Resistance Exercise Associated with Camu-Camu (Myrciaria dubia) and Creatine Supplementation Modulates Antioxidant Response and Cardiac Parameters in Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Camu-Camu and Creatine
2.2. Proximate Composition of Camu-Camu
2.3. Estimation of Total Phenolic Concentration
2.4. Antioxidant Actizvity
2.4.1. Determination of Antioxidant Capacity by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Method
2.4.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
2.4.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Ascorbic Acid Quantification
2.6. Mineral Content
2.7. Animals and Experimental Diets
2.8. Resistance Exercise Training
2.9. Euthanasia
2.10. Ethical Aspects
2.11. Murinometric Measurements and Food Intake Assessment
2.12. Oxidative Stress Analysis
2.13. Mineral Microanalysis
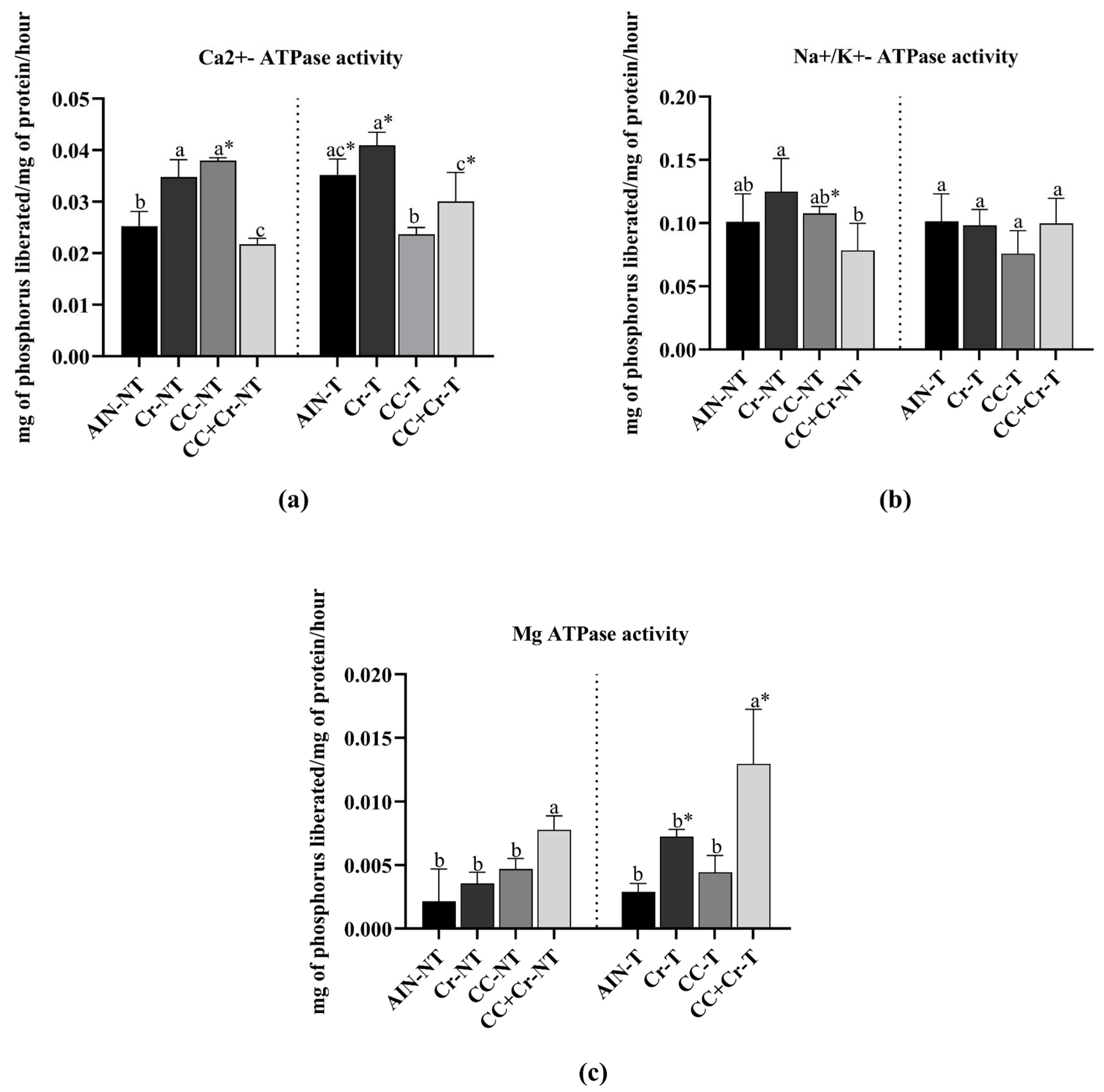
2.14. Determination of Ca2+-ATPase, Na+/K+-ATPase, and Mg2+-ATPase Activities
2.15. Histological Analyses
2.16. Statistical Analyses
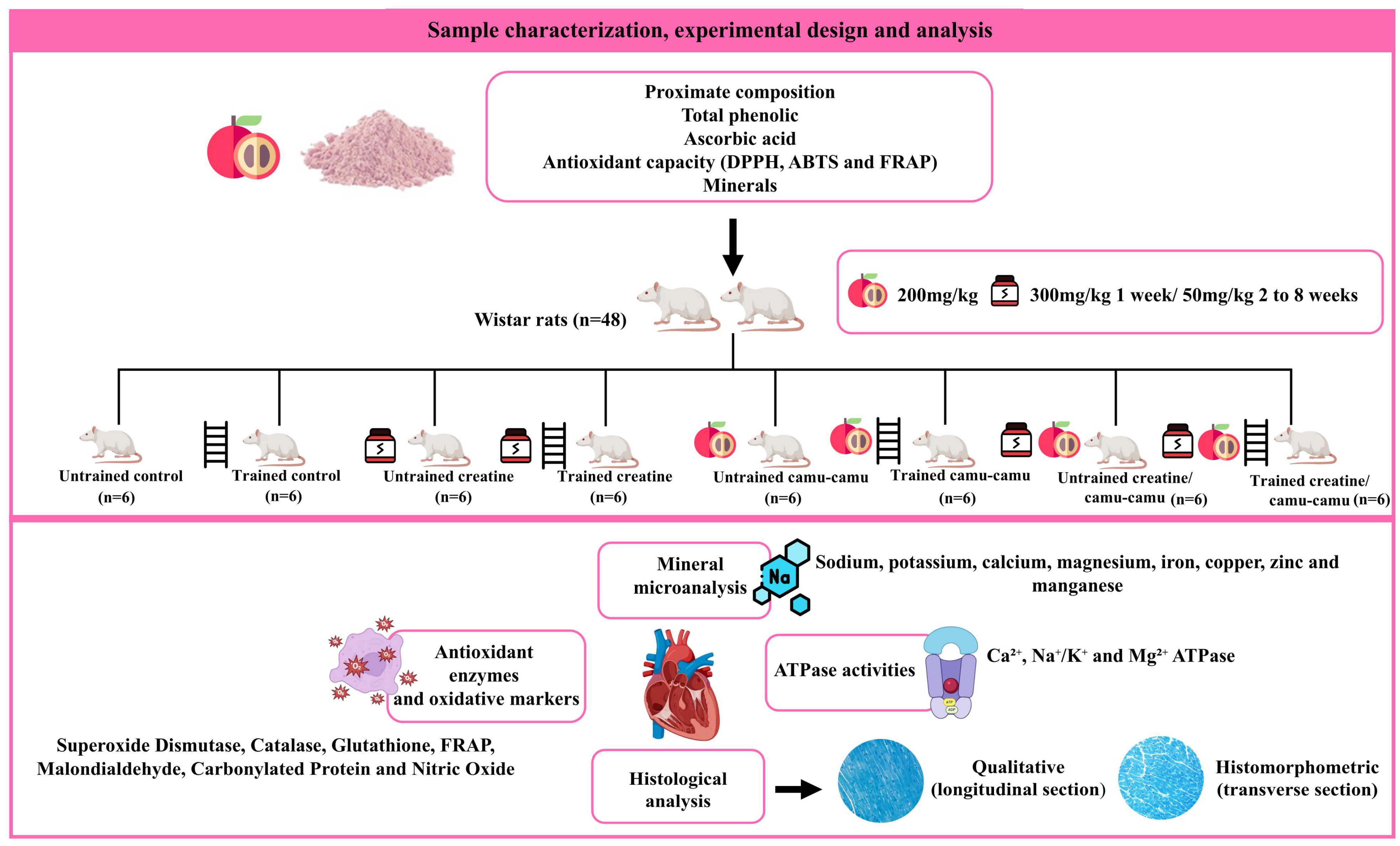
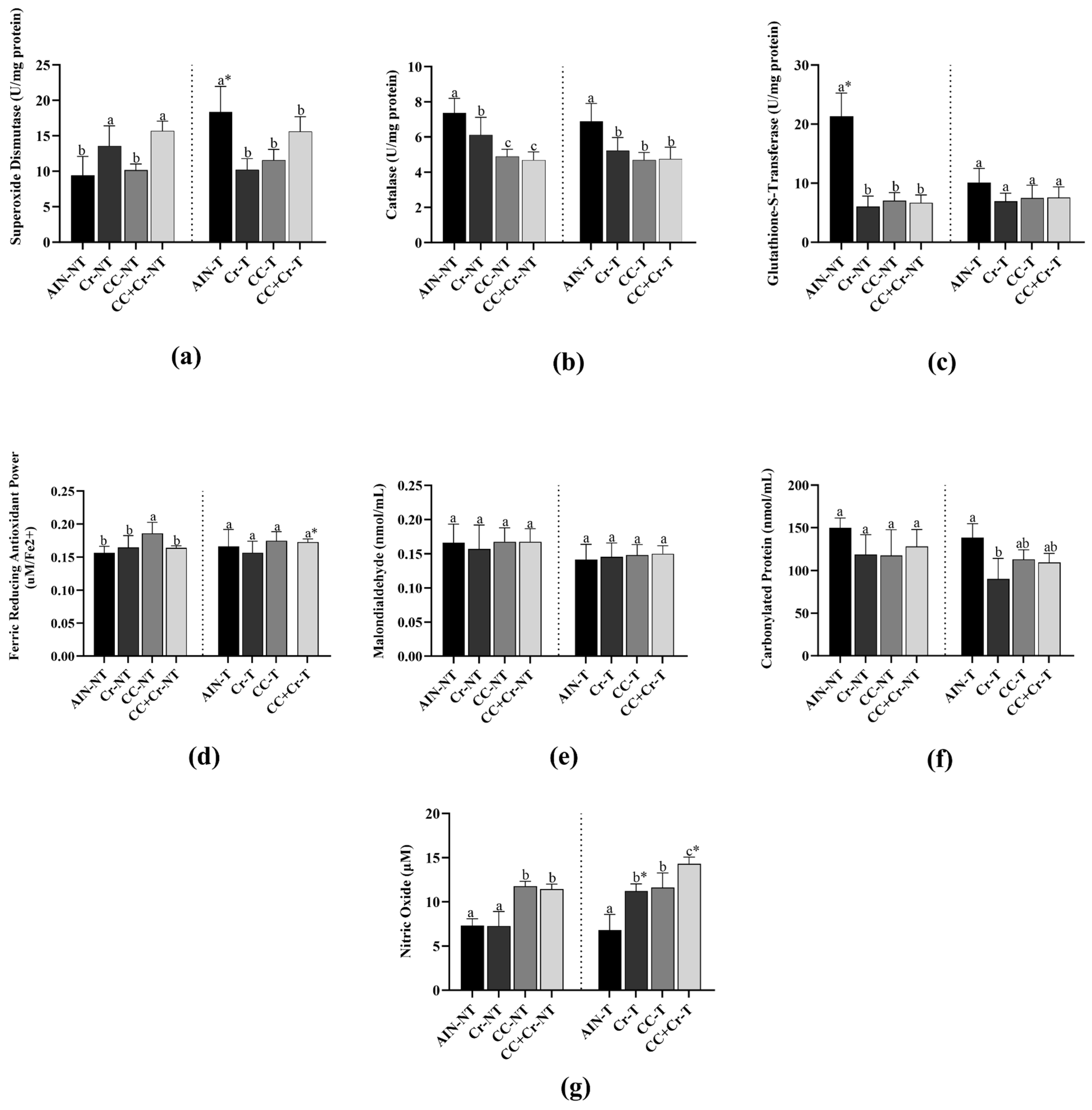
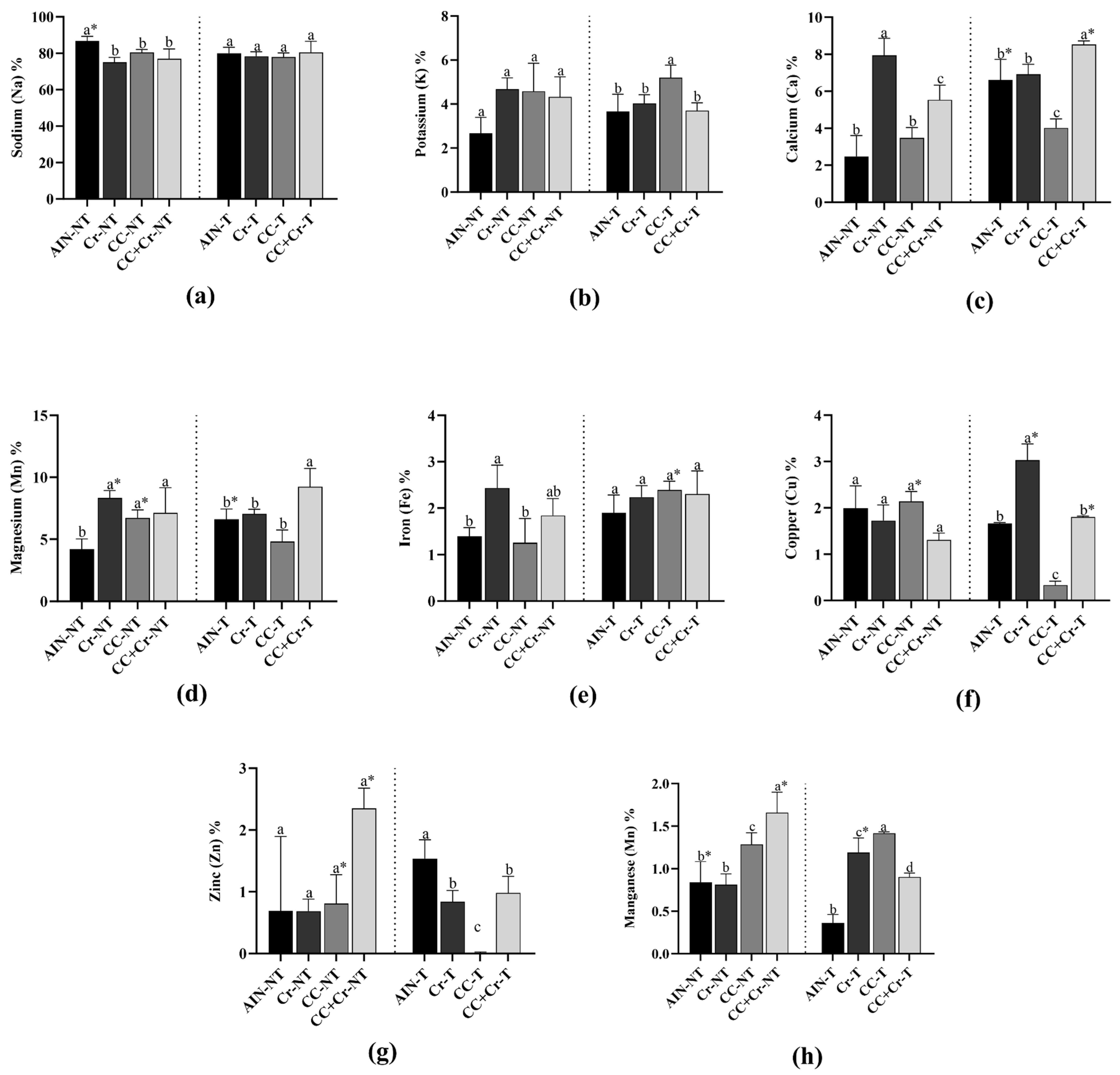
3. Results
3.1. Proximate Composition of Camu-Camu
3.2. Concentration of Total Phenolic Compounds, Vitamin C Content and Antioxidant Activity by DPPH, ABSTS, and FRAP in Powdered Camu-Camu Pulp
3.3. Mineral Content in Powdered Camu-Camu
3.4. Murinometric and Food Intake Measurements
3.5. Antioxidant Enzymes and Oxidative Markers
3.6. Mineral Microanalysis
3.7. Content of Ca2+-ATPase, Na+/K+-ATPase, and Mg2+-ATPase Activities
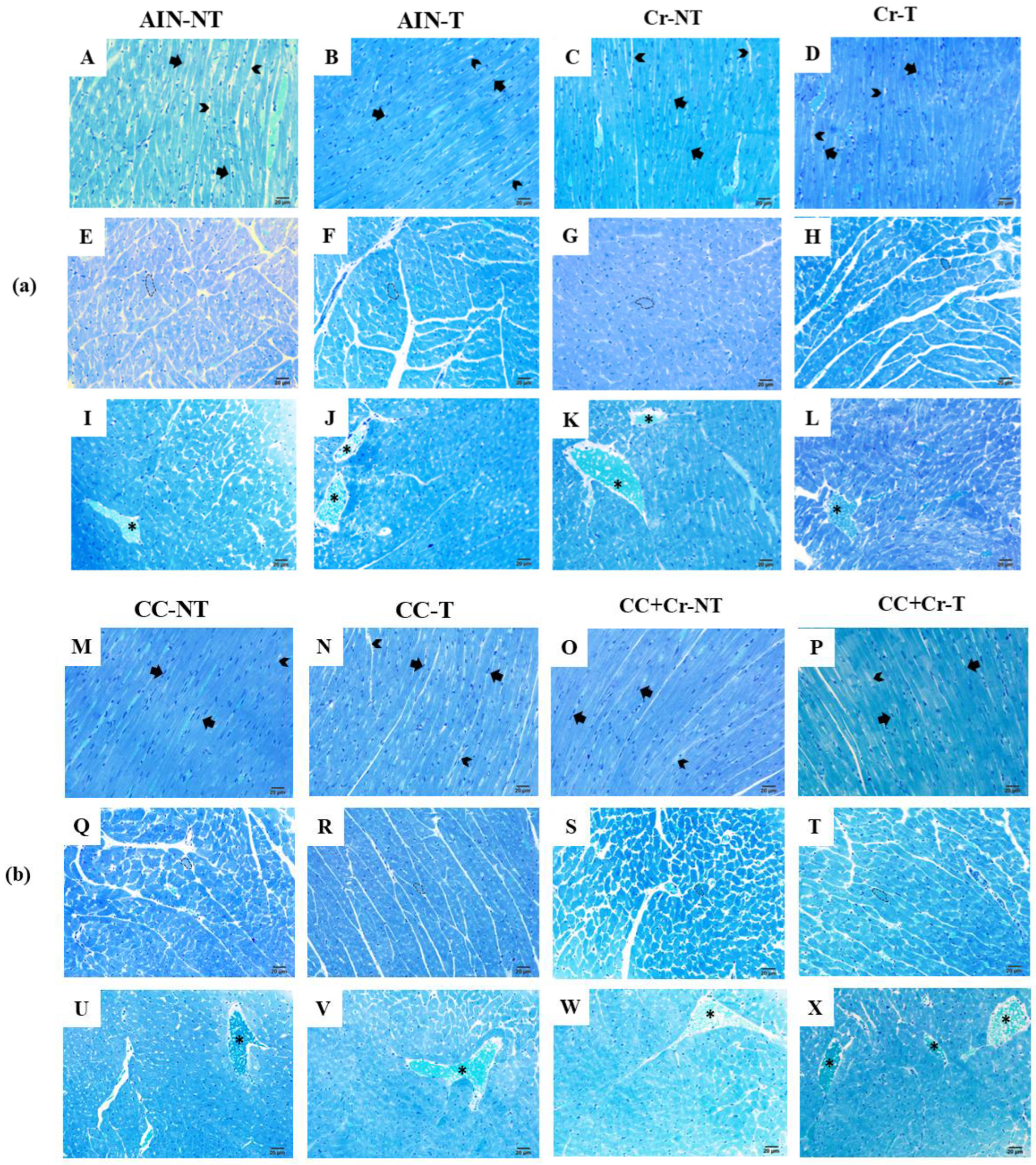
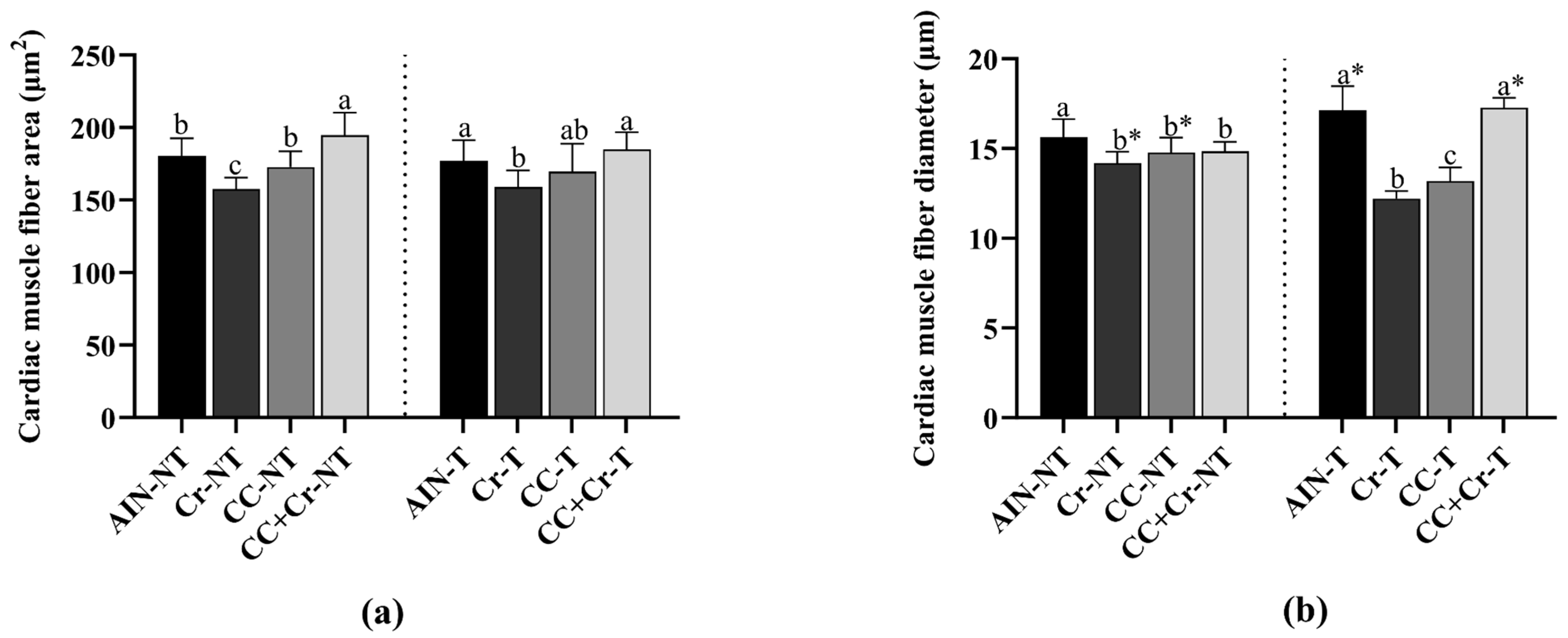
3.8. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momma, H.; Kawakami, R.; Honda, T.; Sawada, S.S. Muscle-Strengthening Activities Are Associated with Lower Risk and Mortality in Major Non-Communicable Diseases: A Systematic Review and Meta-Analysis of Cohort Studies. Br. J. Sports Med. 2022, 56, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Paluch, A.E.; Boyer, W.R.; Franklin, B.A.; Laddu, D.; Lobelo, F.; Lee, D.; McDermott, M.M.; Swift, D.L.; Webel, A.R.; Lane, A. Resistance Exercise Training in Individuals with and Without Cardiovascular Disease: 2023 Update: A Scientific Statement from the American Heart Association. Circulation 2024, 149, e217–e231. [Google Scholar] [CrossRef]
- Lim, C.; Nunes, E.A.; Currier, B.S.; McLeod, J.C.; Thomas, A.C.Q.; Phillips, S.M. An Evidence-Based Narrative Review of Mechanisms of Resistance Exercise–Induced Human Skeletal Muscle Hypertrophy. Med. Sci. Sports Exerc. 2022, 54, 1546–1559. [Google Scholar] [CrossRef]
- Vargas-Mendoza, N.; Ángeles-Valencia, M.; Madrigal-Santillán, E.O.; Morales-Martínez, M.; Tirado-Lule, J.M.; Solano-Urrusquieta, A.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Fregoso-Aguilar, T.; Morales-González, Á.; et al. Effect of Silymarin Supplementation on Physical Performance, Muscle and Myocardium Histological Changes, Bodyweight, and Food Consumption in Rats Subjected to Regular Exercise Training. Int. J. Mol. Sci. 2020, 21, 7724. [Google Scholar] [CrossRef]
- Bagheri, R.; Kargarfard, M.; Sadeghi, R.; Scott, D.; Camera, D.M. Effects of 16 Weeks of Two Different High-Protein Diets with Either Resistance or Concurrent Training on Body Composition, Muscular Strength and Performance, and Markers of Liver and Kidney Function in Resistance-Trained Males. J. Int. Soc. Sports Nutr. 2023, 20, 2236053. [Google Scholar] [CrossRef]
- Every, D.W.; Nunes, E.A.; Beaudry, K.M.; Phillips, S.M. Hormones, Hypertrophy, and Hype: An Evidence-Guided Primer on Endogenous Endocrine Influences on Exercise-Induced Muscle Hypertrophy. Exerc. Sport Sci. Rev. 2024, 52, 117–125. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Spanos, M.; Li, G.; Lu, R.; Bei, Y.; Xiao, J. Exercise Training Maintains Cardiovascular Health: Signaling Pathways Involved and Potential Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 306. [Google Scholar] [CrossRef]
- Melo, S.F.S.; Júnior, N.D.S.; Baraúna, V.G.; Oliveira, E.M. Cardiovascular Adaptations Induced by Resistance Training in Animal Models. Int. J. Med. Sci. 2018, 15, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.R.; Veras, A.S.C.; Tebar, W.R.; Rufino, J.C.; Batista, V.R.G.; Teixeira, G.R. Strength Training for Arterial Hypertension Treatment: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Sci. Rep. 2023, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.D.S.M.; Alves, R.E.; De Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Maria, L.G.; Edvan, A.C.; Bala, R.; Pollyana, C.C.; Antonia, R.V.D.S.; Sara, T.M.S.; Railin, R.D.O. Qualitative Evaluation and Biocompounds Present in Different Parts of Camu-Camu (Myrciaria dubia) Fruit. Afr. J. Food Sci. 2017, 11, 124–129. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; da Costa, S.C.; Madrona, G.S. Camu-Camu Bioactive Compounds Extraction by Ecofriendly Sequential Processes (Ultrasound Assisted Extraction and Reverse Osmosis). Ultrason. Sonochem. 2020, 64, 105017. [Google Scholar] [CrossRef]
- Noguera, N.H.; Noguera, D.C.L.H.; Machado, A.P.D.F.; Reguengo, L.M.; Nascimento, R.P. Emerging Berries from the Brazilian Amazon and Atlantic Forest Biomes: New Sources of Bioactive Compounds with Potential Health Benefits. Food Funct. 2024, 15, 5752–5784. [Google Scholar] [CrossRef]
- Do, N.Q.; Zheng, S.; Oh, S.; Nguyen, Q.T.N.; Fang, M.; Kim, M.; Choi, J.; Kim, M.J.; Jeong, J.; Yi, T.H. Anti-Allergic Effects of Myrciaria dubia (Camu-Camu) Fruit Extract by Inhibiting Histamine H1 and H4 Receptors and Histidine Decarboxylase in RBL-2H3 Cells. Antioxidants 2021, 11, 104. [Google Scholar] [CrossRef]
- Abrantes, L.C.S.; Oliveira, L.A.; Dias, K.A.; Fialho, T.C.; Martins, K.V.C.; Paes, S.S.; Della Lucia, C.M. Effect of Consumption of Brazilian Berries on Intestinal Health: A Systematic Review of In Vivo Studies. Crit. Rev. Food Sci. Nutr. 2025, 1–15. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Han, L.; Bian, J.; He, C.; El-Omar, E.; Gong, L.; Wang, M. The Potential Roles of Dietary Anthocyanins in Inhibiting Vascular Endothelial Cell Senescence and Preventing Cardiovascular Diseases. Nutrients 2022, 14, 2836. [Google Scholar] [CrossRef]
- Bublitz, M.; Musgaard, M.; Poulsen, H.; Thøgersen, L.; Olesen, C.; Schiøtt, B.; Morth, J.P.; Møller, J.V.; Nissen, P. Ion Pathways in the Sarcoplasmic Reticulum Ca2+-ATPase. J. Biol. Chem. 2013, 288, 10759–10765. [Google Scholar] [CrossRef]
- Fedosova, N.U.; Habeck, M.; Nissen, P. Structure and Function of Na,K-ATPase—The Sodium-Potassium Pump. Compr. Physiol. 2021, 12, 2659–2679. [Google Scholar] [CrossRef] [PubMed]
- Mishima, M.D.V.; Ladeira, L.C.M.; da Silva, B.P.; Toledo, R.C.L.; de Oliveira, T.V.; Costa, N.M.B.; Martino, H.S.D. Cardioprotective Action of Chia (Salvia hispanica L.) in Ovariectomized Rats Fed a High-Fat Diet. Food Funct. 2021, 12, 3069–3082. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.F.A.; Stringheta, P.C.; de Oliveira, E.B.; Mendonça, A.C.; Pinheiro-Sant’Ana, H.M. Teor de Vitamina C, β-Caroteno e Minerais em Camu-Camu Cultivado em Diferentes Ambientes. Cienc. Rural. 2016, 46, 567–572. [Google Scholar] [CrossRef]
- Shattock, M.J.; Ottolia, M.; Bers, D.M.; Blaustein, M.P.; Boguslavskyi, A.; Bossuyt, J.; Bridge, J.H.; Chen-Izu, Y.; Clancy, C.E.; Edwards, A.; et al. Na+/Ca2+ Exchange and Na+/K+-ATPase in the Heart. J. Physiol. 2015, 593, 1361–1382. [Google Scholar] [CrossRef]
- Zapata, S.M.; Dufour, J.P. Camu-Camu (Myrciaria dubia (H.B.K.) McVaugh): Chemical Composition of Fruit. J. Sci. Food Agric. 1993, 61, 349–351. [Google Scholar] [CrossRef]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef]
- Forbes, S.C.; Candow, D.G.; Neto, J.H.F.; Kennedy, M.D.; Forbes, J.L.; Machado, M.; Bustillo, E.; Gomez-Lopez, J.; Zapata, A.; Antonio, J. Creatine Supplementation and Endurance Performance: Surges and Sprints to Win the Race. J. Int. Soc. Sports Nutr. 2023, 20, 2204071. [Google Scholar] [CrossRef]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef]
- Clarke, H.; Kim, D.H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 19th ed.; AOAC: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bloor, S.J. Overview of Methods for Analysis and Identification of Flavonoids. Methods Enzymol. 2001, 335, 3–14. [Google Scholar] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Leite-Legatti, A.V.; Batista, Â.G.; Dragano, N.R.V.; Marques, A.C.; Malta, L.G.; Riccio, M.F.; Machado, A.R.T.; Carvalho-Silva, L.B.; Gois Ruiz, A.L.T.; de Carvalho, J.E.; et al. Jaboticaba Peel: Antioxidant Compounds, Antiproliferative and Antimutagenic Activities. Food Res. Int. 2012, 49, 596–603. [Google Scholar] [CrossRef]
- Oyaizu, M. Antioxidative Activity of Browning Products of Glucosamine Fractionated by Organic Solvent and Thin-Layer Chromatography. Nippon Shokuhin Kogyo Gakkaishi 1986, 44, 307–315. [Google Scholar]
- Campos, F.M.; Ribeiro, S.M.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of Methodology to Analyze Ascorbic and Dehydroascorbic Acid in Vegetables. Química Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Fontelles, M.J.; Simões, M.G.; Almeida, J.C.D.; Fontelles, R.G.S. Metodologia da pesquisa: Diretrizes para o cálculo do tamanho da amostra. Rev. Para. Med. 2010, 24, 57–64. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Brannon, T.A.; Adams, G.R.; Conniff, C.L.; Baldwin, K.M. Effects of Creatine Loading and Training on Running Performance and Biochemical Properties of Rat Skeletal Muscle. Med. Sci. Sports Exerc. 1997, 29, 489–495. [Google Scholar] [CrossRef]
- McGuire, M.; Bradford, A.; MacDermott, M. Contractile Properties of the Diaphragm in Creatine-Fed Rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 782–783. [Google Scholar] [CrossRef]
- Young, R.E.; Young, J.C. The Effect of Creatine Supplementation on Mass and Performance of Rat Skeletal Muscle. Life Sci. 2007, 81, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Brochot, A.; Pomié, N.; Wemelle, E.; Druart, C.; Régnier, M.; Delzenne, N.M.; de Vos, W.M.; Knauf, C.; Cani, P.D. Camu-Camu Reduces Obesity and Improves Diabetic Profiles of Obese and Diabetic Mice: A Dose-Ranging Study. Metabolites 2022, 12, 301. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Trottier, J.; Dudonné, S.; Le Barz, M.; Feutry, P.; Pilon, G.; Barbier, O.; Desjardins, Y.; et al. Treatment with Camu Camu (Myrciaria dubia) Prevents Obesity by Altering the Gut Microbiota and Increasing Energy Expenditure in Diet-Induced Obese Mice. Gut 2019, 68, 453–464. [Google Scholar] [CrossRef]
- Gidi, P.C. Efeito da Farinha de Camu-Camu (Myrciaria dubia) Liofilizada sobre a Glicemia de Ratos Machos da Linhagem Wistar. In IV Congresso de Iniciação Científica do INPA-CONIC; INPA: Manaus, Brazil, 2015. [Google Scholar]
- Hornberger, T.A., Jr.; Farrar, R.P. Physiological Hypertrophy of the FHL Muscle Following 8 Weeks of Progressive Resistance Exercise in the Rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Teixeira, L.G.; Aguilar, E.C.; Matoso, R.O.; Soares, F.L.; Ferreira, A.V.; Alvarez-Leite, J.I. Differences in Adipose Tissue Inflammation and Oxidative Status in C57BL/6 and ApoE-/- Mice Fed High Fat Diet. Anim. Sci. J. 2012, 83, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Novelli, E.L.; Diniz, Y.S.; Galhardi, C.M.; Ebaid, G.M.; Rodrigues, H.G.; Mani, F.; Fernandes, A.A.; Cicogna, A.C.; Novelli Filho, J.L. Anthropometrical Parameters and Markers of Obesity in Rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Hegsted, D.M. Protein quality and its detennination. In Food Proteins; Whitaker, R., Tannenbaum, S.R., Eds.; AVI Publishing Co.: Westport, CT, USA, 1977; pp. 347–362. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene Expression of Antioxidative Enzymes in the Human Heart: Increased Expression of Catalase in the End-Stage Failing Heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Habig, W.; Pabst, M.; Jakoby, W. Glutathione S-Transferases: The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Levine, R.L. Carbonyl assay for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 246–257. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, L.C.M.; Dos Santos, E.C.; Valente, G.E.; Da Silva, J.; Santos, T.A.; Dos Santos Costa Maldonado, I.R. Could Biological Tissue Preservation Methods Change Chemical Elements Proportion Measured by Energy Dispersive X-Ray Spectroscopy? Biol. Trace Elem. Res. 2020, 196, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Hjertén, S.; Pan, H. Purification and Characterization of Two Forms of a Low-Affinity Ca2+-ATPase from Erythrocyte Membranes. Biochim. Biophys. Acta Biomembr. 1983, 728, 281–288. [Google Scholar] [CrossRef]
- Bonting, S.L.; Caravaggio, L.L.; Hawkins, N.M. Studies on Sodium-Potassium-Activated Adenosinetriphosphatase. IV. Correlation with Cation Transport Sensitive to Cardiac Glycosides. Arch. Biochem. Biophys. 1962, 98, 413–419. [Google Scholar] [CrossRef]
- Ohnishi, T.; Suzuki, T.; Suzuki, Y.; Ozawa, K. A Comparative Study of Plasma Membrane Mg2+-ATPase Activities in Normal, Regenerating and Malignant Cells. Biochim. Biophys. Acta Biomembr. 1982, 684, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cerri, P.S.; Sasso-Cerri, E. Staining Methods Applied to Glycol Methacrylate Embedded Tissue Sections. Micron 2003, 34, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, T.; Koizumi, R.; Myoda, T.; Sagane, Y.; Niwa, K.; Watanabe, T.; Minami, K. Data on a Single Oral Dose of Camu Camu (Myrciaria dubia) Pericarp Extract on Flow-Mediated Vasodilation and Blood Pressure in Young Adult Humans. Data Brief 2017, 16, 993–999. [Google Scholar] [CrossRef]
- Assen, A.H.; Belete, A.; Gebre-Mariam, T. Preparation and Physicochemical Characterization of Acid Modified Cassava Starch and Its Evaluation as Directly Compressible Tablet Excipient. Ethiop. Pharm. J. 2013, 29, 27–42. [Google Scholar] [CrossRef]
- Ordoñez, M.; Herrera, A.O. Morphologic and Stability Cassava Starch Matrices for Encapsulating Limonene by Spray Drying. Powder Technol. 2013, 253, 89–97. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Chen, W. Trends of Spray Drying: A Critical Review on Drying of Fruit and Vegetable Juices. Trends Food Sci. Technol. 2017, 65, 49–67. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Xie, Y.; Gu, Y.; Li, Z.; Zhang, L.; Hei, Y. Effects of Exercise on Different Antioxidant Enzymes and Related Indicators: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2025, 15, 12518. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ojaghi, A.; Jourkesh, M.; Ojaghi, M.; Mirheidar, L.; Neshati, A. The Effect of Eight-Week Resistance Training on Oxidative Stress in Isoproterenol-Induced Myocardial Infarction in Rats. Arch. Med. Sci. Atheroscler. Dis. 2024, 9, 177–182. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Quindry, J.C. Mechanisms of Exercise-Induced Cardioprotection. Physiology 2014, 29, 27–38. [Google Scholar] [CrossRef]
- Morrison, D.; Hughes, J.; Della Gatta, P.A.; Mason, S.; Lamon, S.; Russell, A.P.; Wadley, G.D. Vitamin C and E Supplementation Prevents Some of the Cellular Adaptations to Endurance-Training in Humans. Free Radic. Biol. Med. 2015, 89, 852–862. [Google Scholar] [CrossRef]
- Zhou, Y.; Baker, J.S.; Chen, X.; Wang, Y.; Chen, H.; Davison, G.W.; Yan, X. High-Dose Astaxanthin Supplementation Suppresses Antioxidant Enzyme Activity during Moderate-Intensity Swimming Training in Mice. Nutrients 2019, 11, 1244. [Google Scholar] [CrossRef]
- Langley, P.C.; Pergolizzi, J.V., Jr.; Taylor, R., Jr.; Ridgway, C. Antioxidant and Associated Capacities of Camu Camu (Myrciaria dubia): A Systematic Review. J. Altern. Complement. Med. 2015, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E. Effects of Dietary Antioxidants on Training and Performance in Female Runners. Eur. J. Sport Sci. 2014, 14, 160–168. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants Prevent Health-Promoting Effects of Physical Exercise in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Rahimi, R. Creatine Supplementation Decreases Oxidative DNA Damage and Lipid Peroxidation Caused by a Single Session of Resistance Exercise. J. Strength Cond. Res. 2011, 25, 3448–3455. [Google Scholar] [CrossRef]
- Louis, M.; Van Beneden, R.; Dehoux, M.; Thissen, J.P.; Francaux, M. Creatine Increases IGF—I and Myogenic Regulatory Factor mRNA in C(2)C(12) Cells. FEBS Lett. 2004, 557, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Rosene, J.M. Effects of Oral Creatine and Resistance Training on Myogenic Regulatory Factor Expression. Med. Sci. Sports Exerc. 2003, 35, 923–929. [Google Scholar] [CrossRef]
- Jones, A.M.; Wilkerson, D.P.; Fulford, J. Influence of dietary creatine supplementation on muscle phosphocreatine kinetics during knee-extensor exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, 1078–1087. [Google Scholar] [CrossRef]
- Gülcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Sestili, P.; Martinelli, C.; Colombo, E.; Barbieri, E.; Potenza, L.; Sartini, S.; Fimognari, C. Creatine as an Antioxidant. Amino Acids 2011, 40, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D.; Kang, C.; Zhang, T. The Role of Mitochondria in Redox Signaling of Muscle Homeostasis. J. Sport Health Sci. 2020, 9, 386–393. [Google Scholar] [CrossRef]
- Jackson, M.J.; Pollock, N.; Staunton, C.; Jones, S.; McArdle, A. Redox Control of Signalling Responses to Contractile Activity and Ageing in Skeletal Muscle. Cells 2022, 11, 1698. [Google Scholar] [CrossRef]
- Dias, K.A.; da Conceição, A.R.; Oliveira, L.A.; Pereira, S.M.S.; Paes, S.D.S.; Monte, L.F.; Sarandy, M.M.; Novaes, R.D.; Gonçalves, R.V.; Della Lucia, C.M. Effects of Curcumin Supplementation on Inflammatory Markers, Muscle Damage, and Sports Performance during Acute Physical Exercise in Sedentary Individuals. Oxidative Med. Cell. Longev. 2021, 2021, 9264639. [Google Scholar] [CrossRef]
- Sahin, E.; Orhan, C.; Erten, F.; Er, B.; Acharya, M.; Morde, A.A.; Sahin, K. Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats. Antioxidants 2021, 10, 1692. [Google Scholar] [CrossRef]
- Bai, K.Y.; Liu, G.H.; Fan, C.H.; Kuo, L.T.; Hsu, W.H.; Yu, P.A.; Chen, C.L. 12-Week Curcumin Supplementation May Relieve Postexercise Muscle Fatigue in Adolescent Athletes. Front. Nutr. 2023, 9, 1078108. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.J.; Burbeary, R.; Hennis, P.J.; James, R.M.; Saward, C.; Colledge, A.; Scott, R.; Gilpin, S.; McMahon, R.; Varley, I. Turmeric Supplementation Improves Markers of Recovery in Elite Male Footballers: A Pilot Study. Front. Nutr. 2023, 10, 1175622. [Google Scholar] [CrossRef]
- Mancini, N.; Moscatelli, F.; Di Padova, M.; Mancini, S.; Basta, A.; Guerriero, M.A.; Dipace, A.; Limone, P.; Colella, D.; Grosu, V.T. The Relationship between Reaction Time and Agility Performance in Young Athletes: A Study Using Perception–Action Technological Devices. J. Phys. Educ. Sport. 2024, 29, 2205–2215. [Google Scholar]
- Gonzalez, A.M.; Trexler, E.T. Effects of Citrulline Supplementation on Exercise Performance in Humans: A Review of the Current Literature. J. Strength Cond. Res. 2020, 34, 1480–1495. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Zabriskie, H.A.; Erickson, J.L.; Molling, P.E.; Kerksick, C.M.; Jagim, A.R. Multi-Ingredient Pre-Workout Supplements, Safety Implications, and Performance Outcomes: A Brief Review. J. Int. Soc. Sports Nutr. 2018, 15, 41. [Google Scholar] [CrossRef]
- Oral, O. Nitric Oxide and Its Role in Exercise Physiology. J. Sports Med. Phys. Fit. 2021, 61, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Chaabi, M.; Anselm, E.; Lobstein, A.; Schini-Kerth, V.B. The Red Wine Extract-Induced Activation of Endothelial Nitric Oxide Synthase Is Mediated by a Great Variety of Polyphenolic Compounds. Mol. Nutr. Food Res. 2010, 54, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, T.; Haufe, V.; Blechschmidt, S. Voltage-Gated Sodium Channels in the Mammalian Heart. Glob. Cardiol. Sci. Pract. 2014, 4, 449–463. [Google Scholar]
- Green, H.J.; Chin, E.R.; Ball-Burnett, M.; Ranney, D. Increases in Human Skeletal Muscle Na+-K+-ATPase Concentration with Short-Term Training. Am. J. Physiol. 1993, 264, 1538–1541. [Google Scholar] [CrossRef]
- Carmeliet, E. K+ Channels and Control of Ventricular Repolarization in the Heart. Fundam. Clin. Pharmacol. 1993, 7, 19–28. [Google Scholar] [CrossRef]
- Melo, S.F.; Barauna, V.G.; Neves, V.J.; Fernandes, T.; Lara, L.S.; Mazzotti, D.R.; Oliveira, E.M. Exercise Training Restores the Cardiac MicroRNA-1 and -214 Levels Regulating Ca2+ Handling after Myocardial Infarction. BMC Cardiovasc. Disord. 2015, 15, 166. [Google Scholar] [CrossRef]
- Do, N.Q.; Zheng, S.; Park, B.; Nguyen, Q.T.N.; Choi, B.R.; Fang, M.; Kim, M.; Jeong, J.; Choi, J.; Yang, S.J.; et al. Camu-Camu Fruit Extract Inhibits Oxidative Stress and Inflammatory Responses by Regulating NFAT and Nrf2 Signaling Pathways in High Glucose-Induced Human Keratinocytes. Molecules 2021, 26, 3174. [Google Scholar] [CrossRef]
- de Groof, A.J.; Fransen, J.A.; Errington, R.J.; Willems, P.H.; Wieringa, B.; Koopman, W.J. The Creatine Kinase System Is Essential for Optimal Refill of the Sarcoplasmic Reticulum Ca2+ Store in Skeletal Muscle. J. Biol. Chem. 2002, 277, 5275–5284. [Google Scholar] [CrossRef]
- De la Cal, C.; Di Croce, D.E.; Takara, D. Changes in Masseter Muscle Structure, Membrane Lipid Peroxidation, and Ca-ATPase Activity as Effects of Different Local Anesthetics. Acta Odontol. Latinoam. 2024, 37, 105–113. [Google Scholar] [CrossRef]
- Dergousova, E.A.; Petrushanko, I.Y.; Klimanova, E.A.; Mitkevich, V.A.; Ziganshin, R.H.; Lopina, O.D.; Makarov, A.A. Enhancement of Na,K-ATPase Activity as a Result of Removal of Redox Modifications from Cysteine Residues of the α1 Subunit: The Effect of Reducing Agents. Mol. Biol. 2018, 52, 247–250. [Google Scholar] [CrossRef]
- Aboalgasm, H.; Petersen, M.; Gwanyanya, A. Improvement of Cardiac Ventricular Function by Magnesium Treatment in Chronic Streptozotocin-Induced Diabetic Rat Heart. Cardiovasc. J. Afr. 2021, 32, 141–148. [Google Scholar] [PubMed]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Neufer, P.D. The Bioenergetics of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029678. [Google Scholar] [CrossRef]
- Tardy, A.L.; Pouteau, E.; Marquez, D.; Yilmaz, C.; Scholey, A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients 2020, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.G.; Quinzi, F.; Folino, K.; Greco, F.; Oranges, F.P.; Cerulli, C.; Emerenziani, G.P. Effects of Magnesium Supplementation on Muscle Soreness in Different Type of Physical Activities: A Systematic Review. J. Transl. Med. 2024, 22, 629. [Google Scholar] [CrossRef]
- García-Chacón, J.M.; Marín-Loaiza, J.C.; Osorio, C. Camu Camu (Myrciaria dubia (Kunth) McVaugh): An Amazonian Fruit with Biofunctional Propertie—A Review. ACS Omega. 2023, 8, 5169–5183. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Komoda, H.; Uchida, T.; Node, K. Tropical Fruit Camu-Camu (Myrciaria dubia) Has Anti-Oxidative and Anti-Inflammatory Properties. J. Cardiol. 2008, 52, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.C.G.; de Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse Oxidativo: Conceito, Implicações e Fatores Modulatórios. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative Stress and Potential Interventions to Reduce Oxidative Stress in Overweight and Obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Homeostase do Cobre e Cuproptose na Saúde e na Doença. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Nazem, M.R.; Asadi, M.; Jabbari, N.; Allameh, A. Effects of Zinc Supplementation on Superoxide Dismutase Activity and Gene Expression, and Metabolic Parameters in Overweight Type 2 Diabetes Patients: A Randomized, Double-Blind, Controlled Trial. Clin. Biochem. 2019, 69, 15–20. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Steffen, J.; Eide, D.J. Cytosolic Superoxide Dismutase (SOD1) Is Critical for Tolerating the Oxidative Stress of Zinc Deficiency in Yeast. PLoS ONE 2009, 4, e7061. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Regulation of Increased Blood Flow (Hyperemia) to Muscles during Exercise: A Hierarchy of Competing Physiological Needs. Physiol. Rev. 2015, 95, 549–601. [Google Scholar] [CrossRef]
- Balestrino, M. The Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Gowayed, M.A.; Mahmoud, S.A.; El-Sayed, Y.; Abu-Samra, N.; Kamel, M.A. Enhanced Mitochondrial Biogenesis Is Associated with the Ameliorative Action of Creatine Supplementation in Rat Soleus and Cardiac Muscles. Exp. Ther. Med. 2020, 19, 384–392. [Google Scholar] [CrossRef] [PubMed]
| Compounds | AIN-93M (g/kg of Diet) |
|---|---|
| Corn starch | 449.2 |
| Albumin 1 | 156.5 |
| Maltodextrin | 155.0 |
| Sucrose | 100.0 |
| Soybean oil | 40.0 |
| Cellulose | 50.0 |
| Mineral mixture | 35.0 |
| Vitamin mix | 10.0 |
| L-cystine | 1.8 |
| Choline bitartrate | 2.5 |
| Creatine * | - |
| Camu-camu * | - |
| Proximate Composition | % |
|---|---|
| Moisture | 3.29 |
| Lipids | 1.24 |
| Total ash | 0.15 |
| Proteins | 0.42 |
| Total carbohydrates | 94.90 |
| Fiber | 0.80 |
| TEV kcal | 398.74 |
| ED | 3.98 |
| Parameter | Value/g Sample |
|---|---|
| Total phenolic (mg GAE) | 46.26 ± 1.49 |
| Ascorbic acid (mg) | 6.47 ± 0.12 |
| DPPH (µM Trolox) | 419.64 ± 6.41 |
| ABTS (µmol TE) | 335.48 ± 2.76 |
| FRAP (µmol TE) | 155.42 ± 2.49 |
| Minerals | Content (mg/100 g) |
|---|---|
| Phosphorus (P) | 8.0 ± 0.00 |
| Potassium (K) | 24.0 ± 0.57 |
| Calcium (Ca) | 9.0 ± 1.00 |
| Magnesium (Mg) | 4.0 ± 0.00 |
| Copper (Cu) | 0.0177 ± 0.00 |
| Iron (Fe) | 0.2836 ± 0.11 |
| Zinc (Zn) | 0.1196 ± 0.04 |
| Manganese (Mn) | 0.0951 ± 0.00 |
| Sodium (Na) | 34.0 ± 1.00 |
| Parameters | AIN-NT | AIN-T | Cr-NT | Cr-T | CC-NT | CC-T | CC + Cr-NT | CC + Cr-T |
|---|---|---|---|---|---|---|---|---|
| Weight gain (grams) | 79.42 ± 8.09 | 78.08 ± 7.81 | 81.66 ± 13.53 | 80.36 ± 7.42 | 79.10 ± 11.22 | 83.14 ± 7.52 | 85.23 ± 15.19 | 80.85 ± 12.37 |
| WC gain (centimeters) | 1.05 ± 0.12 | 1.25 ± 0.21 | 1.50 ± 0.41 | 1.35 ± 0.88 | 1.03 ± 0.21 | 1.61 ± 0.67 | 1.28 ± 0.32 | 0.96 ± 0.47 |
| BMI (g/cm2) | 0.62 ± 0.04 | 0.63 ± 0.03 | 0.63 ± 0.05 | 0.65 ± 0.07 | 0.68 ± 0.01 | 0.69 ± 0.02 | 0.66 ± 0.05 | 0.69 ± 0.04 |
| TFI (grams) | 801.40 ± 33.93 | 841.30 ± 95.97 | 718.60 ± 49.30 | 812.90 ± 101.30 | 762.20 ± 62.96 | 811.80 ± 60.82 | 757.30 ± 106.00 | 820.30 ± 59.15 |
| FEC | 9.98 ± 0.51 | 9.32 ± 1.84 | 11.24 ± 2.13 | 9.60 ± 2.09 | 10.52 ± 2.14 | 10.58 ± 1.89 | 11.16 ± 0.94 | 9.93 ± 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fialho, T.C.; Abrantes, L.C.S.; Martins, K.V.C.; Carvalho, R.P.R.; Ramírez-López, C.J.; Sousa, A.F.R.d.; Guimarães-Ervilha, L.O.; Oliveira, L.A.; Lacerda, G.F.; Moreira, A.J.B.; et al. Resistance Exercise Associated with Camu-Camu (Myrciaria dubia) and Creatine Supplementation Modulates Antioxidant Response and Cardiac Parameters in Wistar Rats. Nutrients 2025, 17, 3587. https://doi.org/10.3390/nu17223587
Fialho TC, Abrantes LCS, Martins KVC, Carvalho RPR, Ramírez-López CJ, Sousa AFRd, Guimarães-Ervilha LO, Oliveira LA, Lacerda GF, Moreira AJB, et al. Resistance Exercise Associated with Camu-Camu (Myrciaria dubia) and Creatine Supplementation Modulates Antioxidant Response and Cardiac Parameters in Wistar Rats. Nutrients. 2025; 17(22):3587. https://doi.org/10.3390/nu17223587
Chicago/Turabian StyleFialho, Thaís Cupertino, Lívia Carvalho Sette Abrantes, Karina Vitória Cipriana Martins, Renner Philipe Rodrigues Carvalho, Camilo José Ramírez-López, Alex Filipe Ramos de Sousa, Luiz Otávio Guimarães-Ervilha, Lívya Alves Oliveira, Gabrieli Fernandes Lacerda, Ana Júlia Brandão Moreira, and et al. 2025. "Resistance Exercise Associated with Camu-Camu (Myrciaria dubia) and Creatine Supplementation Modulates Antioxidant Response and Cardiac Parameters in Wistar Rats" Nutrients 17, no. 22: 3587. https://doi.org/10.3390/nu17223587
APA StyleFialho, T. C., Abrantes, L. C. S., Martins, K. V. C., Carvalho, R. P. R., Ramírez-López, C. J., Sousa, A. F. R. d., Guimarães-Ervilha, L. O., Oliveira, L. A., Lacerda, G. F., Moreira, A. J. B., Costa, S. F. F., Lana, V. S. d., Machado-Neves, M., Natali, A. J., Forte, P., Leite, L. B., Carvalho, I. M. M., Martino, H. S. D., da Silva, R. C., & Lucia, C. M. D. (2025). Resistance Exercise Associated with Camu-Camu (Myrciaria dubia) and Creatine Supplementation Modulates Antioxidant Response and Cardiac Parameters in Wistar Rats. Nutrients, 17(22), 3587. https://doi.org/10.3390/nu17223587










