah, P.B.T.; Cha, Y.S. Vitamin D and Metabolic Diseases: Growing Roles of Vitamin D. J. Obes. Metab. Syndr. 2018, 27, 223–232. [Google Scholar] [CrossRef]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Burne, T.H.J.; McGrath, J.J. Vitamin D, Effects on Brain Development, Adult Brain Function and the Links between Low Levels of Vitamin D and Neuropsychiatric Disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Peng, Q.; Zhang, X.; Guo, J.; Tong, H.; Li, S. Vitamin A Promotes the Repair of Mice Skeletal Muscle Injury through RARα. Nutrients 2023, 15, 3674. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, R. The Role of Obesity in Type 2 Diabetes Mellitus—An Overview. Int. J. Mol. Sci. 2024, 25, 1882. [Google Scholar] [CrossRef] [PubMed]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal Muscle Energy Metabolism in Obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of Diabetes on Cognitive Function and Brain Structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef]
- Purnamasari, D.; Tetrasiwi, E.N.; Kartiko, G.J.; Astrella, C.; Husam, K.; Laksmi, P.W. Sarcopenia and Chronic Complications of Type 2 Diabetes Mellitus. Rev. Diabet. Stud. 2022, 18, 157–165. [Google Scholar] [CrossRef]
- Rajput, M.; Malik, I.A.; Methi, A.; Cortés Silva, J.A.; Fey, D.; Wirths, O.; Fischer, A.; Wilting, J.; von Arnim, C.A.F. Cognitive decline and neuroinflammation in a mouse model of obesity: An accelerating role of ageing. Brain Behav. Immun. 2025, 125, 226–239. [Google Scholar] [CrossRef]
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the Brain: Oxidative Stress, Inflammation, and Autophagy. Oxid. Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef]
- Morrison, C.D. Leptin signaling in brain: A link between nutrition and cognition? Biochim. Biophys. Acta 2009, 1792, 401–408. [Google Scholar] [CrossRef]
- Kostka, M.; Morys, J.; Małecki, A.; Nowacka-Chmielewska, M. Muscle-Brain Crosstalk Mediated by Exercise-Induced Myokines—Insights from Experimental Studies. Front. Physiol. 2024, 15, 1488375. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Yan, Z.; Cui, Y.; Liu, Y.; Cui, S.; Wang, Y.; Liu, T. Unveiling the Muscle-Brain Axis: A Bidirectional Mendelian Randomization Study Investigating the Causal Relationship between Sarcopenia-Related Traits and Brain Aging. Arch. Gerontol. Geriatr. 2024, 123, 105412. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Wang, S.M.; Kang, D.W.; Um, Y.H.; Yoon, H.M.; Lee, S.; Choe, Y.S.; Kim, R.E.Y.; Kim, D.; Lee, C.U.; et al. Development of a Prediction Model for Cognitive Impairment of Sarcopenia Using Multimodal Neuroimaging in Non-Demented Older Adults. Alzheimer’s Dement. 2024, 20, 4868–4878. [Google Scholar] [CrossRef] [PubMed]
- Inyushkin, A.N.; Poletaev, V.S.; Inyushkina, E.M.; Kalberdin, I.S.; Inyushkin, A.A. Irisin/BDNF Signaling in the Muscle-Brain Axis and Circadian System: A Review. J. Biomed. Res. 2023, 38, 1–16. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of Lysosomal Dynamics and Autophagy by CTSB/Cathepsin B. Autophagy 2016, 12, 2504–2505. [Google Scholar] [CrossRef]
- Li, H.; Meng, Q.; Xiao, F.; Chen, S.; Du, Y.; Yu, J.; Li, W. Myokines and Muscle–Brain Crosstalk: The Effects of Exercise on Neurodegenerative Diseases. Front. Neurosci. 2022, 16, 836735. [Google Scholar]
- Piquet, M.; Martínez, M.C.; Romacho, T. Inter-Organ Crosstalk in the Development of Obesity-Associated Insulin Resistance. Handb. Exp. Pharmacol. 2022, 274, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, J.; Lu, C.; Lu, A.; Wang, C. Exercise Regulates Myokines in Aging-Related Diseases through Muscle-Brain Crosstalk. Gerontology 2024, 70, 193–209. [Google Scholar] [CrossRef]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 435092. [Google Scholar] [CrossRef] [PubMed]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Prigent-Tessier, A.; Garnier, P. Molecular Mechanisms Underlying Physical Exercise-Induced Brain BDNF Overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; Zhang, M.; Bai, X.; Feng, Y.; Li, J.; Liu, Y.; Li, J. Decreased Peripheral Brain-Derived Neurotrophic Factor Levels in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar]
- Huang, L.; Yan, S.; Luo, L.; Yang, L. Irisin Regulates the Expression of BDNF and Glycometabolism in Diabetic Rats. Mol. Med. Rep. 2018, 19, 1074. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Fei, A. BDNF Contributes to the Skeletal Muscle Anti-Atrophic Effect of Exercise Training through AMPK-PGC1α Signaling in Heart Failure Mice. Arch. Med. Sci. 2018, 15, 214. [Google Scholar] [CrossRef]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin Ameliorates Age-Associated Sarcopenia and Metabolic Dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef]
- Li, H.; Wang, F.; Yang, M.; Sun, J.; Zhao, Y.; Tang, D. The Effect of Irisin as a Metabolic Regulator and Its Therapeutic Potential for Obesity. Int. J. Endocrinol. 2021, 2021, 6572342. [Google Scholar] [CrossRef] [PubMed]
- Villamil-Parra, W.; Moscoso-Loaiza, L. Effects of Physical Exercise on Irisin and BDNF Concentrations, and Their Relationship with Cardiometabolic and Mental Health of Individuals with Metabolic Syndrome: A Systematic Review. Exp. Gerontol. 2024, 198, 112640. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Wei, P.; Zou, T.; You, J. Fibroblast Growth Factor 21: A Fascinating Perspective on the Regulation of Muscle Metabolism. Int. J. Mol. Sci. 2023, 24, 16951. [Google Scholar] [CrossRef]
- Mashili, F.L.; Cederberg, A.; Carlsson, L.; Fredriksson, J.M.; Peacock, S.M.; Bengtsson, T.; Zierath, J.R. Elevated skeletal muscle FGF21 expression in response to insulin and mitochondrial dysfunction in type 2 diabetes. Cell Metab. 2011, 13, 29–39. [Google Scholar] [CrossRef]
- Geng, L.; Liao, B.; Jin, L.; Huang, Z.; Triggle, C.R.; Ding, H.; Zhang, J.; Huang, Y.; Lin, Z.; Xu, A. Exercise Alleviates Obesity-Induced Metabolic Dysfunction via Enhancing FGF21 Sensitivity in Adipose Tissues. Cell Rep. 2019, 26, 2738–2752.e4. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Wang, X.; Deng, P.; He, W.; Zheng, H.; Zhao, L.; Gao, H. Integration of FGF21 Signaling and Metabolomics in High-Fat Diet-Induced Obesity. J. Proteome Res. 2021, 20, 3900–3912. [Google Scholar] [CrossRef]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.; Matosin, N.; Kaul, D.; Philipsen, A.; Gassen, N.C. The Role of Cathepsins in Memory Functions and the Pathophysiology of Psychiatric Disorders. Front. Psychiatry 2020, 11, 718. [Google Scholar] [CrossRef]
- Ellingsgaard, H.; Hojman, P.; Pedersen, B.K. Exercise and Health—Emerging Roles of IL-6. Curr. Opin. Physiol. 2019, 10, 49–54. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; Yin, H.; Zhang, X.; Wang, D.; Ma, C.; Li, J.; Zhao, Y.; Li, X. Interleukin-6 Mediated Exercise-Induced Alleviation of Adiposity and Hepatic Steatosis in Mice. BMJ Open Diabetes Res. Care 2021, 9, e001431. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin Reduces Akt/TORC1/P70S6K Signaling, Inhibiting Myoblast Differentiation and Myotube Size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Allen, D.L.; Hittel, D.S.; McPherron, A.C. Expression and Function of Myostatin in Obesity, Diabetes, and Exercise Adaptation. Med. Sci. Sports Exerc. 2011, 43, 1828. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Jiang, N.; Liu, Y.; Luo, S.; Li, C.; Zhao, H.; Han, Y.; Chen, W.; Li, L.; et al. Myostatin: A Potential Therapeutic Target for Metabolic Syndrome. Front. Endocrinol. 2023, 14, 1181913. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and Its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of Myostatin in Mice Improves Insulin Sensitivity via Irisin-Mediated Cross Talk between Muscle and Adipose Tissues. Int. J. Obes. 2015, 40, 434. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Perakakis, N.; Triantafyllou, G.A.; Kulkarni, S.S.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and Role of Irisin in Glucose Homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF Mediates Adaptive Brain and Body Responses to Energetic Challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.Y.; Lim, Y. Annona Muricate Extract Supplementation Contributes to Improve Aberrant Multi-Organ Energy Metabolism via Muscle-Brain Connectivity in Diabetic Mice. Nutrients 2023, 15, 2559. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.-J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic Variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related Behavior. Science 2006, 314, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Wei, W.P.; Yang, P.; Zhang, R.; Sheng, Y.; Shi, Y.Q. Relationship between Circulating Irisin Levels and Overweight/Obesity: A Meta-Analysis. World J. Clin. Cases 2019, 7, 1444. [Google Scholar] [CrossRef]
- Ansari, S.; Djalali, M.; Honarvar, N.M.; Mazaherioun, M.; Zarei, M.; Agh, F.; Gholampour, Z.; Javanbakht, M.H. The Effect of N-3 Polyunsaturated Fatty Acids Supplementation on Serum Irisin in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Endocrinol. Metab. 2017, 15, e40614. [Google Scholar] [CrossRef] [PubMed]
- Nadimi, H.; Djazayery, A.; Javanbakht, M.H.; Dehpour, A.; Ghaedi, E.; Derakhshanian, H.; Mohammadi, H.; Zarei, M.; Djalali, M. The Effect of Vitamin D Supplementation on Serum and Muscle Irisin Levels, and FNDC5 Expression in Diabetic Rats. Rep. Biochem. Mol. Biol. 2019, 8, 236. [Google Scholar]
- Lin, K.; Lin, J.; Lin, H.; Chen, Y.; Wang, M.; Hsiao, P.; Tsou, P.; Lee, M.; Chen, C. Associations between circulating irisin levels and cognitive function in patients with type 2 diabetes. J. Diabetes Complicat. 2019, 33, 107414. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, H.; Liang, J.; Zhang, Q.; Sun, J.; Yang, H.; Yan, H.; Zhang, S.; Li, Y.; Jin, Y.; et al. Increased FGF-21 Improves Ectopic Lipid Deposition in the Liver and Skeletal Muscle. Nutrients 2024, 16, 1254. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.G.; Jornayvaz, F.R.; Petersen, M.C.; Pesta, D.; Guigni, B.A.; Serr, J.; Zhang, D.; Kahn, M.; Samuel, V.T.; Jurczak, M.J.; et al. Cellular Mechanisms by Which FGF21 Improves Insulin Sensitivity in Male Mice. Endocrinology 2013, 154, 3099–3109. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, D.C.Y.; Karpisek, M.; Stejskal, D.; Zhou, Z.-G.; Liu, F.; Wong, R.L.C.; Chow, W.-S.; Tso, A.W.K.; Lam, K.S.L.; et al. Serum FGF21 Levels Are Increased in Obesity and Are Independently Associated with the Metabolic Syndrome in Humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef]
- Mráz, M.; Bartlová, M.; Lacinová, Z.; Michalský, D.; Kasalický, M.; Haluzíková, D.; Matoulek, M.; Doležalová, R.; Humenanská, V.; Haluzík, M. Serum concentrations of fibroblast growth factor 21 in patients with obesity and type 2 diabetes mellitus. Clin. Endocrinol. 2011, 75, 470–475. [Google Scholar] [CrossRef]
- Flippo, K.H.; and Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The Fasting Polypeptide FGF21 Can Enter Brain from Blood; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, S.H.; Min, Y.K.; Yang, H.M.; Lee, J.B.; Lee, M.S. Acute Exercise Induces FGF21 Expression in Mice and in Healthy Humans. PLoS ONE 2013, 8, e63517. [Google Scholar] [CrossRef]
- Li, B.F.; Huang, S.; Wu, K.; Liu, Y. Altered Cathepsin B Expression as a Diagnostic Marker of Skeletal Muscle Insulin Resistance in Type 2 Diabetes. ACS Biomater. Sci. Eng. 2023, 9, 2731–2740. [Google Scholar] [CrossRef]
- Araujo, T.F.; Cordeiro, A.V.; Vasconcelos, D.A.A.; Vitzel, K.F.; Silva, V.R.R. The Role of Cathepsin B in Autophagy during Obesity: A Systematic Review. Life Sci. 2018, 209, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-Induced Skeletal Muscle Atrophy. J. Appl. Physiol. 2005, 98, 911–917. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, F.Y.; Hsiao, Y.H. Myostatin Is Associated With Cognitive Decline in an Animal Model of Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 1984–1991. [Google Scholar] [CrossRef]
- Desgeorges, M.M.; Devillard, X.; Toutain, J.; Castells, J.; Divoux, D.; Arnould, D.F.; Haqq, C.; Bernaudin, M.; Durieux, A.C.; Touzani, O.; et al. Pharmacological Inhibition of Myostatin Improves Skeletal Muscle Mass and Function in a Mouse Model of Stroke. Sci. Rep. 2017, 7, 14000. [Google Scholar] [CrossRef] [PubMed]
- McNeish, D.M.; Bethune, A.J.; Xu, J.; Widjaja, E.; Halliday, G.M.; Hodges, J.R.; Leyton, C.E. Associations Between Circulating Levels of Myostatin and Plasma β-Amyloid 42/40 in a Biracial Cohort of Older Adults. J. Gerontol. Ser. A 2023, 78, 1560–1567. [Google Scholar] [CrossRef]
- Hofmann, M.; Schober-Halper, B.; Oesen, S.; Franzke, B.; Tschan, H.; Bachl, N.; Strasser, E.-M.; Quittan, M.; Hellweg, R.; Freiberger, E.; et al. Effects of Elastic Band Resistance Training and Nutritional Supplementation on Muscle Quality and Serum Myokine Levels in Elderly Individuals. Exp. Gerontol. 2016, 79, 42–51. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Nitzan, O.; Deans, C.; Lloyd, D.J. Mechanisms by Which Skeletal Muscle Myokines Ameliorate Insulin Resistance. Int. J. Mol. Sci. 2022, 23, 4636. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, M. Effects of Aerobic Exercise on Global Cognitive Function and Sleep in Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Geriatr. Nurs. 2023, 51, 9–16. [Google Scholar] [CrossRef]
- Blomstrand, P.; Tesan, D.; Nylander, E.M.; Ramstrand, N. Mind Body Exercise Improves Cognitive Function More than Aerobic- and Resistance Exercise in Healthy Adults Aged 55 Years and Older—An Umbrella Review. Eur. Rev. Aging Phys. Act. 2023, 20, 15. [Google Scholar] [CrossRef]
- Choi, J.W.; Jo, S.W.; Kim, D.E.; Paik, I.Y.; Balakrishnan, R. Aerobic Exercise Attenuates LPS-Induced Cognitive Dysfunction by Reducing Oxidative Stress, Glial Activation, and Neuroinflammation. Redox Biol. 2024, 71, 103101. [Google Scholar] [CrossRef]
- Akalp, O.; Feeney, J.; O’Keeffe, S.T.; Walsh, N.; O’Shea, D.; Kenny, R.A.; Kearney, P.M.; Lawlor, B.; Ronan, L.; Laird, E. Effects of Exercise Training on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2024, 25, 898–903. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Zeng, Z.; Wu, L.; Zhang, Y.; Guo, Y.; Lv, J.; Wang, C.; Fan, J.; Chen, N. Lifelong Aerobic Exercise Alleviates Sarcopenia by Activating Autophagy and Inhibiting Protein Degradation via the AMPK/PGC-1α Signaling Pathway. Metabolites 2021, 11, 323. [Google Scholar] [CrossRef]
- McGlory, C.; Devries, M.C.; Phillips, S.M. Skeletal Muscle and Resistance Exercise Training; the Role of Protein Synthesis in Recovery and Remodeling. J. Appl. Physiol. 2016, 122, 541. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Pinho, R.A.; Aguiar, A.S.; Radák, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, 529. [Google Scholar] [CrossRef]
- Li, N.; Shi, H.; Guo, Q.; Gan, Y.; Zhang, Y.; Jia, J.; Zhang, L.; Zhou, Y. Aerobic Exercise Prevents Chronic Inflammation and Insulin Resistance in Skeletal Muscle of High-Fat Diet Mice. Nutrients 2022, 14, 3730. [Google Scholar] [CrossRef]
- Lei, Y.; Gan, M.; Qiu, Y.; Chen, Q.; Wang, X.; Liao, T.; Zhao, M.; Chen, L.; Zhang, S.; Zhao, Y.; et al. The Role of Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Atrophy: From Molecular Mechanisms to Therapeutic Insights. Cell. Mol. Biol. Lett. 2024, 29, 59. [Google Scholar] [CrossRef]
- Park, S.S.; Seo, Y.K.; Kwon, K.S. Sarcopenia Targeting with Autophagy Mechanism by Exercise. BMB Rep. 2019, 52, 64. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Pai, M.-C.; Ukropec, J.; Ukropcová, B. Distinctive Effects of Aerobic and Resistance Exercise Modes on Neurocognitive and Biochemical Changes in Individuals with Mild Cognitive Impairment. Curr. Alzheimer Res. 2019, 16, 316–332. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, W.; Chen, Q.; Zhang, Y.; Kuo, C.H.; Liu, J. Synergistic Effects of Exercise and Nutritional Interventions on Skeletal Muscle Health and Cognitive Performance in Metabolic Disorders: A Systematic Review. Nutrients 2023, 15, 1450. [Google Scholar] [CrossRef]
- Cadore, E.L.; Izquierdo, M. How to Simultaneously Optimize Muscle Strength, Power, and Hypertrophy in Aging Adults: Exercise Prescription Considerations. Sports Med. 2022, 52, 249–267. [Google Scholar] [CrossRef]
- Konopka, A.R.; Harber, M.P. Skeletal Muscle Strength and Hypertrophy Adaptations in Older Adults with Metabolic Disease: Exercise Tolerance and Mechanistic Considerations. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 1024–1034. [Google Scholar] [CrossRef]
- Chen, G.; Batignani, G.; Mandard, S.; Marin, J.J.; Strom, S. Roles of Vitamin A Metabolism in the Development of Hepatic Insulin Resistance. Int. Sch. Res. Not. Hepatol. 2013, 2013, 534972. [Google Scholar] [CrossRef]
- Amengual, J.; García-Carrizo, F.J.; Arreguín, A.; Mušinović, H.; Granados, N.; Palou, A.; Bonet, M.L.; Ribot, J. Retinoic Acid Increases Fatty Acid Oxidation and Irisin Expression in Skeletal Muscle Cells and Impacts Irisin In Vivo. Cell. Physiol. Biochem. 2018, 46, 187–202. [Google Scholar] [CrossRef]
- Biyong, E.F.; Alfos, S.; Dumetz, F.; Helbling, J.C.; Aubert, A.; Brossaud, J.; Foury, A.; Moisan, M.P.; Layé, S.; Richard, E.; et al. Dietary Vitamin A Supplementation Prevents Early Obesogenic Diet-Induced Microbiota, Neuronal and Cognitive Alterations. Int. J. Obes. 2020, 45, 588–598. [Google Scholar] [CrossRef]
- Grineva, E.N.; Karonova, T.; Micheeva, E.; Belyaeva, O.; Nikitina, I.L. Vitamin D Deficiency Is a Risk Factor for Obesity and Diabetes Type 2 in Women at Late Reproductive Age. Aging 2013, 5, 575–581. [Google Scholar] [CrossRef]
- Braga, M.; Simmons, Z.; Norris, K.C.; Ferrini, M.G.; Artaza, J.N. Vitamin D Induces Myogenic Differentiation in Skeletal Muscle Derived Stem Cells. Endocr. Connect. 2017, 6, 139. [Google Scholar] [CrossRef]
- Sanesi, L.; Dicarlo, M.; Pignataro, P.; Zerlotin, R.; Pugliese, F.; Columbu, C.; Carnevale, V.; Tunnera, S.; Scillitani, A.; Grano, M.; et al. Vitamin D Increases Irisin Serum Levels and the Expression of Its Precursor in Skeletal Muscle. Int. J. Mol. Sci. 2023, 24, 4129. [Google Scholar] [CrossRef]
- Anjum, I.; Jaffery, S.S.; Fayyaz, M.; Samoo, Z.; Anjum, S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus 2018, 10, e2960. [Google Scholar] [CrossRef]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G.; et al. Brain Vitamin D Forms, Cognitive Decline, and Neuropathology in Community-Dwelling Older Adults. Alzheimer’s Dement. 2023, 19, 2389–2396. [Google Scholar] [CrossRef]
- Calder, P.C.; Grimble, R.F. Polyunsaturated Fatty Acids, Inflammation and Immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef]
- Baynes, H.W.; Mideksa, S.; Ambachew, S. The Role of Polyunsaturated Fatty Acids (n-3 PUFAs) on the Pancreatic β-Cells and Insulin Action. Adipocyte 2018, 7, 81. [Google Scholar] [CrossRef]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A Systematic Review, Meta-Analysis and Meta-Regression of the Effect of Protein Supplementation on Resistance Training–Induced Gains in Muscle Mass and Strength in Healthy Adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef]
- Kimball, S.R.; Jefferson, L.S. Signaling Pathways and Molecular Mechanisms through Which Branched—Chain Amino Acids Mediate Translational Control of Protein Synthesis. J. Nutr. 2006, 136, 227S–231S. [Google Scholar] [CrossRef]
- Phillips, S.M. A Brief Review of Critical Processes in Exercise-Induced Muscular Hypertrophy. Sports Med. 2014, 44, 71–77. [Google Scholar] [CrossRef]
- Leuchtmann, A.B.; Schmidt-Trucksäss, A.; Knechtle, B.; Ruhs, H.; Knechtle, P.; Schäfer, J. Exercise-Induced Irisin, Myostatin and IL-6 Responses: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1681. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscles and Their Myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef]
- Moberg, M.; Apró, W.; Ohlsson, I.; Pontén, M.; Villanueva, A.; Ekblom, B.; Blomstrand, E. Absence of leucine in an essential amino acid supplement reduces activation of mTORC1 signalling following resistance exercise in young females. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Et Metab. 2014, 39, 183–194. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Conte Camerino, D.; Tricarico, D.; Pierno, S.; Desaphy, J.F.; Liantonio, A.; Pusch, M.; Burdi, R.; Camerino, C.; Fraysse, B.; De Luca, A. Taurine and skeletal muscle disorders. Neurochem. Res. 2004, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Spriet, L.L.; Whitfield, J. Taurine and skeletal muscle function. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 96–101. [Google Scholar] [CrossRef]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine Supplementation in the Aging Population: Effects on Skeletal Muscle, Bone and Brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of Creatine Supplementation on Cognitive Function: A Systematic Review of Randomized Controlled Trials. Psychopharmacology 2018, 235, 2811–2827. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; A Swinton, P.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-Alanine Supplementation to Improve Exercise Capacity and Performance: A Systematic Review and Meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. Carnosine and Its Possible Roles in Nutrition and Health. Adv. Food Nutr. Res. 2009, 57, 87–154. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, H.; Song, G.; Yang, Y.; Zou, X.; Han, P.; Li, S. Resveratrol Improves Muscle Atrophy by Modulating Mitochondrial Quality Control in STZ-Induced Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, e1700941. [Google Scholar] [CrossRef] [PubMed]
- Boondam, Y.; Saefoong, C.; Niltup, N.; Monteil, A.; Kitphati, W. The Cognitive Restoration Effects of Resveratrol: Insight Molecular through Behavioral Studies in Various Cognitive Impairment Models. ACS Pharmacol. Transl. Sci. 2024, 2024, 3334–3357. [Google Scholar] [CrossRef]
- Kim, O.Y.; Chung, J.Y.; Song, J. Effect of Resveratrol on Adipokines and Myokines Involved in Fat Browning: Perspectives in Healthy Weight against Obesity. Pharmacol. Res. 2019, 148, 104411. [Google Scholar] [CrossRef]
- Thaung Zaw, J.J.; Howe, P.R.; Wong, R.H. Long-Term Effects of Resveratrol on Cognition, Cerebrovascular Function and Cardio-Metabolic Markers in Postmenopausal Women: A 24-Month Randomised, Double-Blind, Placebo-Controlled, Crossover Study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Bahramzadeh, A.; Bolandnazar, K.; Meshkani, R. Resveratrol as a Potential Protective Compound against Skeletal Muscle Insulin Resistance. Heliyon 2023, 9, 2405–8440. [Google Scholar] [CrossRef]
- Kodali, M.; Parihar, V.K.; Hattiangady, B.; Mishra, V.; Shuai, B.; Shetty, A.K. Resveratrol Prevents Age-Related Memory and Mood Dysfunction with Increased Hippocampal Neurogenesis and Microvasculature and Reduced Glial Activation. Sci. Rep. 2015, 5, 8075. [Google Scholar] [CrossRef]
- Shojaei, S.; Panjehshahin, M.R.; Shafiee, S.M.; Khoshdel, Z.; Borji, M.; Ghasempour, G.; Owji, A.A. Differential Effects of Resveratrol on the Expression of Brain-Derived Neurotrophic Factor Transcripts and Protein in the Hippocampus of Rat Brain. Iran. J. Med. Sci. 2017, 42, 32. [Google Scholar]
- Guo, J.; Li, Z.; Yao, Y.; Fang, L.; Yu, M.; Wang, Z. Curcumin in the Treatment of Inflammation and Oxidative Stress Responses in Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Front. Neurol. 2024, 15, 1380353. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Tian, M.; Wang, L.; Yu, G.; Liu, B.; Li, Y. Curcumin Preserves Cognitive Function and Improve Serum HDL in Chronic Cerebral Hypoperfusion Aging-Rats. Mol. Neurodegener. 2012, 7, S3. [Google Scholar] [CrossRef]
- Saud Gany, S.L.; Chin, K.Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a Therapeutic Agent for Sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef]
- Zou, T.; Li, S.; Wang, B.; Wang, Z.; Liu, Y.; You, J. Curcumin Improves Insulin Sensitivity and Increases Energy Expenditure in High-Fat-Diet-Induced Obese Mice Associated with Activation of FNDC5/Irisin. Nutrition 2021, 90, 111263. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Butler, A.E.; Moallem, S.A.; Sahebkar, A. The Effects of Curcumin on Brain-Derived Neurotrophic Factor Expression in Neurodegenerative Disorders. Curr. Med. Chem. 2024, 31, 5937–5952. [Google Scholar] [CrossRef]
- Sarraf, P.; Parohan, M.; Javanbakht, M.H.; Ranji-Burachaloo, S.; Djalali, M. Short-Term Curcumin Supplementation Enhances Serum Brain-Derived Neurotrophic Factor in Adult Men and Women: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutr. Res. 2019, 69, 1–8. [Google Scholar] [CrossRef]
- Enayati, A.; Soghi, A.; Butler, A.E.; Rizzo, M.; Sahebkar, A. The Effect of Curcumin on the Gut-Brain Axis: Therapeutic Implications. J. Neurogastroenterol. Motil. 2023, 29, 409. [Google Scholar] [CrossRef]
- Yi, H.S. Sclerostin as a Putative Myokine in Sarcopenia. Endocrinol. Metab. 2022, 37, 430–431. [Google Scholar] [CrossRef]
- da Costa Teixeira, L.A.; Avelar, N.C.P.; Peixoto, M.F.D.; Parentoni, A.N.; dos Santos, J.M.; Pereira, F.S.M.; Danielewicz, A.L.; Leopoldino, A.A.O.; Costa, S.P.; Arrieiro, A.N.; et al. Inflammatory Biomarkers at Different Stages of Sarcopenia in Older Women. Sci. Rep. 2023, 13, 10367. [Google Scholar] [CrossRef]
- Ishibashi, C.; Nakanishi, K.; Nishida, M.; Shinomiya, H.; Shinzawa, M.; Kanayama, D.; Yamamoto, R.; Kudo, T.; Nagatomo, I.; Yamauchi-Takihara, K. Myostatin as a Plausible Biomarker for Early Stage of Sarcopenic Obesity. Sci. Rep. 2024, 14, 28629. [Google Scholar] [CrossRef]
- Eo, H.; Lee, H.-J.; Lim, Y. Ameliorative Effect of Dietary Genistein on Diabetes Induced Hyper-Inflammation and Oxidative Stress during Early Stage of Wound Healing in Alloxan Induced Diabetic Mice. Biochem. Biophys. Res. Commun. 2016, 478, 1021–1027. [Google Scholar] [CrossRef]
- Rostás, I.; Pótó, L.; Mátrai, P.; Hegyi, P.; Tenk, J.; Garami, A.; Illés, A.; Solymár, M.; Pétervári, E.; Szűcs, Á.; et al. In Middle-Aged and Old Obese Patients, Training Intervention Reduces Leptin Level: A Meta-Analysis. PLoS ONE 2017, 12, e0182801. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, A.E.; Ard, J.D.; Gudzune, K.A.; Herman, W.H. Obesity Management for the Treatment of Type 2 Diabetes. In Diabetes in America; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2024. [Google Scholar]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in aging: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef]
- Davison, G.W.; George, L.; Jackson, S.K.; Young, I.S.; Davies, B.; Bailey, D.M.; Peters, J.R.; Ashton, T. Exercise, free radicals, and lipid peroxidation in type 1 diabetes mellitus. Free. Radic. Biol. Med. 2002, 33, 1543–1551. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.Y.; Lim, Y. Solanum Melongena Extract Supplementation Protected Skeletal Muscle and Brain Damage by Regulation of BDNF/PGC1α/Irisin Pathway via Brain Function-Related Myokines in High-Fat Diet Induced Obese Mice. J. Nutr. Biochem. 2024, 124, 109537. [Google Scholar] [CrossRef] [PubMed]
| Circulating Factor | Origin (Expression Trend) | Target Organ | Effects on the Brain | Mechanisms of Action | Main References |
|---|---|---|---|---|---|
| Brain-derived neurotrophic factor | Skeletal muscle, brain (↑ → ↓) | Hippocampus, skeletal muscle |
|
| [33,34,35,36,37,38] |
| Irisin | Skeletal muscle, hippocampus (↑ → ↓) | Hippocampus, skeletal muscle |
|
| [39,40,41] |
| Fibroblast growth factor 21 (FGF21) | Liver, skeletal muscle, adipose tissue (↑ → ↓) | Hypothalamus Adipose tissue, skeletal muscle |
|
| [42,43,44,45,46] |
| CTSB | Skeletal muscle (↓) | Hippocampus, skeletal muscle |
|
| [4,47] |
| IL-6 | Skeletal muscle, adipose tissue, liver (Kupffer cells), macrophages (↑) | Hippocampus, skeletal muscle |
|
| [48,49,50] |
| Myostatin | Skeletal muscle (↑) | Brain (cortex, hippocampus), skeletal muscle |
|
| [51,52,53,54,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Lim, Y. Can Myokines Serve as Supporters of Muscle–Brain Connectivity in Obesity and Type 2 Diabetes? Potential of Exercise and Nutrition Interventions. Nutrients 2025, 17, 3615. https://doi.org/10.3390/nu17223615
Lee H, Lim Y. Can Myokines Serve as Supporters of Muscle–Brain Connectivity in Obesity and Type 2 Diabetes? Potential of Exercise and Nutrition Interventions. Nutrients. 2025; 17(22):3615. https://doi.org/10.3390/nu17223615
Chicago/Turabian StyleLee, Heaji, and Yunsook Lim. 2025. "Can Myokines Serve as Supporters of Muscle–Brain Connectivity in Obesity and Type 2 Diabetes? Potential of Exercise and Nutrition Interventions" Nutrients 17, no. 22: 3615. https://doi.org/10.3390/nu17223615
APA StyleLee, H., & Lim, Y. (2025). Can Myokines Serve as Supporters of Muscle–Brain Connectivity in Obesity and Type 2 Diabetes? Potential of Exercise and Nutrition Interventions. Nutrients, 17(22), 3615. https://doi.org/10.3390/nu17223615







