Polyphenol-Related Gut Metabotype Signatures Linked to Quality of Life in Postmenopausal Women: A Randomized, Placebo-Controlled Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Polyphenol-Rich Supplement (PPs)
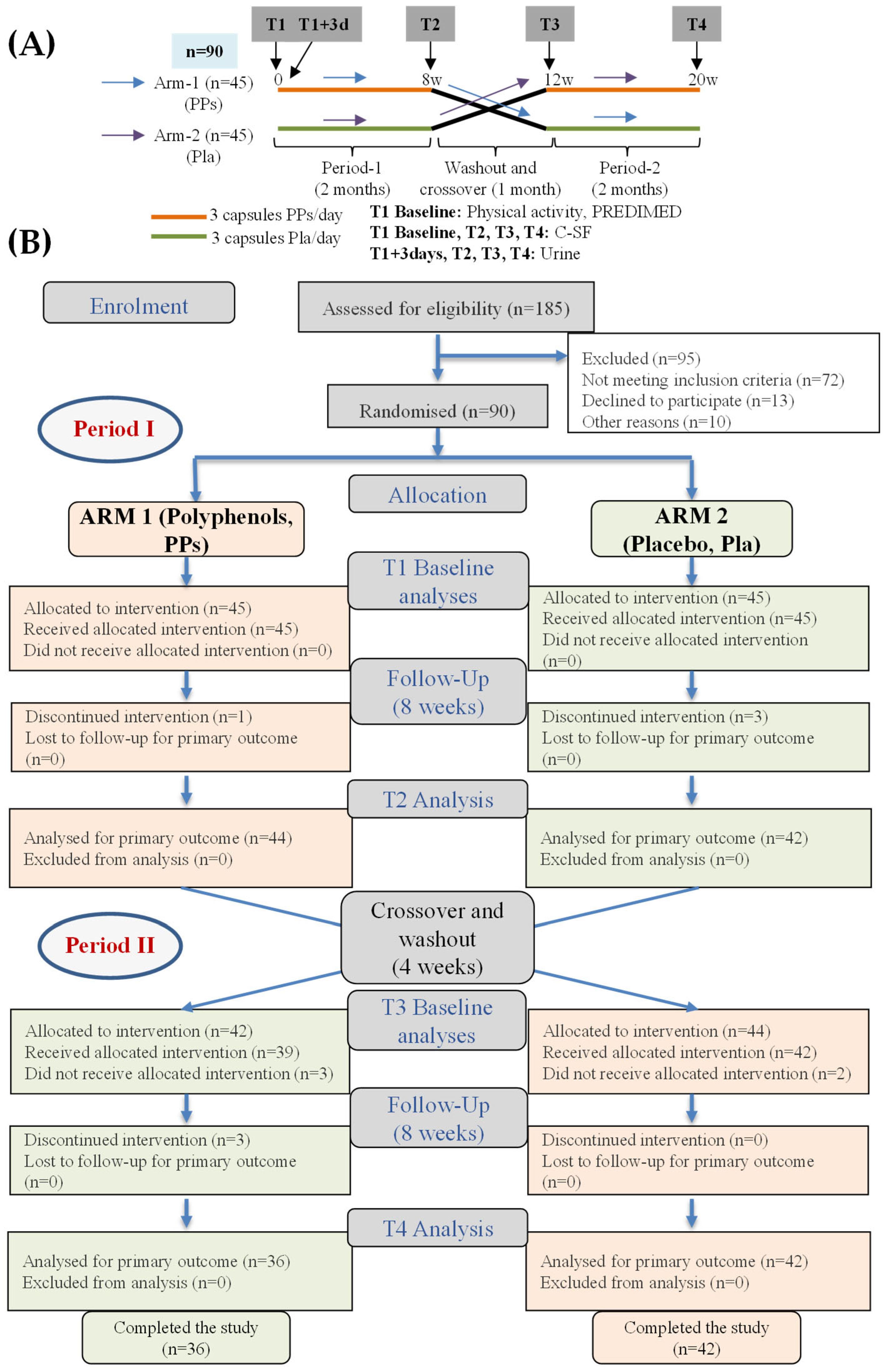
2.3. Study Design and Participants
2.4. Cervantes Scale—Short Form (C-SF)
2.5. Sampling Procedure, Processing, and Analysis
2.6. Identification and Quantification of Metabolites
2.7. Statistical Analyses
3. Results
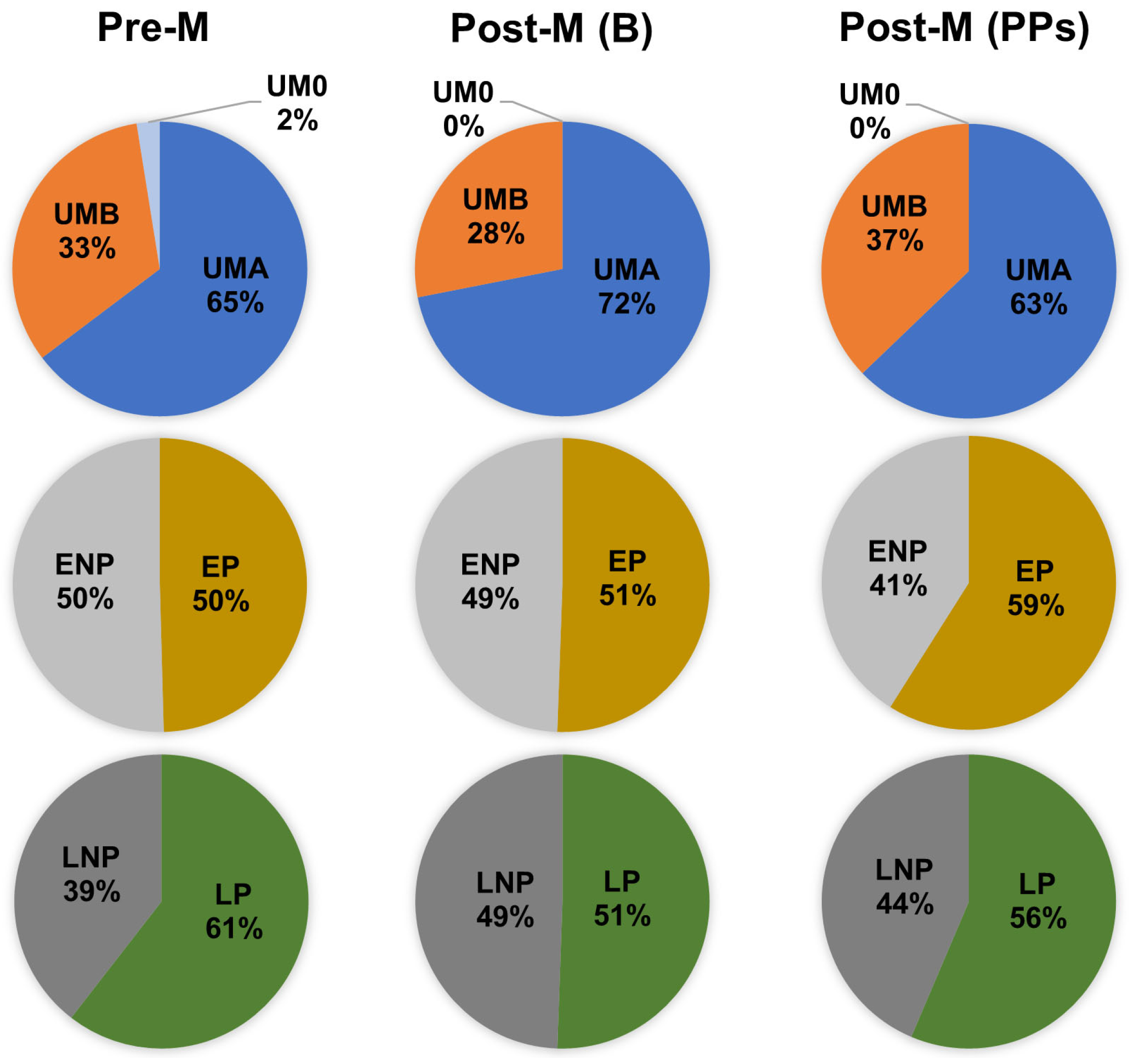
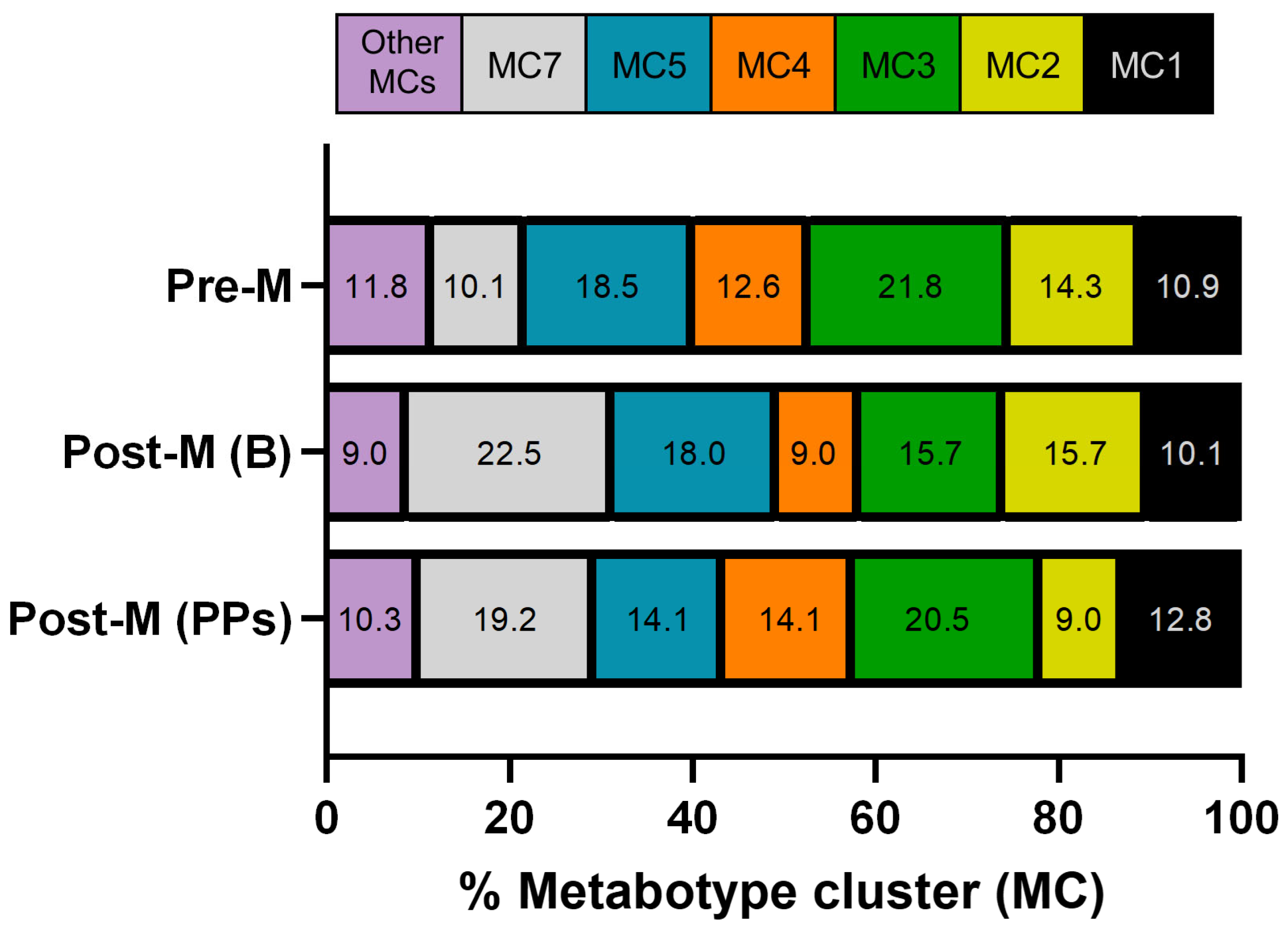
3.1. Characteristics of Participants, Metabotypes, and Metabotype Clusters Distribution at Baseline
3.2. Effect of Polyphenol-Rich Extracts Intake on the Distribution of Metabotypes and Metabotype Clusters in Postmenopausal Women
3.3. Quantitative Production of Metabolites in Premenopausal and Postmenopausal Women at Baseline
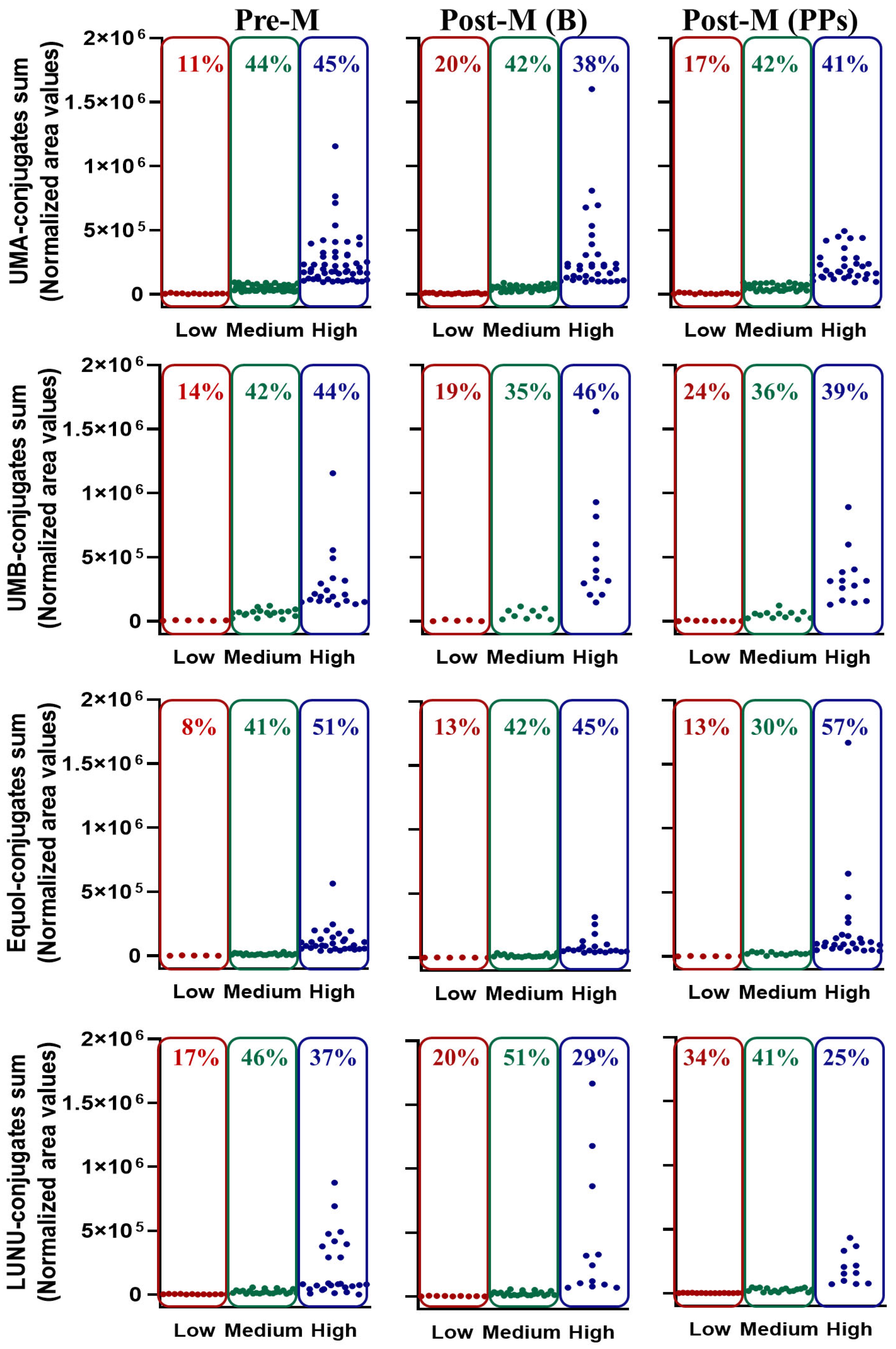
3.4. Distribution of Low-, Medium-, and High-Metabolite Producers in Premenopausal and Postmenopausal Women at Baseline
3.5. Effect of Chronic PPs Intake on Metabolite Production in Postmenopausal Women
3.6. Distribution of Low, Medium, and High Producers Following PPs Intake in Postmenopausal Women
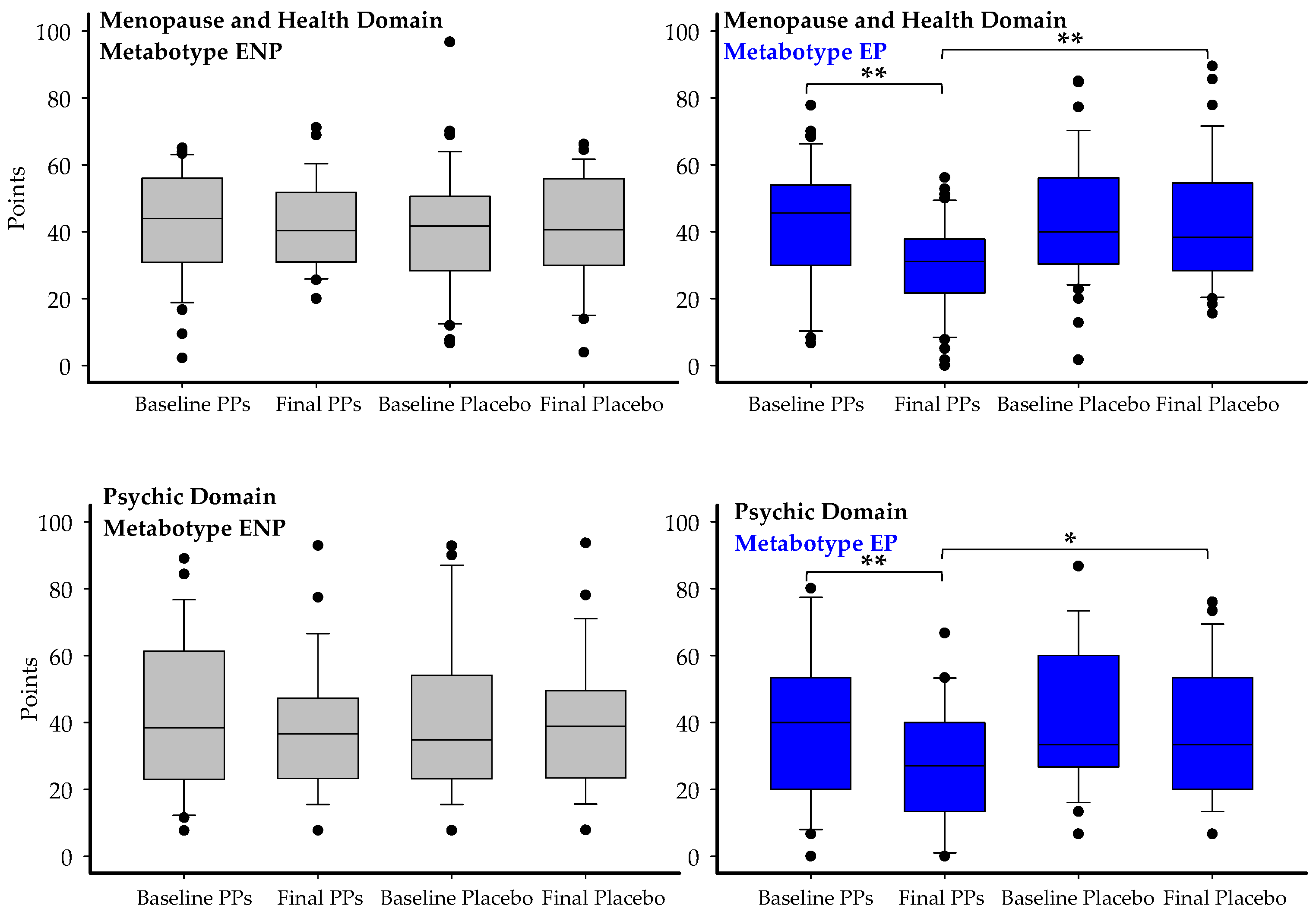
3.7. Impact of Chronic PPs Intake on the Quality of Life of Postmenopausal Women
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| C-SF | Cervantes Scale short form |
| EA | ellagic acid |
| EIC | extracted ion chromatogram |
| EP | equol producer |
| ENP | equol non-producer |
| HPLC-ESI-IT-MS/MS | High-Performance Liquid Chromatography coupled to Electrospray Ionization-Ion Trap Tandem Mass Spectrometry |
| LUNU | lunularin |
| LP | lunularin producer |
| LNP | lunularin non-producer |
| MC | metabotype cluster |
| MS | mass spectrometer |
| ODMA | O-desmethylangolensin |
| Pla | placebo |
| QoL | quality of life |
| RSV | trans-resveratrol |
| PPs | polyphenol-rich plant extract mixture |
| Pre-M | premenopausal |
| Post-M | postmenopausal |
| SD | standard deviation |
| SEM | standard error of the mean |
| UPLC-ESI-QTOF-MS | ultra-high performance liquid chromatography coupled with electrospray ionization and quadrupole time-of-flight mass spectrometry |
| Uro | urolithin |
| UMA | metabotype A |
| UMB | metabotype B |
| UM0 | metabotype 0 |
References
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The Menopause Transition: Signs, Symptoms, and Management Options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef]
- Rees, M.; Abernethy, K.; Bachmann, G.; Bretz, S.; Ceausu, I.; Durmusoglu, F.; Erkkola, R.; Fistonic, I.; Gambacciani, M.; Geukes, M.; et al. The essential menopause curriculum for healthcare professionals: A European Menopause and Andropause Society (EMAS) position statement. Maturitas 2022, 158, 70–77. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Duquet, M.; Prud’homme, D.; Giroux, I. Diet and Depression During Peri- and Post-Menopause: A Scoping Review. Nutrients 2025, 17, 2846. [Google Scholar] [CrossRef]
- Palacios, S.; Ferrer-Barriendos, J.; Parrilla, J.J.; Castelo-Branco, C.; Manubens, M.; Alberich, X.; Marti, A. Health-related quality of life in the Spanish women through and beyond menopause. Development and validation of the Cervantes Scale. Med. Clin. 2004, 122, 205–211. [Google Scholar] [CrossRef]
- Coronado, P.J.; Sánchez-Borrego, R.; Ruiz, M.A.; Baquedano, L.; Sánchez, S.; Argudo, C.; Fernández-Abellán, M.; González, S.; Iglesias, E.; Calleja, J.; et al. Psychometric attributes of the Cervantes short-form questionnaire for measuring health-related quality of life in menopausal women. Maturitas 2016, 84, 55–62. [Google Scholar] [CrossRef]
- Coronado, P.J.; Monroy, M.; Fasero, M.; Sánchez-Borrego, R.; Palacios, S.; Rejas, J.; Ruiz, M.A. Population-based norms for the Cervantes-SF short-form questionnaire assessing health-related quality of life in menopause. Maturitas 2021, 146, 34–41. [Google Scholar] [CrossRef]
- Jiang, K.; Bai, Y.; Hou, R.; Chen, G.; Liu, L.; Ciftci, O.N.; Farag, M.A.; Liu, L. Advances in dietary polyphenols: Regulation of inflammatory bowel disease (IBD) via bile acid metabolism and the gut-brain axis. Food Chem. 2025, 472, 142932. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- Espín, J.C.; Jarrín-Orozco, M.P.; Osuna-Galisteo, L.; Ávila-Gálvez, M.A.; Romo-Vaquero, M.; Selma, M.V. Perspective on the Coevolutionary Role of Host and Gut Microbiota in Polyphenol Health Effects: Metabotypes and Precision Health. Mol. Nutr. Food Res. 2024, 68, e2400526. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Cryan, J.F.; Espín, J.C.; Muguerza, B. Interplay between (poly)phenols, gut microbiota, and biological rhythms: Outlook for a new paradigm in brain health. Crit. Rev. Food Sci. Nutr. 2025, 13, 1–16. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.A.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef] [PubMed]
- Kanadys, W.; Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Drop, B.; Malm, M.; Kanecki, K. Effects of Soy Isoflavones on Biochemical Markers of Bone Metabolism in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 5346. [Google Scholar] [CrossRef] [PubMed]
- Bajerska, J.; Łagowska, K.; Mori, M.; Reguła, J.; Skoczek-Rubińska, A.; Toda, T.; Mizuno, N.; Yamori, Y. A Meta-Analysis of Randomized Controlled Trials of the Effects of Soy Intake on Inflammatory Markers in Postmenopausal Women. J. Nutr. 2022, 152, 5–15. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, Q.; Huang, X.; Liang, Z.; Hu, H. Effect of Isoflavones on Blood Lipid Alterations in Postmenopausal Females: A Systematic Review and Meta-Analysis of Randomized Trials. Adv. Nutr. 2023, 14, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Liu, X.X.; Bai, Y.Y.; Wang, X.J.; Sun, K.; Chen, J.Z.; Hui, R.T. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: A meta-analysis of randomized placebo-controlled trials. Am. J. Clin. Nutr. 2010, 91, 480–486. [Google Scholar] [CrossRef]
- Abshirini, M.; Omidian, M.; Kord-Varkaneh, H. Effect of soy protein containing isoflavones on endothelial and vascular function in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Menopause 2020, 27, 1425–1433. [Google Scholar] [CrossRef]
- Gençtürk, N.; Bilgiç, F.Ş.; Kaban, H.U. The effect of soy isoflavones given to women in the climacteric period on menopausal symptoms and quality of life: Systematic review and meta-analysis of randomized controlled trials. Explore 2024, 20, 103012. [Google Scholar] [CrossRef]
- Salvio, G.; Ciarloni, A.; Gianfelice, C.; Lacchè, F.; Sabatelli, S.; Giacchetti, G.; Balercia, G. The Effects of Polyphenols on Bone Metabolism in Postmenopausal Women: Systematic Review and Meta-Analysis of Randomized Control Trials. Antioxidants 2023, 12, 1830. [Google Scholar] [CrossRef]
- Wu, W.; Meng, T.; Jin, F.; Li, J.; Huang, J.; Guo, Z.; Yu, M.; Zhou, Y. Effects of resveratrol on postmenopausal women: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1588284. [Google Scholar] [CrossRef]
- Rodrigues Uggioni, M.L.; Ronsani, L.; Motta, S.; Denoni Júnior, J.C.; Marçal, F.; Dagostin Ferraz, S.; Rosa, M.I.; Colonetti, T. Effects of resveratrol on the lipid profile of post-menopause women: Systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103827. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Romo-Vaquero, M.; Selma, M.V.; Espín, J.C. Unveiling metabotype clustering in resveratrol, daidzein, and ellagic acid metabolism: Prevalence, associated gut microbiomes, and their distinctive microbial networks. Food Res. Int. 2023, 173, 113470. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61, 1500900. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltrán, D.; Aguilar-Aguilar, E.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Lunularin Producers versus Non-producers: Novel Human Metabotypes Associated with the Metabolism of Resveratrol by the Gut Microbiota. Agric. Food Chem. 2022, 70, 10521–10531. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Örem, A.; Zafrilla, P.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, 1600830. [Google Scholar] [CrossRef] [PubMed]
- Meroño, T.; Peron, G.; Gargari, G.; González-Domínguez, R.; Miñarro, A.; Vegas-Lozano, E.; Hidalgo-Liberona, N.; Del Bo’, C.; Bernardi, S.; Kroon, P.A.; et al. The relevance of urolithins-based metabotyping for assessing the effects of a polyphenol-rich dietary intervention on intestinal permeability: A post-hoc analysis of the MaPLE trial. Food Res. Int. 2022, 159, 111632. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Loygorri, J.I.; Villarejo-Zori, B.; Viedma-Poyatos, Á.; Zapata-Muñoz, J.; Benítez-Fernández, R.; Frutos-Lisón, M.D.; Tomás-Barberán, F.A.; Espín, J.C.; Area-Gómez, E.; Gomez-Duran, A.; et al. Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat. Commun. 2024, 15, 830. [Google Scholar] [CrossRef]
- Wu, J.; Oka, J.; Ezaki, J.; Ohtomo, T.; Ueno, T.; Uchiyama, S.; Toda, T.; Uehara, M.; Ishimi, Y. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: A double-blind, randomized, controlled trial. Menopause 2007, 14, 866–874. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-de-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Meoro, A.; Selma, M.V.; Espín, J.C. Pharmacological Therapy Determines the Gut Microbiota Modulation by a Pomegranate Extract Nutraceutical in Metabolic Syndrome: A Randomized Clinical Trial. Mol. Nutr. Food Res. 2021, 65, e2001048. [Google Scholar] [CrossRef]
- Takahashi, A.; Anzai, Y.; Tanji, N.; Imaizumi, H.; Fujita, M.; Hayashi, M.; Abe, K.; Ohira, H. Association of equol with obesity in postmenopausal women. Menopause 2021, 28, 807–810. [Google Scholar] [CrossRef]
- Lucas, R.; Alcántara, D.; Morales, J.C. A concise synthesis of glucuronide metabolites of urolithin-B, resveratrol, and hydroxytyrosol. Carbohydr. Res. 2009, 344, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Miguel, V.; Merino, G.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; Álvarez, A.I.; Espín, J.C. The Gut Microbiota Ellagic Acid-Derived Metabolite Urolithin A and Its Sulfate Conjugate Are Substrates for the Drug Efflux Transporter Breast Cancer Resistance Protein (ABCG2/BCRP). J. Agric. Food Chem. 2013, 61, 4352–4359. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.A.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, e1801239. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Fernández-Jarne, E.; Serrano-Martínez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef]
- Estrugo, C.P.; Rodríguez, M.T.; de Guevara, N.M.; Gómez, J.G.; Ridocci, F.; Moro-Martín, M.T.; Guinot, M.; Saz-Leal, P.; Nieto Magro, C. Combination of Soy Isoflavones, 8-Prenylnaringenin and Melatonin Improves Hot Flashes and Health-Related Quality of Life Outcomes in Postmenopausal Women: Flavie Study. J. Menopausal Med. 2023, 29, 73–83. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef] [PubMed]
- Thaung Zaw, J.J.; Howe, P.R.C.; Wong, R.H.X. Long-term resveratrol supplementation improves pain perception, menopausal symptoms, and overall well-being in postmenopausal women: Findings from a 24-month randomized, controlled, crossover trial. Menopause 2020, 28, 40–49. [Google Scholar] [CrossRef]
- Wong, R.H.X.; Evans, H.M.; Howe, P.R.C. Resveratrol supplementation reduces pain experience by postmenopausal women. Menopause 2017, 24, 916–922. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef]
- López-Ríos, L.; Barber, M.A.; Wiebe, J.; Machín, R.P.; Vega-Morales, T.; Chirino, R. Influence of a new botanical combination on quality of life in menopausal Spanish women: Results of a randomized, placebo-controlled pilot study. PLoS ONE 2021, 16, e0255015. [Google Scholar] [CrossRef]
- Garcia-Yu, I.A.; Garcia-Ortiz, L.; Gomez-Marcos, M.A.; Rodriguez-Sanchez, E.; Tamayo-Morales, O.; Maderuelo-Fernandez, J.A.; Recio-Rodriguez, J.I. Cocoa-Rich Chocolate and Quality of Life in Postmenopausal Women: A Randomized Clinical Trial. Nutrients 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Yamaguchi, T.; Oishi, S.; Misawa, K.; Suzuki, A.; Hibi, M.; Terauchi, M. Supplementation with cassis polyphenol has no effect on menopausal symptoms in healthy middle-aged women: A randomized, double-blind, parallel-group, placebo-controlled trial. Nutr. Res. 2024, 126, 14–22. [Google Scholar] [CrossRef]
- Park, S.L.; Kim, M.S.; Kim, T.H. Gut Microbiome and Estrogen. J. Menopausal Med. 2025, 31, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Xian, W.; Yang, S.; Deng, Y.; Yang, Y.; Chen, C.; Li, W.; Yang, R. Distribution of Urolithins Metabotypes in Healthy Chinese Youth: Difference in Gut Microbiota and Predicted Metabolic Pathways. J. Agric. Food Chem. 2021, 69, 13055–13065. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Yoshikata, R.; Myint, K.Z.Y.; Taguchi, J. Comparison of blood and urine concentrations of equol by LC-MS/MS method and factors associated with equol production in 466 Japanese men and women. PLoS ONE 2024, 19, e0288946. [Google Scholar] [CrossRef]
- Frankenfeld, C.L.; Maskarinec, G.; Franke, A.A. Metabolomics profiles of premenopausal women are different based on O-desmethylangolensin metabotype. Br. J. Nutr. 2022, 128, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Daily, J.W.; Ko, B.S.; Ryuk, J.; Liu, M.; Zhang, W.; Park, S. Equol Decreases Hot Flashes in Postmenopausal Women: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2019, 22, 127–139. [Google Scholar] [CrossRef]
- Leonard, L.M.; Choi, M.S.; Cross, T.L. Maximizing the Estrogenic Potential of Soy Isoflavones through the Gut Microbiome: Implication for Cardiometabolic Health in Postmenopausal Women. Nutrients 2022, 14, 553. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, C.; Okada, Y.; Shigeta, M.; Nomura, Y.; Sekizawa, A.; Yoshimura, Y. Influence of equol production capacity on female lower urinary tract symptoms. J. Obstet. Gynaecol. Res. 2025, 51, e16266. [Google Scholar] [CrossRef] [PubMed]
- Cotillard, A.; Kennedy, S.P.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef]
| Compound | RT | m/z- | MS/MS | λmax | mg/g Extract | mg/Capsule |
|---|---|---|---|---|---|---|
| Pomegranate | ||||||
| Punicalin | 12.76 | 781 | 721/601/299 | 258/380 | 11.47 ± 0.92 | 8.03 ± 0.65 |
| Punicalagin α | 15.08 | 1083 | 781/721/601 | 258/372 | 48.15 ± 8.75 | 33.71 ± 6.13 |
| Punicalagin β | 17.08 | 1083 | 781/721/601 | 258/372 | 48.20 ± 6.24 | 32.16 ± 2.28 |
| Ellagic acid | 25.68 | 301 | 257/229/185 | 254/360 | 42.81 ± 1.81 | 29.97 ± 1.27 |
| Σ Ellagitannins and ellagic acid | 103.9 ± 10.32 | |||||
| Resveratrol | ||||||
| trans-Resveratrol | 30.84 | 227 | 185/157 | 306 | 63.42 ± 4.80 | 44.39 ± 3.36 |
| Red clover | ||||||
| Daidzein | 32.33 | 253 | 225/209 | 248/304 | 5.20 ± 0.61 | 3.64 ± 0.43 |
| Genistein | 37.15 | 269 | 241/225 | 260/324 | 4.86 ± 0.15 | 3.41 ± 0.10 |
| Formononetin | 40.80 | 267 | 252/235/211 | 250/304 | 43.81 ± 1.60 | 30.67 ± 1.12 |
| Biochanin A | 43.15 | 283 | 268/251/227 | 260/332 | 25.34 ± 10.63 | 17.74 ± 7.44 |
| Σ Isoflavones | 55.45 ± 9.10 | |||||
| Total phenolics | 203.7 ± 22.8 | |||||
| Characteristics | Values 1 | |
|---|---|---|
| Pre-M (n = 120) | Post-M (n = 90) | |
| Age (years, y) | 40.2 ± 4.0; 41.0 (30−48) | 53.2 ± 3.3; 54.0 (45−59) |
| BMI (kg/m2) | 23.7 ± 3.8; 23.0 (18.3−36.2) | 26.0 ± 4.3; 25.1 (19.0−37.4) |
| Normal weight | 85 (70.8%) | 45 (50.0%) |
| Overweight | 27 (22.5%) | 32 (35.6%) |
| Obese | 8 (6.7%) | 13 (14.4%) |
| Menopause onset (y) | - | 49.7 ± 3.7 (40−56) |
| Postmenopausal duration (y) | - | 3.9 ± 3.5 (0.3−14) |
| Domains of the Cervantes Scale: | ||
| Global | 36.7 ± 16.3; 34.1 (5.6−77.2) | |
| Menopause and health | - | 45.0 ± 19.3; 42.8 (2.2−96.7) |
| Psychic | 36.7 ± 27.7; 33.3 (0−100) | |
| Sexuality | 44.6 ± 24.6; 40 (0−100) | |
| Couple relationship | 14.7 ± 20.10; 10 (0−100) | |
| Individual metabotypes: | ||
| UMA, UMB, UM0 | (64.7%, 32.8%, 2.5%) | (71.9%, 28.1%, 0%) |
| EP, ENP | (49.6%, 50.4%) | (50.6%, 49.4%) |
| LP, LNP | (60.5%, 39.5%) | (50.6%, 49.4%) |
| Metabotype clusters (MCs): | ||
| MC1: UMB+ENP+LP | 10.9% | 10.1% |
| MC2: UMA+ENP+LP | 14.3% | 15.7% |
| MC3: UMA+EP+LP | 21.8% | 15.7% |
| MC4: UMB+EP+LP | 12.6% | 9.0% |
| MC5: UMA+ENP+LNP | 18.5% | 18.0% |
| MC6: UMB+ENP+LNP | 6.0% | 5.6% |
| MC7: UMA+EP+LNP | 10.1% | 22.5% |
| MC8: UMB+EP+LNP | 3.4% | 3.4% |
| MC9: UM0+EP+LNP | 0.8% | 0% |
| MC10: UM0+EP+LNP | 0.8% | 0% |
| MC11: UM0+EP+LP | 0.8% | 0% |
| MC12: UM0+ENP+LP | 0% | 0% |
| Domains and Subdomains | Placebo (Pla) | Polyphenols (PPs) | p-Value (PPs vs. Pla) | 95% CI (LSM) (PPs-Pla) | Cohen’s d |
|---|---|---|---|---|---|
| Global | |||||
| All (n = 78) | 0.49 ± 1.4; −1.5% | −3.4 ± 1.5; 9.5% * | 0.023 | [−7.9, 0.21] | −0.30 |
| Metabotypes | |||||
| UMB (n = 29) | −1.1 ± 2.3; 3% | −5.1 ± 2.1; 12.8% * | 0.037 | [−7.1, −0.9] | −0.41 |
| EP (n = 46) | −2.1 ± 2.2; 5.2% | −6.2 ± 2.1; 16.6% * | 0.039 | [−7.2, −1.2] | −0.28 |
| MCs | |||||
| MC4: UMB+EP+LP (n = 11) | −1.6 ± 1.9; 5% | −6.1 ± 1.8; 15.3% * | 0.048 | [−8.8, −0.3] | −0.58 |
| Menopause and health | |||||
| All (n = 78) | −2.1 ± 2.8; 3.5% | −6.3 ± 1.9; 14.2% * | <0.001 | [−8.9, 0.5] | −0.20 |
| Metabotypes | |||||
| UMA (n = 49) | −2.6 ± 2.4; 5.5% | −4.7 ± 2.1; 10.5% * | 0.012 | [−5.8, −0.6] | −0.38 |
| UMB (n = 29) | −3.1 ± 3.5; 6.2% | −9.3 ± 3.1; 20% *& | 0.006 | [−10.3, −2.1] | −0.52 |
| EP (n = 46) | −4.3 ± 2.6; 5.2% | −10.1 ± 2.3; 24.1% *& | 0.004 | [−9.9, 4.7] | −0.35 |
| MCs | |||||
| MC4: UMB+EP+LP (n = 11) | −4.6 ± 5.1; 8.8% | −20.8 ± 4.3; 41.3% *& | 0.022 | [−24, −11.6] | −0.84 |
| MC3: UMA+EP+LP (n = 16) | −1.6 ± 3.4; 3.4% | −9.7 ± 4.1; 22.5% *& | 0.043 | [−12.6, 2.8] | −0.61 |
| Psychic | |||||
| All (n = 78) | −3.8 ± 2.4; 9.8% | −8.3 ± 2.2; 23.8% *& | 0.053 | [−7.9, −1.7] | −0.41 |
| Metabotypes | |||||
| EP (n = 46) | −3.4 ± 3.1; 8.5% | −9.9 ± 2.9; 28% *& | 0.039 | [−8.8, −3.4] | −0.32 |
| MCs | |||||
| MC3: UMA+EP+LP (n = 16) | 0.48 ± 5.2; 1.3% | −6.7 ± 4.1; 17.8% * | 0.051 | [−11.3, −0.1] | −0.61 |
| Sexuality | 2.7 ± 2.6; −7.5% | −5.1 ± 4.3; 11% | 0.309 | [−12.3, 1.5] | −0.26 |
| Couple relationship | −1.6 ± 2.7; 5.5% | −1.9 ± 2.4; 9% | 0.653 | [−3.8, 2.2] | −0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarrín-Orozco, M.P.; Romo-Vaquero, M.; Carrascosa, C.; Pertegal, M.; Berná, J.; Puigcerver, J.; Saura-Sanmartín, A.; Espinosa-Salinas, I.; García-Nicolás, M.; Ávila-Gálvez, M.Á.; et al. Polyphenol-Related Gut Metabotype Signatures Linked to Quality of Life in Postmenopausal Women: A Randomized, Placebo-Controlled Crossover Trial. Nutrients 2025, 17, 3572. https://doi.org/10.3390/nu17223572
Jarrín-Orozco MP, Romo-Vaquero M, Carrascosa C, Pertegal M, Berná J, Puigcerver J, Saura-Sanmartín A, Espinosa-Salinas I, García-Nicolás M, Ávila-Gálvez MÁ, et al. Polyphenol-Related Gut Metabotype Signatures Linked to Quality of Life in Postmenopausal Women: A Randomized, Placebo-Controlled Crossover Trial. Nutrients. 2025; 17(22):3572. https://doi.org/10.3390/nu17223572
Chicago/Turabian StyleJarrín-Orozco, María P., María Romo-Vaquero, Concepción Carrascosa, Miriam Pertegal, José Berná, Julio Puigcerver, Adrián Saura-Sanmartín, Isabel Espinosa-Salinas, María García-Nicolás, María Á. Ávila-Gálvez, and et al. 2025. "Polyphenol-Related Gut Metabotype Signatures Linked to Quality of Life in Postmenopausal Women: A Randomized, Placebo-Controlled Crossover Trial" Nutrients 17, no. 22: 3572. https://doi.org/10.3390/nu17223572
APA StyleJarrín-Orozco, M. P., Romo-Vaquero, M., Carrascosa, C., Pertegal, M., Berná, J., Puigcerver, J., Saura-Sanmartín, A., Espinosa-Salinas, I., García-Nicolás, M., Ávila-Gálvez, M. Á., & Espín, J. C. (2025). Polyphenol-Related Gut Metabotype Signatures Linked to Quality of Life in Postmenopausal Women: A Randomized, Placebo-Controlled Crossover Trial. Nutrients, 17(22), 3572. https://doi.org/10.3390/nu17223572












