Salivary Total Antioxidant Capacity of Sportive Adolescents—The Effect of Antioxidant Vitamin Intake with Usual Diet and Physical Exercises
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Protocol and Measures
2.2.1. Anthropometric and Blood Pressure Data
2.2.2. Energy Expenditure
2.2.3. Nutritional Assessment
2.2.4. Salivary TAC and pH
2.3. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Nutrient Intake
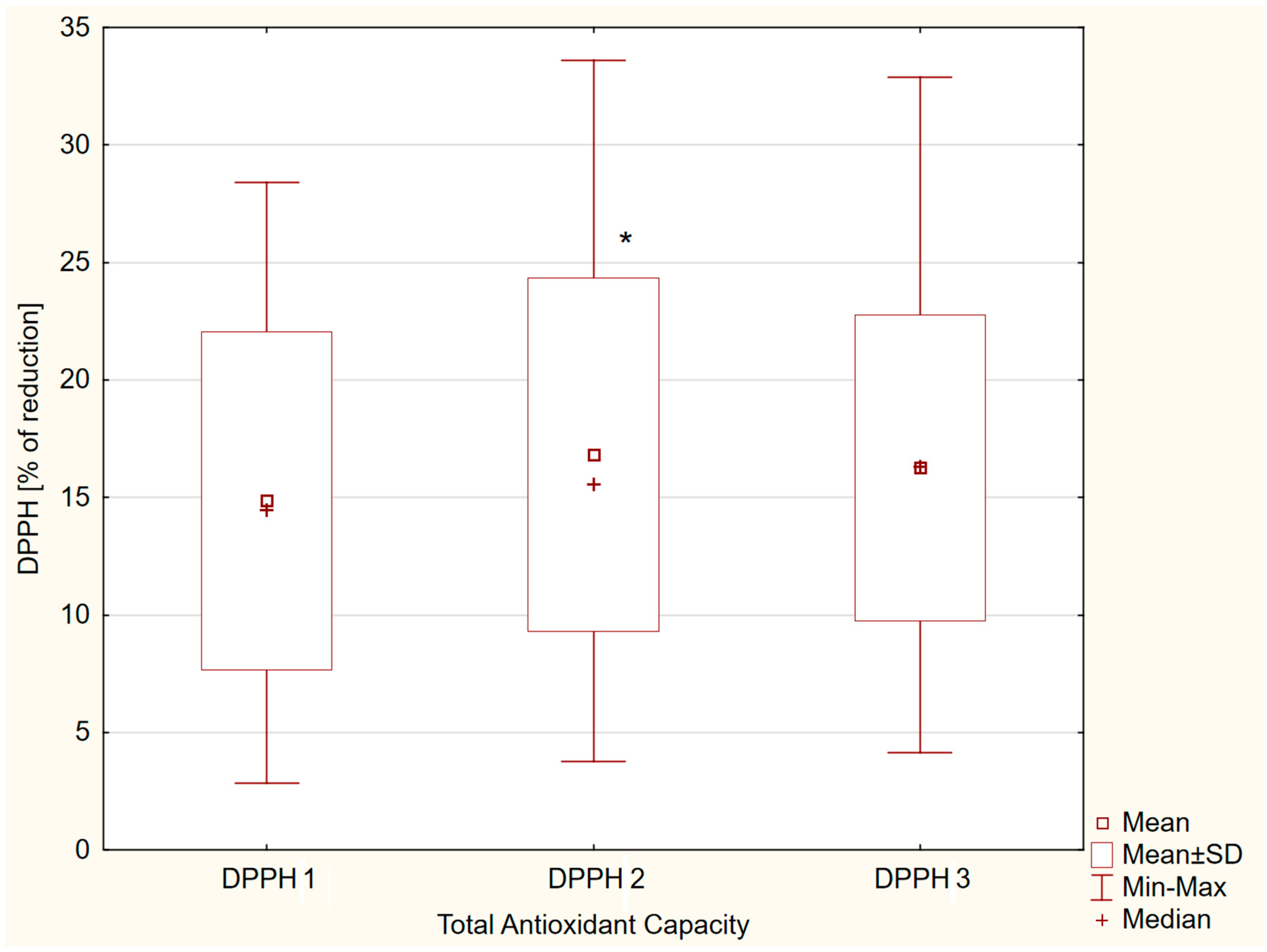
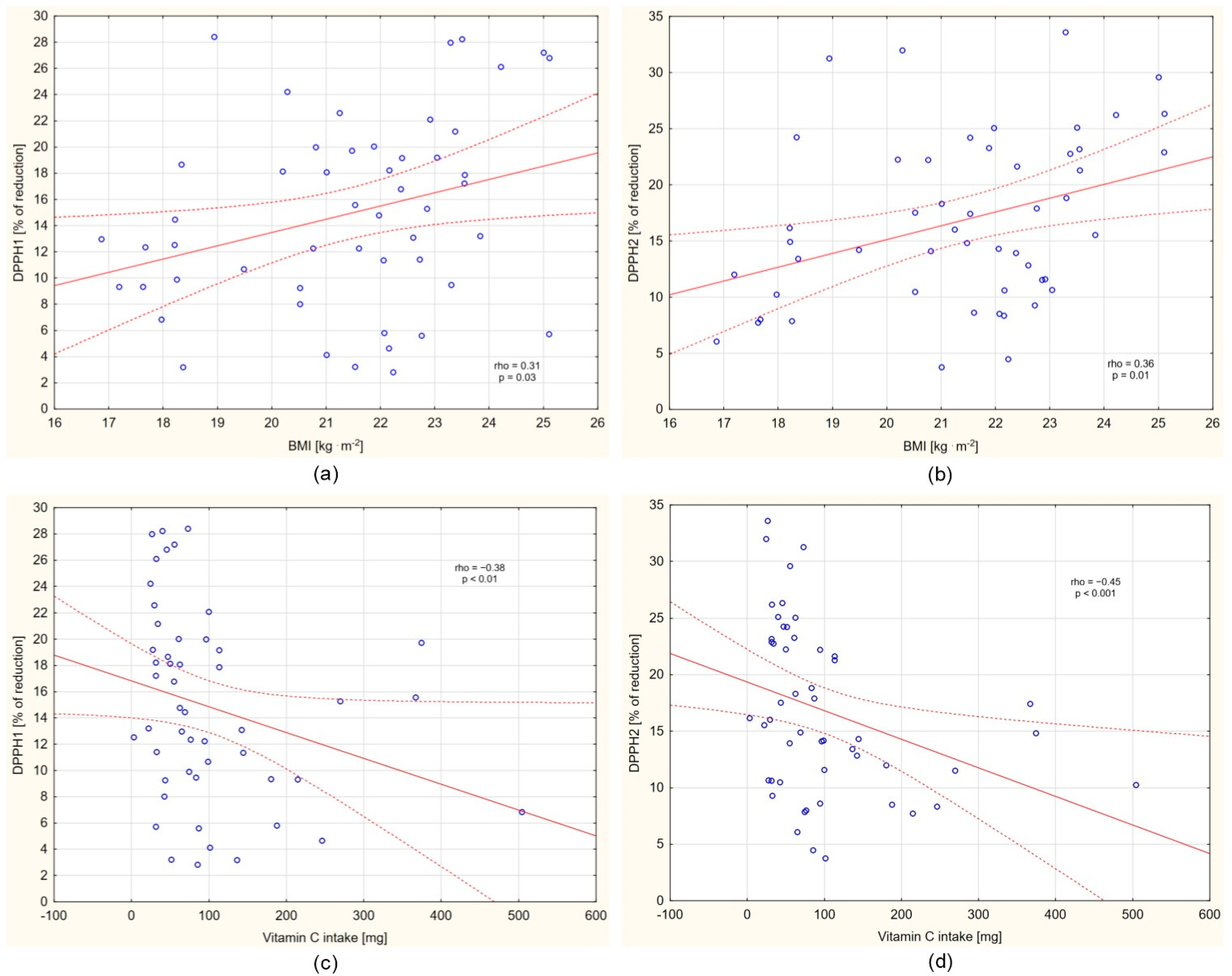
3.3. Analyses of DPPH Changes and Selected Correlates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- González, D.; Marquina, R.; Rondón, N.; Rodriguez-Malaver, A.J.; Reyes, R. Effects of Aerobic Exercise on Uric Acid, Total Antioxidant Activity, Oxidative Stress, and Nitric Oxide in Human Saliva. Res. Sports Med. 2008, 16, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ostachowska-Gasior, A.; Kolarzyk, E.; Szot, W.; Lyszczarz, J. The Relation between Antioxidative Ability and the Diet of Young Swimmers. Rocz. Akad. Med. Bialymst. 2005, 50 (Suppl. S1), 241–244. [Google Scholar]
- Damirchi, A.; Saati Zareei, A.; Sariri, R. Salivary Antioxidants of Male Athletes after Aerobic Exercise and Garlic Supplementation on: A Randomized, Double Blind, Placebo-Controlled Study. J. Oral Biol. Craniofacial Res. 2015, 5, 146–152. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Azimi, S.; Mehdipour, M.; Amini, S.; Elmi, Z.; Namazi, Z. Effect of Cigarette Smoke on Salivary Total Antioxidant Capacity. J. Dent. Res. Dent. Clin. Dent. Prospects 2015, 9, 281–284. [Google Scholar] [CrossRef]
- Neshat, F.; Shirzaiy, M.; Shademan, S. Salivary Total Antioxidant and Lipid Peroxidation Levels in Passive Smoking and Nonsmoking Adolescents. Addict. Health 2020, 12, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Ghorchi, V.; Rezaei, F.; Vaisi-Raygani, A. Evaluation of Total Antioxidant Capacity of Saliva in High School Students. Glob. J. Health Sci. 2015, 8, 89–94. [Google Scholar] [CrossRef]
- Matei, M.N.; Popa, P.Ș.; Covaci, A.M.; Chipirliu, O.; Earar, K.; Stoica, G.; Zaharia, A.E.; Maftei, N.M.; Gurău, G.; Lisă, E.L.; et al. The Impact of Competitive Sports on Oral Health: Exploring Their Relationship with Salivary Oxidative Stress in Children. Healthcare 2023, 11, 2927. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Tonosaki, K.; Fujisawa, S. Salivary Free Radical-Scavenging Activity Is Affected by Physical and Mental Activities. Oral Dis. 2008, 14, 490–496. [Google Scholar] [CrossRef]
- Lin, S.-P.; Li, C.-Y.; Suzuki, K.; Chang, C.-K.; Chou, K.-M.; Fang, S.-H. Green Tea Consumption after Intense Taekwondo Training Enhances Salivary Defense Factors and Antibacterial Capacity. PLoS ONE 2014, 9, e87580. [Google Scholar] [CrossRef]
- Gawron-Skarbek, A.; Chrzczanowicz, J.; Kostka, J.; Nowak, D.; Drygas, W.; Jegier, A.; Kostka, T. Physical Activity, Aerobic Capacity, and Total Antioxidant Capacity in Healthy Men and in Men with Coronary Heart Disease. Oxid. Med. Cell. Longev. 2015, 2015, 197307. [Google Scholar] [CrossRef]
- Zalavras, A.; Fatouros, I.G.; Deli, C.K.; Draganidis, D.; Theodorou, A.A.; Soulas, D.; Koutsioras, Y.; Koutedakis, Y.; Jamurtas, A.Z. Age-Related Responses in Circulating Markers of Redox Status in Healthy Adolescents and Adults during the Course of a Training Macrocycle. Oxid. Med. Cell. Longev. 2015, 2015, 283921. [Google Scholar] [CrossRef]
- Battino, M.; Ferreiro, M.S.; Gallardo, I.; Newman, H.N.; Bullon, P. The Antioxidant Capacity of Saliva. J. Clin. Periodontol. 2002, 29, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Raguzzini, A. Salivary and Urinary Total Antioxidant Capacity as Biomarkers of Oxidative Stress in Humans. Pathol. Res. Int. 2016, 2016, 5480267. [Google Scholar] [CrossRef] [PubMed]
- Chrzczanowicz, J.; Gawron, A.; Zwolinska, A.; de Graft-Johnson, J.; Krajewski, W.; Krol, M.; Markowski, J.; Kostka, T.; Nowak, D. Simple Method for Determining Human Serum 2,2-Diphenyl-1-Picryl-Hydrazyl (DPPH) Radical Scavenging Activity-Possible Application in Clinical Studies on Dietary Antioxidants. Clin. Chem. Lab. Med. 2008, 46, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Masini, S.; Carpeggiani, C.; L’Abbate, A.; Boni, C.; Zucchelli, G.C. In Vivo Total Antioxidant Capacity: Comparison of Two Different Analytical Methods. Clin. Chem. Lab. Med. 2004, 42, 84–89. [Google Scholar] [CrossRef]
- Sies, H. Total Antioxidant Capacity: Appraisal of a Concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [CrossRef]
- Greabu, M.; Battino, M.; Mohora, M.; Totan, A.; Spinu, T.; Totan, C.; Didilescu, A.; Duţa, C. Could Constitute Saliva the First Line of Defence against Oxidative Stress? Rom. J. Intern. Med. 2007, 45, 209–213. [Google Scholar]
- Buczko, P.; Zalewska, A.; Szarmach, I. Saliva and Oxidative Stress in Oral Cavity and in Some Systemic Disorders. J. Physiol. Pharmacol. 2015, 66, 3–9. [Google Scholar]
- Martins, J.R.; Díaz-Fabregat, B.; Ramírez-Carmona, W.; Monteiro, D.R.; Pessan, J.P.; Antoniali, C. Salivary Biomarkers of Oxidative Stress in Children with Dental Caries: Systematic Review and Meta-Analysis. Arch. Oral Biol. 2022, 139, 105432. [Google Scholar] [CrossRef]
- Mohideen, K.; Chandrasekaran, K.; Veeraraghavan, H.; Faizee, S.H.; Dhungel, S.; Ghosh, S. Meta-Analysis of Assessment of Total Oxidative Stress and Total Antioxidant Capacity in Patients with Periodontitis. Dis. Markers 2023, 2023, 9949047. [Google Scholar] [CrossRef]
- Jarosz, M. Standards of Human Nutrition; Food and Nutrition Institute: Warsaw, Poland, 2012. [Google Scholar]
- Stelmach, W.; Kaczmarczyk-Chałas, K.; Bielecki, W.; Drygas, W. The Impact of Income, Education and Health on Lifestyle in a Large Urban Population of Poland (Cindi Programme). Int. J. Occup. Med. Environ. Health 2004, 17, 393–401. [Google Scholar]
- Tomaszewski, P.; Milde, K.; Stupnicki, R. Body Mass Index–Proposed Norms for Children and Youths. Pap. Anthropol. 2013, 22, 203–213. [Google Scholar] [CrossRef]
- Kułaga, Z.; Litwin, M.; Grajda, A.; Gurzkowska, B.; Napieralska, E.; Kułaga, K. Distribution of Blood Pressure in School-Aged Children and Adolescents Reference Population. Stand. Med. 2010, 7, 853–864. [Google Scholar]
- Fox, S.M., 3rd; Naughton, J.P.; Gorman, P.A. Physical Activity and Cardiovascular Health. 3. The Exercise Prescription: Frequency and Type of Activity. Mod. Concepts Cardiovasc. Dis. 1972, 41, 25–30. [Google Scholar] [PubMed]
- Szponar, L.; Wolnicka, K.; Rychlik, E. Album of Photographs of Food Products and Dishes; National Food and Nutrition Institute: Warsaw, Poland, 2000. [Google Scholar]
- Kunachowicz, H.; Nadolna, I.; Przygoda, B.; Iwanow, K. Charts of Nutritive Values of Products and Foods; PZWL: Warsaw, Poland, 2005. [Google Scholar]
- Navazesh, M. Methods for Collecting Saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Gawron-Skarbek, A.; Prymont-Przymińska, A.; Sobczak, A.; Guligowska, A.; Kostka, T.; Nowak, D.; Szatko, F. A Comparison of Native and Non-Urate Total Antioxidant Capacity of Fasting Plasma and Saliva among Middle-Aged and Older Subjects. Redox Rep. 2018, 23, 57–62. [Google Scholar] [CrossRef]
- Atsumi, T.; Iwakura, I.; Kashiwagi, Y.; Fujisawa, S.; Ueha, T. Free Radical Scavenging Activity in the Nonenzymatic Fraction of Human Saliva: A Simple DPPH Assay Showing the Effect of Physical Exercise. Antioxid. Redox Signal. 1999, 1, 537–546. [Google Scholar] [CrossRef]
- Evans, L.W.; Omaye, S.T. Use of Saliva Biomarkers to Monitor Efficacy of Vitamin C in Exercise-Induced Oxidative Stress. Antioxidants 2017, 6, 5. [Google Scholar] [CrossRef]
- Gawron-Skarbek, A.; Guligowska, A.; Prymont-Przymińska, A.; Godala, M.; Kolmaga, A.; Nowak, D.; Szatko, F.; Kostka, T. Dietary Vitamin C, E and β-Carotene Intake Does Not Significantly Affect Plasma or Salivary Antioxidant Indices and Salivary C-Reactive Protein in Older Subjects. Nutrients 2017, 9, 729. [Google Scholar] [CrossRef]
- Logan, D.; Wallace, S.M.; Woodside, J.V.; McKenna, G. The Potential of Salivary Biomarkers of Nutritional Status and Dietary Intake: A Systematic Review. J. Dent. 2021, 115, 103840. [Google Scholar] [CrossRef]
- Zare Javid, A.; Seal, C.J.; Heasman, P.; Moynihan, P.J. Impact of a Customised Dietary Intervention on Antioxidant Status, Dietary Intakes and Periodontal Indices in Patients with Adult Periodontitis. J. Hum. Nutr. Diet. 2014, 27, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Kamodyová, N.; Tóthová, L.; Celec, P. Salivary Markers of Oxidative Stress and Antioxidant Status: Influence of External Factors. Dis. Markers 2013, 34, 313–321. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. The Definition and Measurement of Antioxidants in Biological Systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Bartosz, G. The Second Face of Oxygen, 2nd ed.; PWN: Warsaw, Poland, 2003. [Google Scholar]
- Poljsak, B.; Raspor, P. The Antioxidant and Pro-Oxidant Activity of Vitamin C and Trolox in Vitro: A Comparative Study. J. Appl. Toxicol. 2008, 28, 183–188. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Cahill, L.E.; El-Sohemy, A. Vitamin C Transporter Gene Polymorphisms, Dietary Vitamin C and Serum Ascorbic Acid. J. Nutr. Nutr. 2010, 2, 292–301. [Google Scholar] [CrossRef]
- Grzesiak-Gasek, I.; Kaczmarek, U. Influence of Swimming Training Session on Selected Saliva Components in Youth Swimmers. Front. Physiol. 2022, 13, 869903. [Google Scholar] [CrossRef]
- Mahdivand, A.; Tabar, S.F.; Dehghani, S.; Ebrahimi, F.; Moosavi, S.S.; Ghiri, E.H.; Rezaee, M. The Effect of One Session Concurrent Training on Salivary Total Antioxidant Capacity, IgA and Hormonal in Male Student-Athletes. Int. J. Sport Stud. 2013, 3, 448–455. [Google Scholar]
- Deminice, R.; Sicchieri, T.; Payão, P.O.; Jordão, A.A. Blood and Salivary Oxidative Stress Biomarkers Following an Acute Session of Resistance Exercise in Humans. Int. J. Sports Med. 2010, 31, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.C.; Al Khabbaz, H.J.; Bin Enayeg, S.H.; Bin Zouman, A.H. The Relationship between Examination-Related Academic Stress, Salivary Antioxidant Capacity and Exercise Patterns of Final-Year Saudi Dental Students. Eur. J. Dent. Educ. 2017, 21, e83–e88. [Google Scholar] [CrossRef]
- Munther, S. The Effects of Cigarette Smoking and Exercise on Total Salivary Antioxidant Activity. Saudi Dent. J. 2019, 31, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Simaei, E.; Taati, B. Comparison of Responses of Salivary Antioxidant Markers to Exhaustive Aerobic Exercise in Smoker and Non-Smoker Young Girls. J. Sports Med. Phys. Fit. 2016, 56, 1132–1138. [Google Scholar]
- Babaei, P.; Damirchi, A.; Soltani Tehrani, B.; Nazari, Y.; Sariri, R.; Hoseini, R. Effect of Exercise Training on Saliva Brain Derived Neurotrophic Factor, Catalase and Vitamin C. Med. J. Islam. Repub. Iran 2016, 30, 452. [Google Scholar]
- Alves, R.C.C.; Ferreira, R.O.; Frazão, D.R.; de Souza Né, Y.G.; Mendes, P.F.S.; Marañón-Vásquez, G.; Royes, L.F.F.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. The Relationship between Exercise and Salivary Oxidative Stress: A Systematic Review. Antioxidants 2022, 11, 1489. [Google Scholar] [CrossRef]
- Safabakhsh, D.; Jazaeri, M.; Abdolsamadi, H.; Abassi, E.; Farhadian, M. Comparison of Salivary Interleukin-6, Interleukin-8, C-Reactive Protein Levels and Total Antioxidants Capacity of Obese Individuals with Normal-Weight Ones. Rom. J. Intern. Med. 2022, 60, 215–221. [Google Scholar] [CrossRef]
- Gunjalli, G.; Kumar, K.N.; Jain, S.K.; Reddy, S.K.; Shavi, G.R.; Ajagannanavar, S.L. Total Salivary Anti-Oxidant Levels, Dental Development and Oral Health Status in Childhood Obesity. J. Int. Oral Health 2014, 6, 63–67. [Google Scholar]
- Zalewska, A.; Kossakowska, A.; Taranta-Janusz, K.; Zięba, S.; Fejfer, K.; Salamonowicz, M.; Kostecka-Sochoń, P.; Wasilewska, A.; Maciejczyk, M. Dysfunction of Salivary Glands, Disturbances in Salivary Antioxidants and Increased Oxidative Damage in Saliva of Overweight and Obese Adolescents. J. Clin. Med. 2020, 9, 548. [Google Scholar] [CrossRef]
- Esenlik, E.; Bolat Gümüş, E.; Eroğlu Albayrak, G.; Kumbul Doğuç, D. Does Puberty Affect Oxidative Stress Levels and Antioxidant Activity of Saliva in Patients with Fixed Orthodontic Appliances? J. Orofac. Orthop./Fortschritte Kieferorthopädie 2023, 84, 56–64. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean ± SD Median (Q1–Q3) |
|---|---|
| Age [years] | 15.4 ± 1.6 16.0 (14.0–17.0) |
| Height [m] | 1.76 ± 0.1 1.76 (1.69–1.82) |
| Body Mass [kg] | 66.8 ± 12.4 68.0 (60.0–76.0) |
| Body Mass Index [kg∙m−2] | 21.4 ± 2.2 21.9 (20.2–22.9) |
| Waist Circumference [cm] | 72.2 ± 5.6 72.0 (70.0–75.0) |
| Hip Circumference [cm] | 89.5 ± 8.2 90.0 (82.0–95.0) |
| Waist-to-Hip Ratio | 0.81 ± 0.05 0.81 (0.78–0.83) |
| Systolic Blood Pressure [mmHg] | 124.6 ± 12.8 124.0 (116.0–131.0) |
| Diastolic Blood Pressure [mmHg] | 68.6 ± 9.8 68.0 (62.0–75.0) |
| Heart Rate [beats per minute] | 68.6 ± 9.8 68.0 (62.0–75.0) |
| Exercise-related Energy Expenditure [kcal∙week−1] | 7000 ± 1804 6300 (6000–8400) |
| Parameter | 24 h Mean ± SD Median (Q1–Q3) | Breakfast Mean ± SD Median (Q1–Q3) |
|---|---|---|
| Total energy [kcal∙d−1] | 3008 ± 880 3106 (2317–3434) | 756 ± 588 610 (554–720) |
| Proteins [g] | 104.4 ± 32.0 103.5 (86.1–124.6) | 22.1 ± 9.8 20.6 (16.4–25.6) |
| Animal proteins [g] | 61.4 ± 25.4 60.6 (44.4–78.1) | 11.2 ± 7.8 10.6 (6.2–13.6) |
| Plant proteins [g] | 42.6 ± 11.1 42.5 (35.8–48.4) | 11.0 ± 3.7 10.2 (9.7–12.4) |
| Carbohydrates [g] | 453.9 ± 136.0 456.5 (380.7–522.2) | 92.2 ± 31.9 78.8 (74.4–114.6) |
| Absorbable carbohydrates [g] | 429.6 ± 131.9 437.8 (360.6–495.0) | 88.6 ± 31.1 73.4 (71.1–110.3) |
| Sucrose [g] | 111.3 ± 62.9 105.8 (79.8–132.9) | 11.0 ± 7.9 7.9 (6.4–13.5) |
| Fiber [g] | 24.4 ± 7.9 23.5 (18.4–29.5) | 3.7 ± 2.2 2.7 (2.2–4.3) |
| Fats [g] | 95.0 ± 39.9 96.1 (67.3–115.8) | 34.8 ± 62.4 22.3 (18.2–27.6) |
| Saturated fatty acids [g] | 40.7 ± 17.4 40.6 (27.8–50.7) | 19.4 ± 39.4 12.3 (8.9–16.4) |
| Monounsaturated fatty acids [g] | 36.6 ± 17.9 34.0 (24.1–45.2) | 10.7 ± 17.8 7.5 (5.3–8.4) |
| Polyunsaturated fatty acids [g] | 11.1 ± 4.9 10.8 (7.2–12.8) | 2.6 ± 3.7 1.8 (1.6–2.2) |
| Cholesterol [mg] | 322.8 ± 137.7 298.1 (241.3–362.3) | 102.9 ± 185.2 68.6 (44.2–88.8) |
| Parameter | 24 h Mean ± SD Median (Q1–Q3) | Breakfast Mean ± SD Median (Q1–Q3) |
|---|---|---|
| Vitamin C [mg] | 100.5 ± 99.2 69.0 (39.8–113.4) | 6.3 ± 7.6 2.5 (0.0–10.2) |
| Vitamin E [mg] | 8.8 ± 4.2 8.0 (6.2–10.3) | 2.1 ± 3.7 1.3 (1.1–1.7) |
| β-carotene [µg] | 4757 ± 5968 3355 (1283–5658) | 192 ± 298 111 (76–192) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawron-Skarbek, A.; Wróblewski, A.M.; Chrzczanowicz, J.; Nowak, D.; Kostka, T. Salivary Total Antioxidant Capacity of Sportive Adolescents—The Effect of Antioxidant Vitamin Intake with Usual Diet and Physical Exercises. Nutrients 2025, 17, 3610. https://doi.org/10.3390/nu17223610
Gawron-Skarbek A, Wróblewski AM, Chrzczanowicz J, Nowak D, Kostka T. Salivary Total Antioxidant Capacity of Sportive Adolescents—The Effect of Antioxidant Vitamin Intake with Usual Diet and Physical Exercises. Nutrients. 2025; 17(22):3610. https://doi.org/10.3390/nu17223610
Chicago/Turabian StyleGawron-Skarbek, Anna, Adam Marek Wróblewski, Jacek Chrzczanowicz, Dariusz Nowak, and Tomasz Kostka. 2025. "Salivary Total Antioxidant Capacity of Sportive Adolescents—The Effect of Antioxidant Vitamin Intake with Usual Diet and Physical Exercises" Nutrients 17, no. 22: 3610. https://doi.org/10.3390/nu17223610
APA StyleGawron-Skarbek, A., Wróblewski, A. M., Chrzczanowicz, J., Nowak, D., & Kostka, T. (2025). Salivary Total Antioxidant Capacity of Sportive Adolescents—The Effect of Antioxidant Vitamin Intake with Usual Diet and Physical Exercises. Nutrients, 17(22), 3610. https://doi.org/10.3390/nu17223610







