Longitudinal Microbiome and Metabolome Shifts After Successful Intervention in Impending Stunting in Indonesian Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
- Infants aged 6–12 months with WF, defined as weight increments < P15th of the WHO weight increment table [10];
- Growth chart available for monitoring (weight, length, and head circumference measured at least once at birth);
- Available height and weight data from both father and mother;
- A parent (either mother or father) agreed to participate in this study and signed their informed consent.
- Subjects with a length-for-age z-score (HAZ) below 2 SD;
- Severe acute malnutrition;
- Presence of cow’s milk allergy;
- Presence of lactose intolerance;
- Presence of galactosemia;
- Major congenital anomaly, severe stunting at birth (newborns whose length-for-gestational age was below 10th percentile), thyroid disorder, major gastrointestinal disease, or other severe diseases, e.g., pneumonia or dehydration;
- Conditions that require special diets, e.g., major renal or hepatic dysfunctions;
- Conditions that influence nutritional status, e.g., moderate to severe dehydration, edema, organomegaly;
- Infants with relative WF but a body weight above the median weight for length (considering that they may become overweight);
- History of a low birth weight (less than 2500 g).
- History of premature birth (born after a period of pregnancy of less than 37 weeks).
2.2. Study Design
3. Results
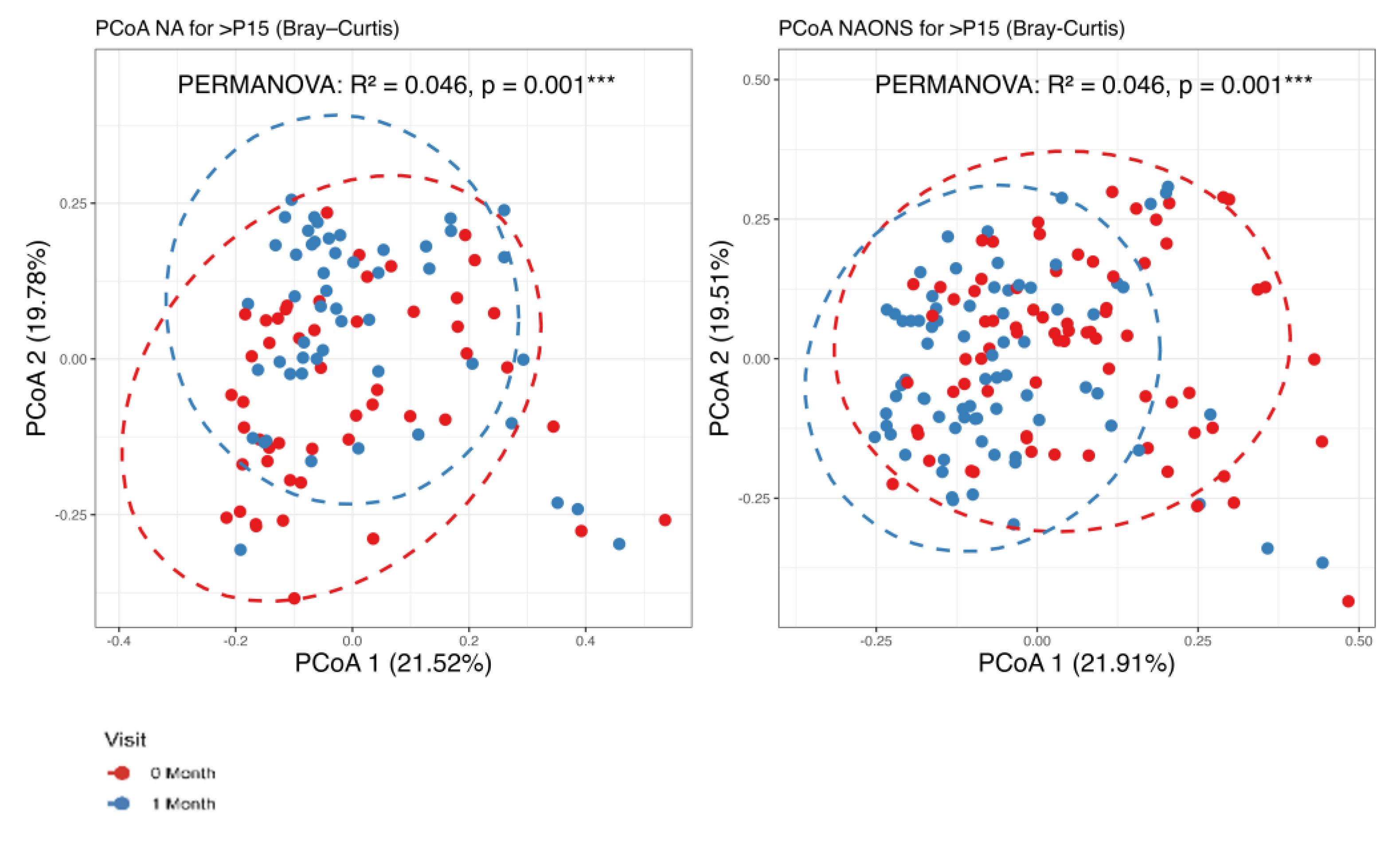
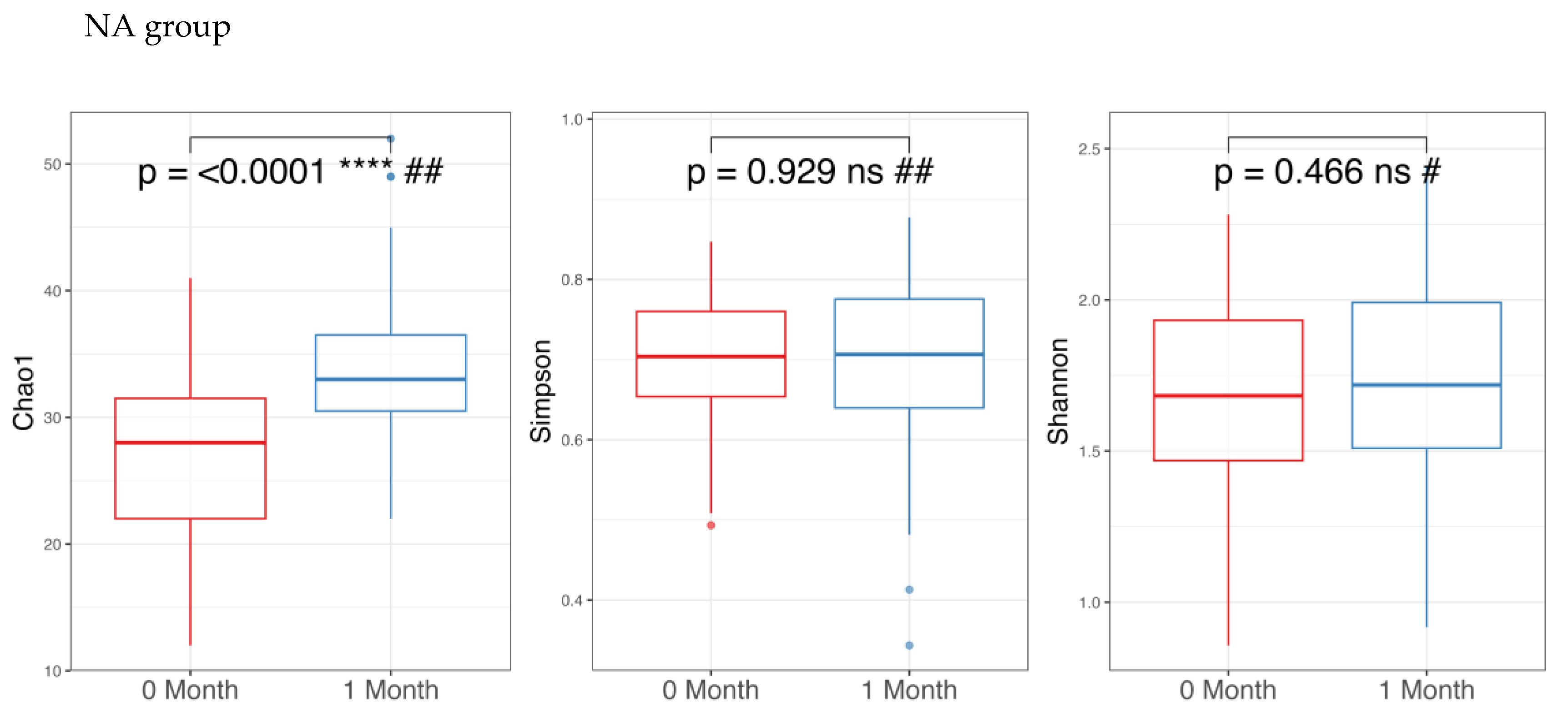
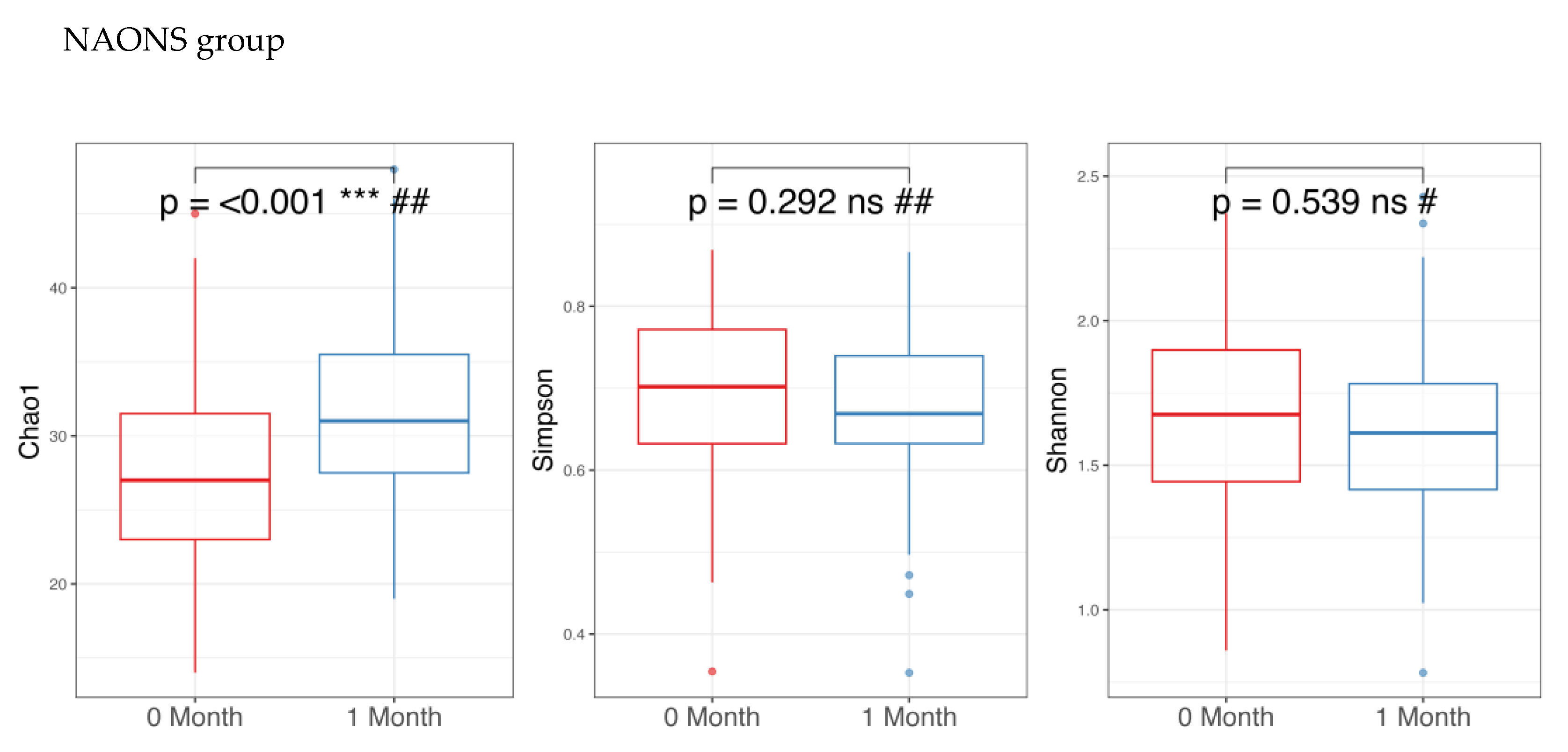
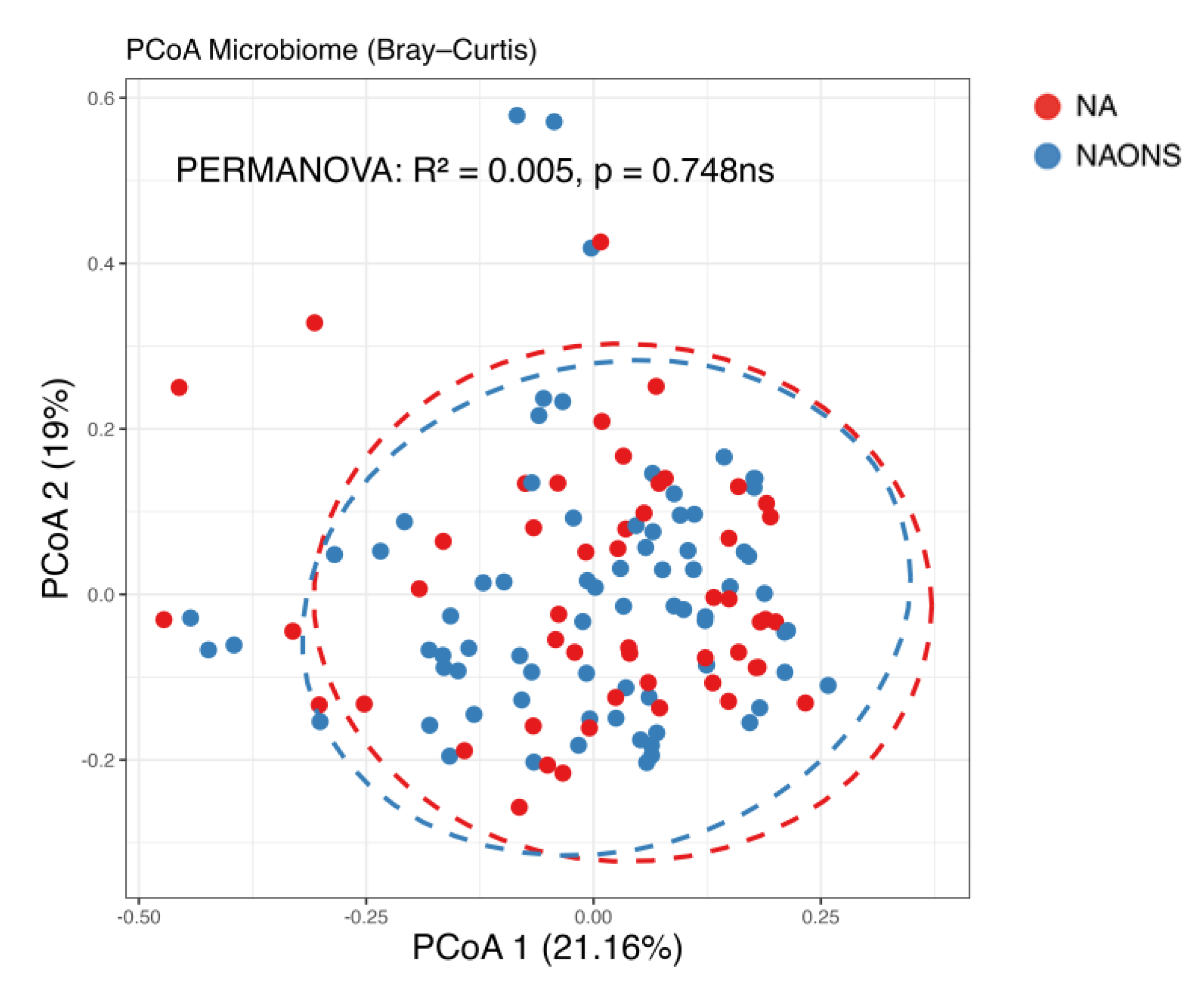
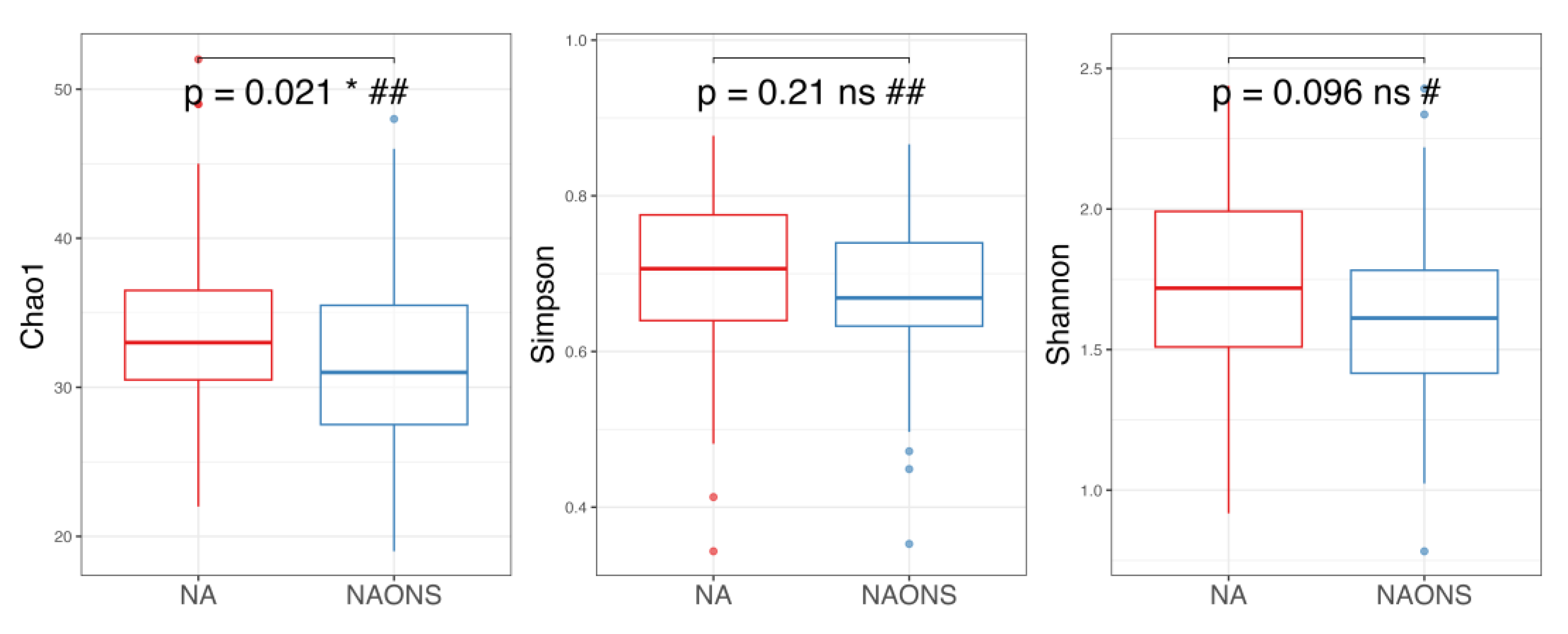
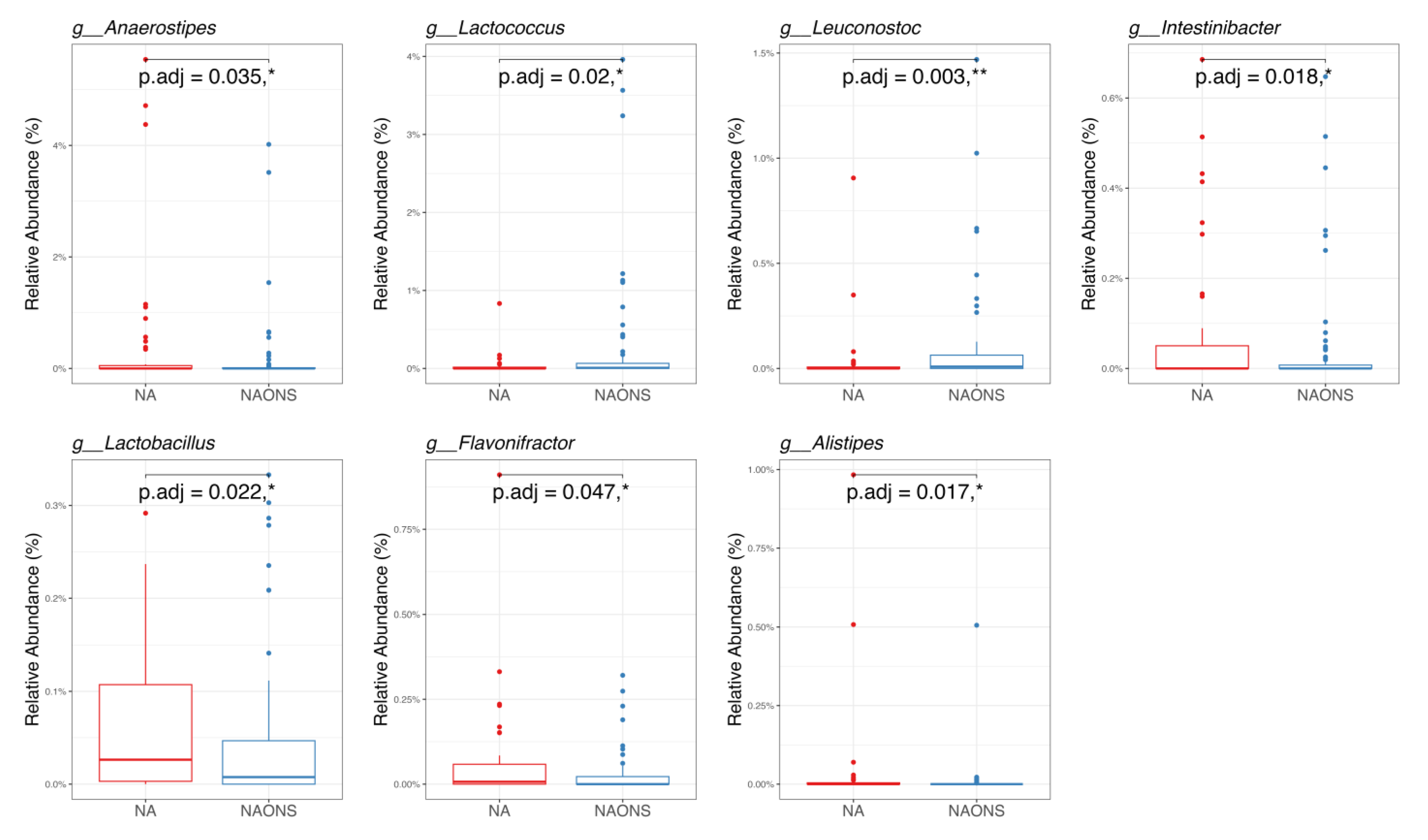
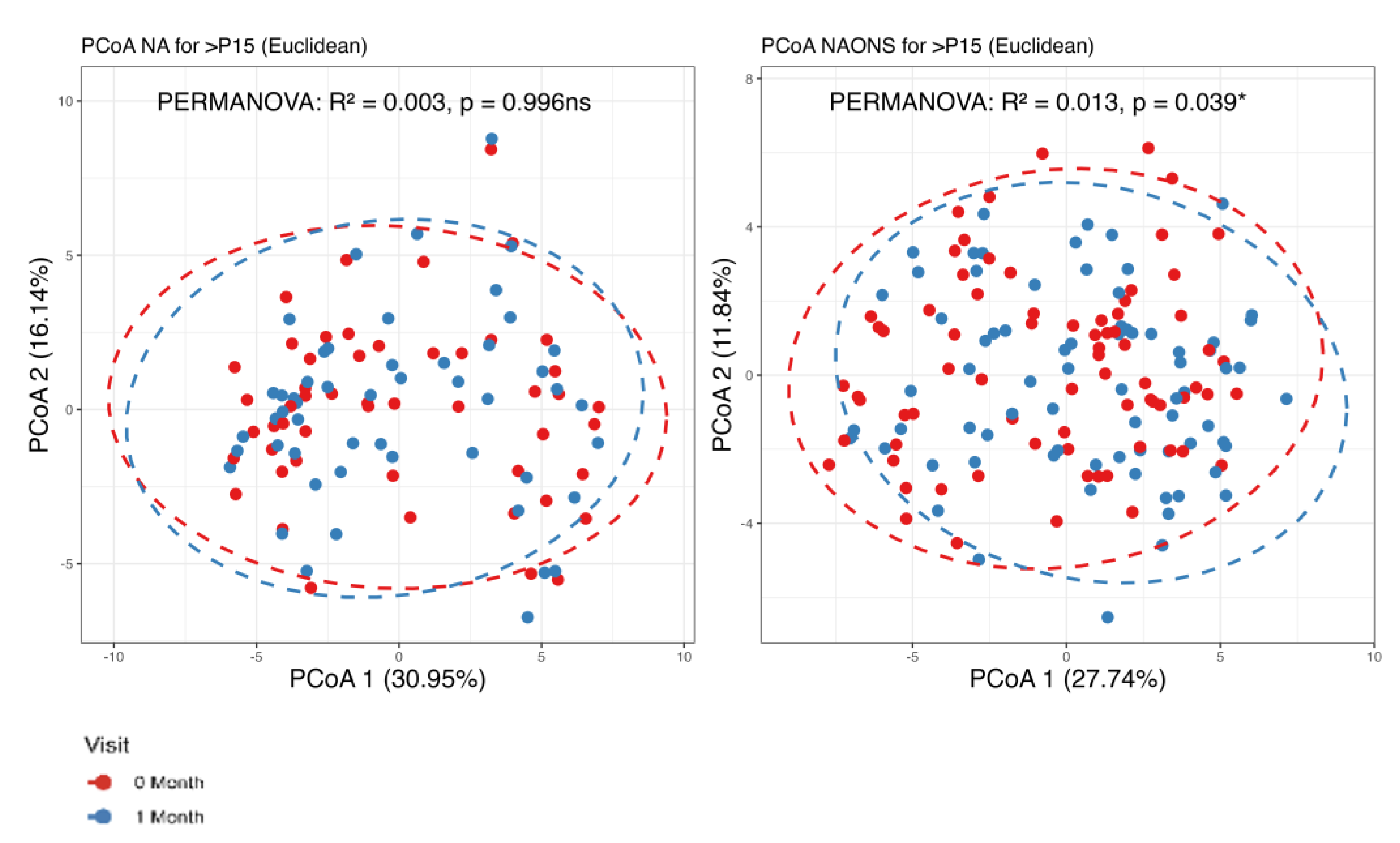
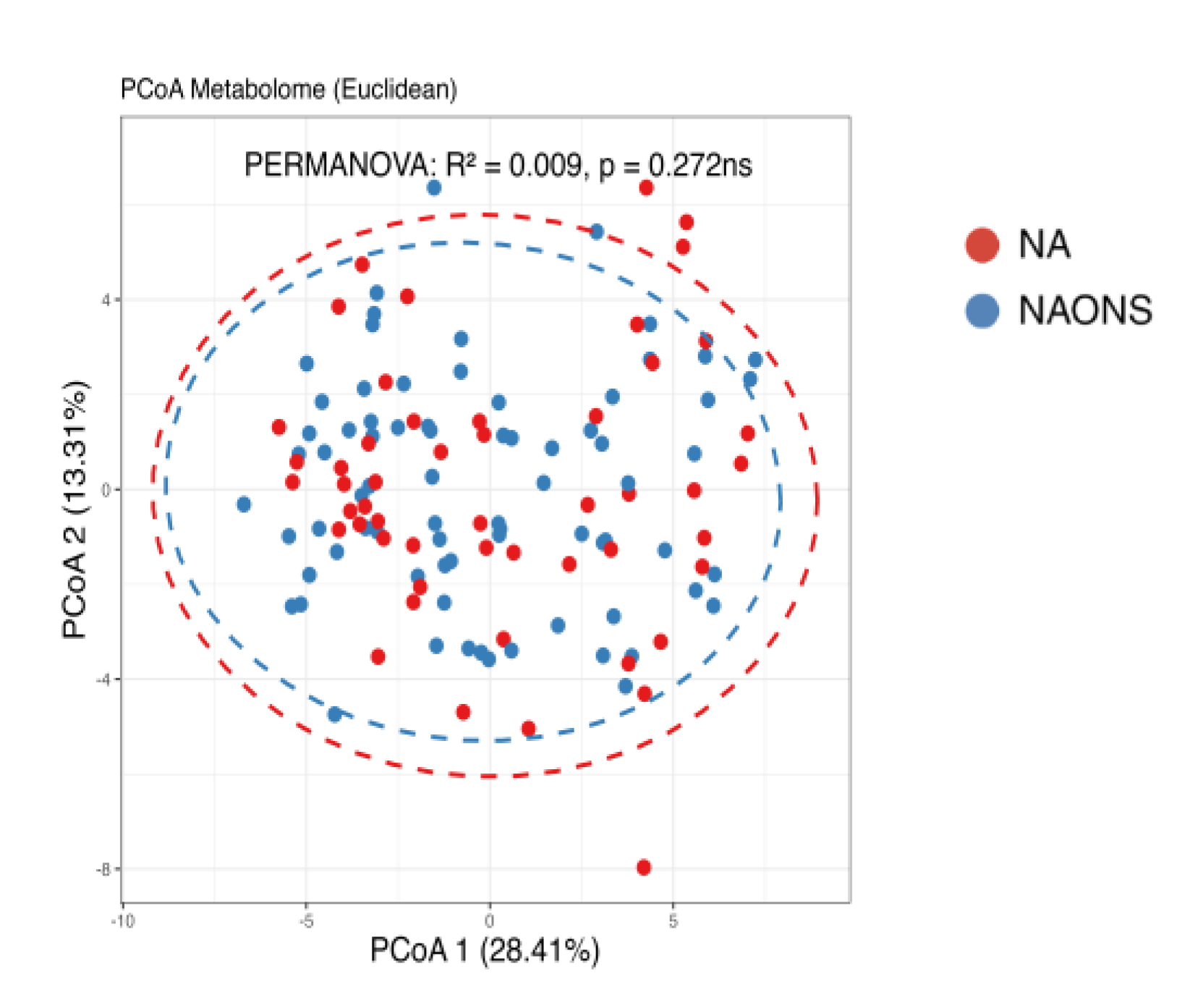
3.1. Microbiome Analysis
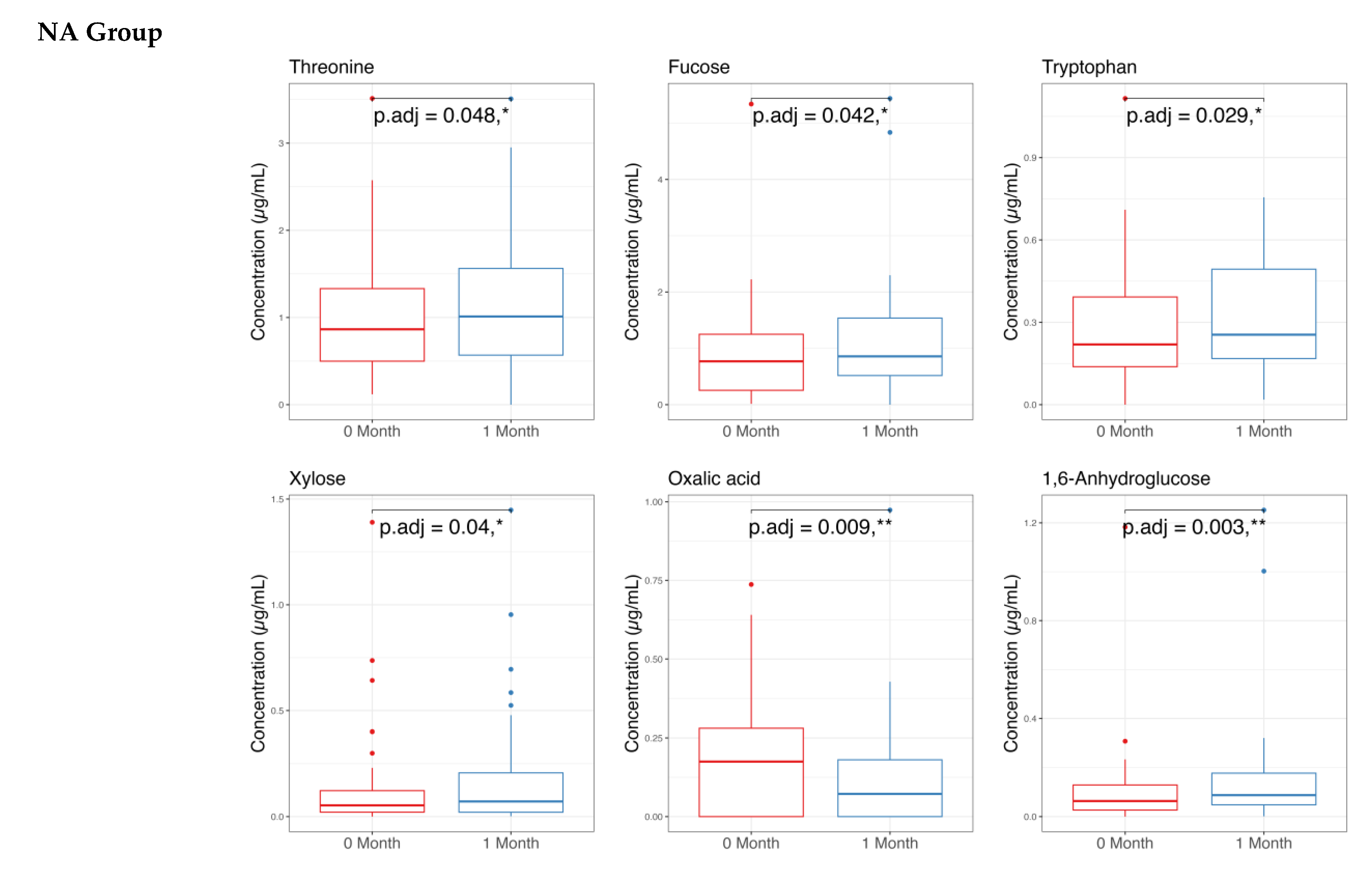
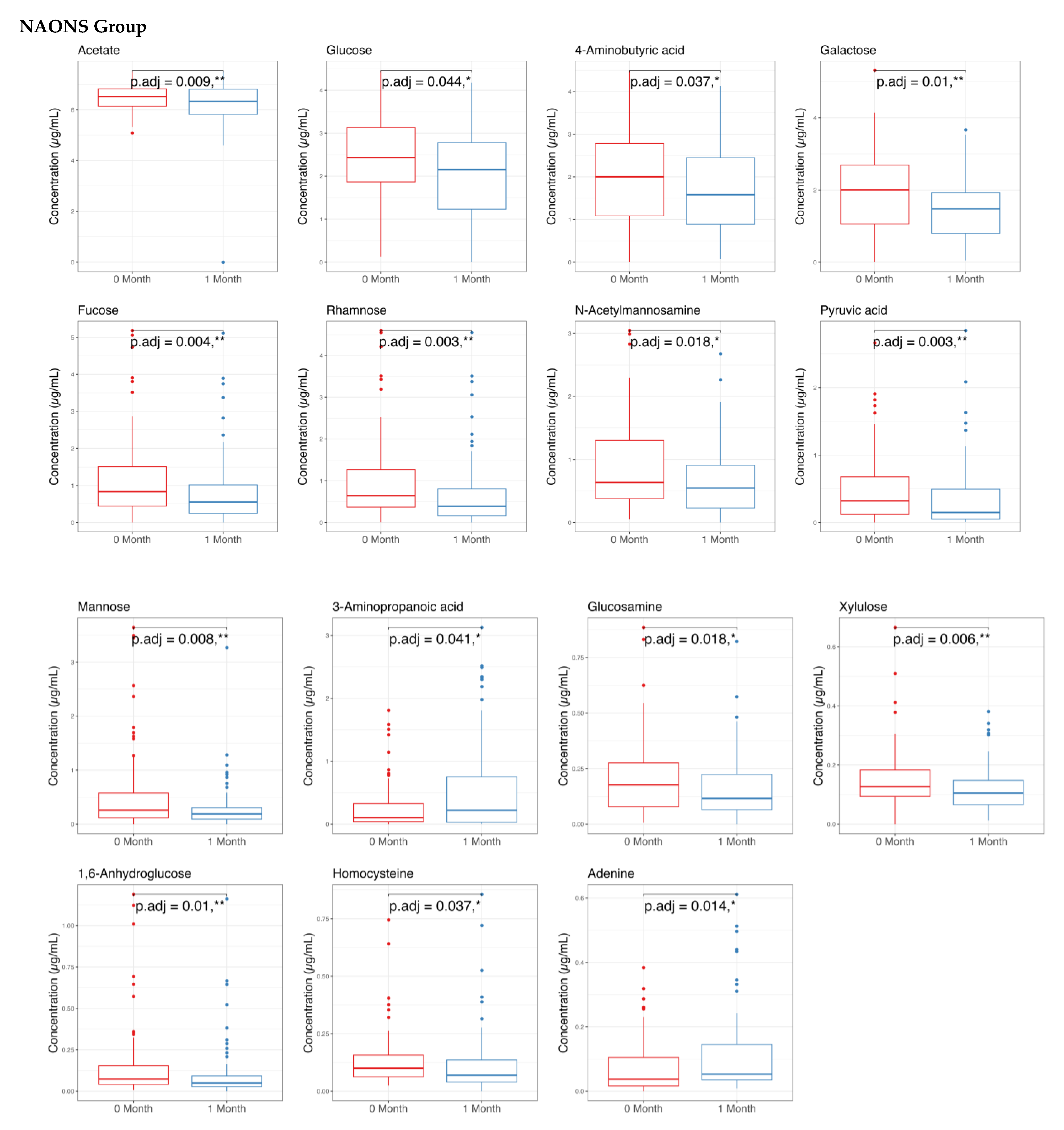
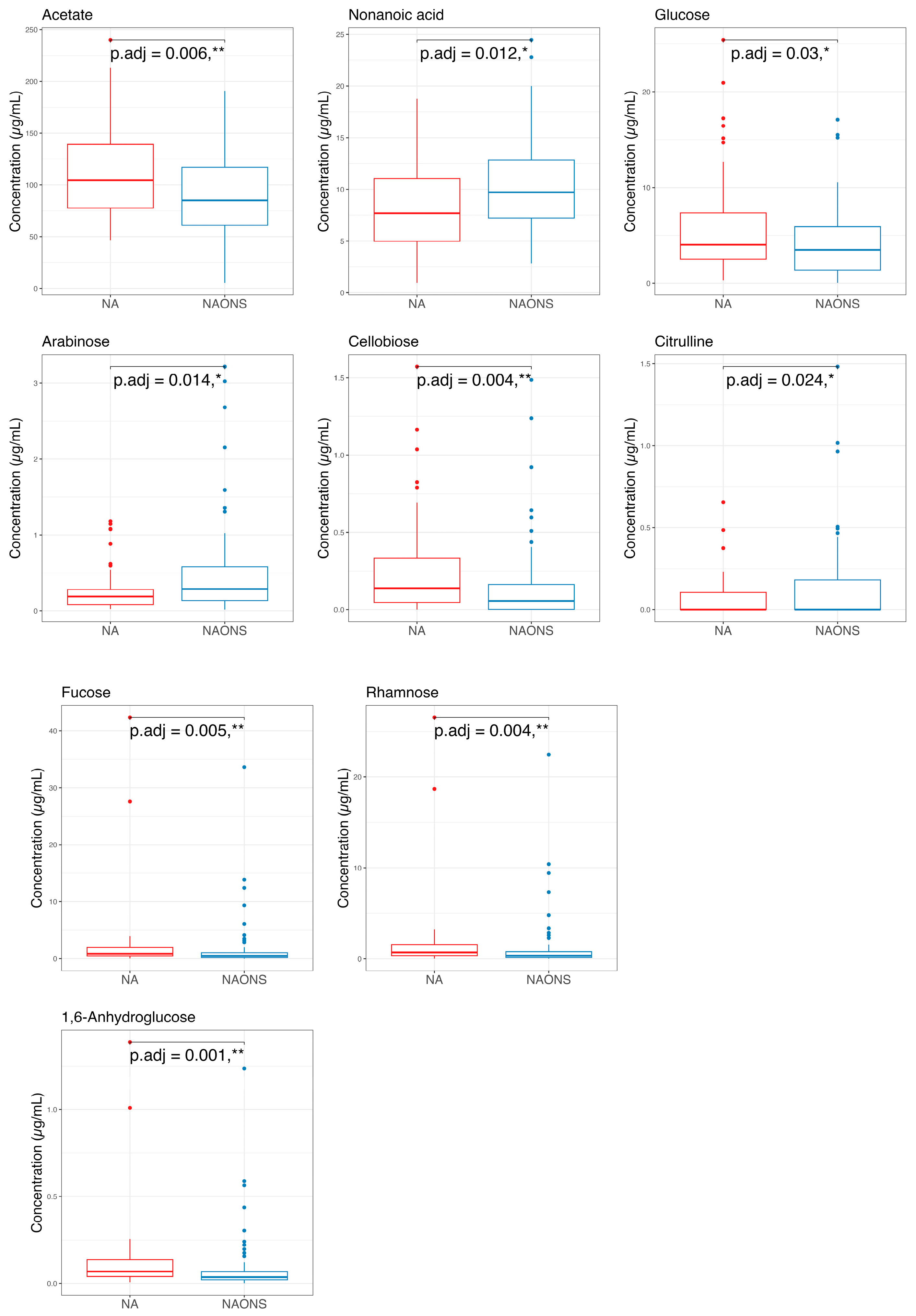
3.2. Stool Metabolome Analysis
4. Discussion
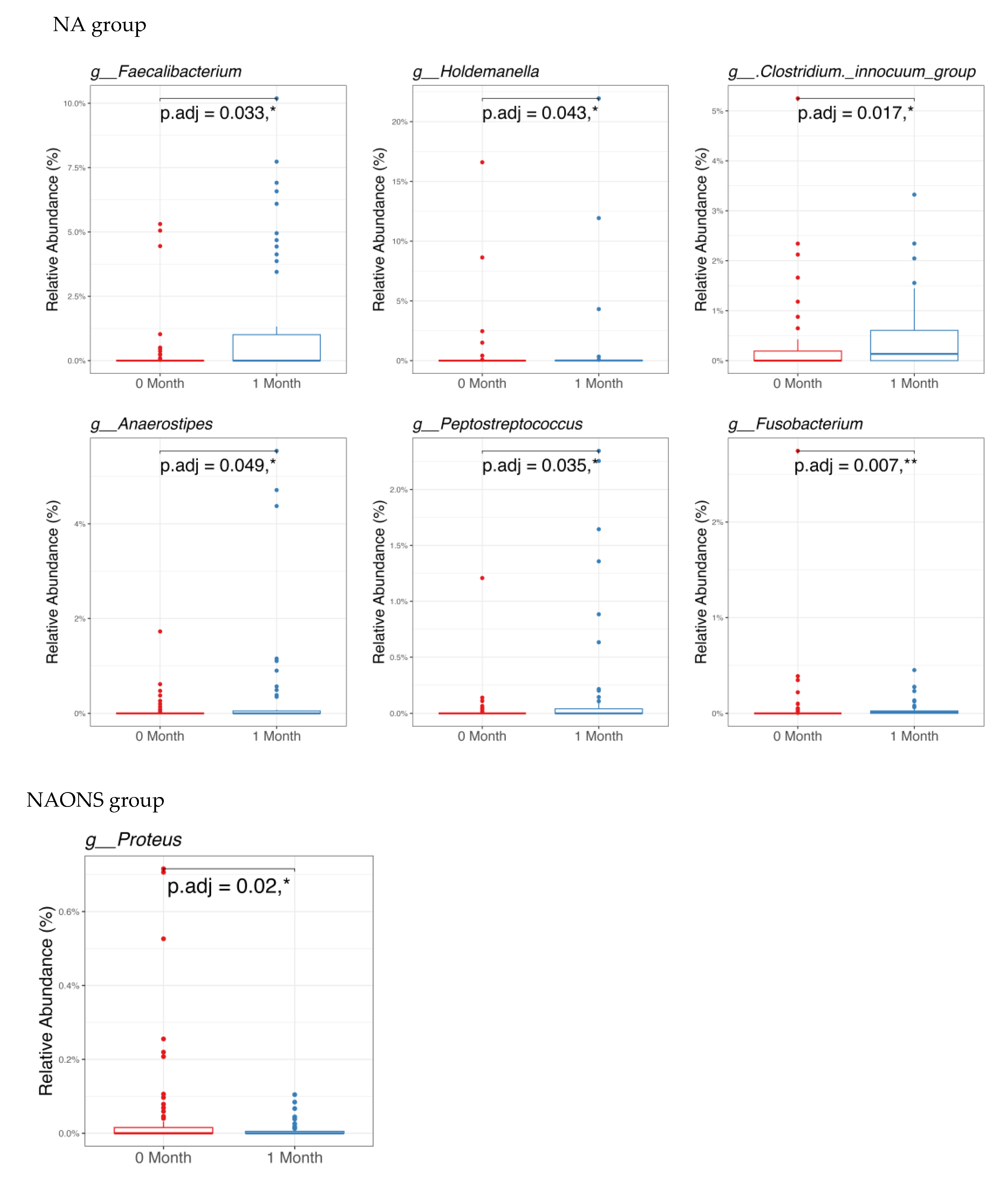
4.1. On Microbiome Changes
- Faecalibacterium: Its elevated levels may contribute to improved gut health and integrity through its anti-inflammatory properties and the production of butyrate [14].
- Holdemanella: Although less extensively studied, elevated levels of this component have been associated with improved gut health [15].
- Clostridium innocuum group: Higher levels have been associated with improved carbohydrate metabolism and the production of beneficial metabolites [16].
- Anaerostipes: Increased butyrate production, resulting from higher levels, may support gut health by serving as an energy source for colonocytes [17].
- Peptostreptococcus: Elevated levels have been associated with enhanced protein metabolism and increased production of short-chain fatty acids [18].
- Fusobacterium: Often associated with inflammation and disease; its decrease suggests a healthier gut environment [19].
- Proteus: Known for its association with urinary tract infections and other inflammatory conditions, it can disrupt gut health and hinder nutrient absorption. The significant decrease in Proteus suggests a healthier gut environment, which is crucial for overcoming weight faltering [20].
4.2. On Stool Metabolome Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Sanctis, V.; Soliman, A.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N. Early and long-term consequences of nutritional stunting: From childhood to adulthood. Acta Biomed. 2021, 92, e2021168. [Google Scholar] [CrossRef]
- De Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern. Child Nutr. 2016, 12, 12–26. [Google Scholar] [CrossRef]
- World Health Organization. Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition; WHO: Geneva, Switzerland, 2017; Available online: https://iris.who.int/bitstream/handle/10665/260202/9789241513647-eng.pdf (accessed on 30 April 2025).
- Graber, E.G. Growth and Weight Faltering in Children. Available online: https://www.msdmanuals.com/professional/pediatrics/growth-and-development/growth-and-weight-faltering-in-children (accessed on 30 April 2025).
- Mercer, E.M.; Ramay, H.R.; Moossavi, S.; Laforest-Lapointe, I.; Reyna, M.E.; Becker, A.B.; Arrieta, M.C. Divergent maturational patterns of the infant bacterial and fungal gut microbiome in the first year of life. Microbiome 2024, 12, 22. [Google Scholar] [CrossRef]
- Yahagi, K. The Early Life of the Gut Microbiota. Nature. 2024. Available online: https://www.nature.com/articles/d42473-024-00330-w (accessed on 30 April 2025).
- Azab, S.M.; de Souza, R.J.; Lamri, A.; Shanmuganathan, M.; Kroezen, Z.; Schulze, K.M.; Desai, D.; Williams, N.C.; Morrison, K.M.; Atkinson, S.A.; et al. Metabolite profiles and the risk of metabolic syndrome in early childhood: A case-control study. BMC Med. 2021, 19, 292. [Google Scholar] [CrossRef]
- Osterman, M.J.K.; Hamilton, B.E.; Martin, J.A.; Driscoll, A.K.; Valenzuela, C.P. Gestational diabetes, the human milk metabolome, and infant growth and adiposity. JAMA Netw. Open. 2024, 7, e2827802. [Google Scholar]
- Tomori, C.; O’Connor, D.L.; Ververs, M.; Orta-Aleman, D.; Paone, K.; Budhathoki, C.; Pérez-Escamilla, R. Critical research gaps in treating growth faltering in infants under 6 months: A systematic review and meta-analysis. PLoS Glob. Public Health 2024, 4, e0001860. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Child Growth Standards: Growth Velocity Based on Weight, Length and Head Circumference: Methods and Development; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Tanjung, C.; Fikri, B.; Prawitasari, T.; Massi, N.; Zainuddin, A.A.; Juliaty, A.; Yullyana, D.S.; Dwitya, S.; Shimojo, N.; Ohno, H.; et al. Comparative analysis of nutritional advice and a combined approach for addressing impending stunting in infants: A clinical trial. Nutrients 2024, 16, 2832. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, A.M.; Wu, M.C. Beta diversity and distance-based analysis of microbiome data. In Statistical Analysis of Microbiome Data; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–127. [Google Scholar] [CrossRef]
- Genomics, C.D. The Use and Types of Alpha-Diversity Metrics in Microbial NGS. Available online: https://www.cd-genomics.com/microbioseq/the-use-and-types-of-alpha-diversity-metrics-in-microbial-ngs.html (accessed on 30 April 2025).
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.-M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Bhattacharjee, D.; Flores, C.; Woelfel-Monsivais, C.; Seekatz, A.M. Diversity and prevalence of Clostridium innocuum in the human gut microbiota. mSphere 2022, 8, e00569-22. [Google Scholar] [CrossRef]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Li, N.; Huang, S.; Jiang, L.; Wang, W.; Li, T.; Zuo, B.; Li, Z.; Wang, J. Differences in the gut microbiota establishment and metabolome characteristics between low- and normal-birth-weight piglets during early-life. Front. Microbiol. 2018, 9, 1798. [Google Scholar] [CrossRef]
- Nguyen, Q.P.; Karagas, M.R.; Madan, J.C.; Dade, E.; Palys, T.J.; Morrison, H.G.; Pathmasiri, W.W.; McRitche, S.; Sumner, S.J.; Frost, H.R.; et al. Associations between the gut microbiome and metabolome in early life. BMC Microbiol. 2021, 21, 238. [Google Scholar] [CrossRef] [PubMed]
- Gough, E.K.; Stephens, D.A.; Moodie, E.E.; Prendergast, A.J.; Stoltzfus, R.J.; Humphrey, J.H.; Manges, A.R. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome 2015, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, D. Significance and roles of Proteus spp. bacteria in natural environments. Microb. Ecol. 2016, 72, 741–758. [Google Scholar] [CrossRef]
- Fontaine, F.; Turjeman, S.; Callens, K.; Koren, O. The intersection of undernutrition, microbiome, and child development in the first years of life. Nat. Commun. 2023, 14, 3554. [Google Scholar] [CrossRef]
- Yoda, M.; Takase, S.; Suzuki, K.; Murakami, A.; Namai, F.; Sato, T.; Fujii, T.; Tochio, T.; Shimosato, T. Development of engineered IL-36γ-hypersecreting Lactococcus lactis to improve the intestinal environment. World J. Microbiol. Biotechnol. 2024, 40, 363. [Google Scholar] [CrossRef]
- Zikmanis, P.; Brants, K.; Kolesovs, S.; Semjonovs, P. Extracellular polysaccharides produced by bacteria of the Leuconostoc genus. World J. Microbiol. Biotechnol. 2020, 36, 161. [Google Scholar] [CrossRef]
- Barker-Tejeda, T.C.; Zubeldia-Varela, E.; Macías-Camero, A.; Alonso, L.; Martín-Antoniano, I.A.; Rey-Stolle, M.F.; Mera-Berriatua, L.; Bazire, R.; Cabrera-Freitag, P.; Shanmuganathan, M.; et al. Comparative characterization of the infant gut microbiome and their maternal lineage by a multi-omics approach. Nat. Commun. 2024, 15, 3004. [Google Scholar] [CrossRef]
- Hu, X.; Yu, C.; He, Y.; Zhu, S.; Wang, S.; Xu, Z.; You, S.; Jiao, Y.; Liu, S.-L.; Bao, H. Integrative metagenomic analysis reveals distinct gut microbial signatures related to obesity. BMC Microbiol. 2024, 24, 119. [Google Scholar] [CrossRef]
- Bai, Z.; Wu, Y.; Gao, D.; Dong, Y.; Pan, Y.; Gu, S. Gut microbiome and metabolome alterations in overweight or obese adult population after weight-loss Bifidobacterium breve BBr60 intervention: A randomized controlled trial. Int. J. Mol. Sci. 2024, 25, 10871. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). β-Alanine. NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C107959&Mask=4 (accessed on 30 April 2025).
- Chemeurope. Beta-Alanine. Available online: https://www.chemeurope.com/en/encyclopedia/Beta-alanine.html (accessed on 30 April 2025).
- Acevedo, N.; Alashkar Alhamwe, B.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and Early-Life Nutrition, Epigenetics, and Allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Van Esch, B.C.A.M.; Porbahaie, M.; Abbring, S.; Garssen, J.; Potaczek, D.P.; Savelkoul, H.F.J.; van Neerven, R.J.J. The Impact of Milk and Its Components on Epigenetic Programming of Immune Function in Early Life and Beyond: Implications for Allergy and Asthma. Front. Immunol. 2020, 11, 2141. [Google Scholar] [CrossRef]
- Zhou, T.; Xiao, L.; Zuo, Z.; Zhao, F. MAMI: A comprehensive database of mother–infant microbiome and probiotic resources. Nucleic Acids Res. 2024, 52, D738–D746. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The association between early-life gut microbiota and long-term health and diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef]
- Thurstans, S.; Sessions, N.; Dolan, C.; Sadler, K.; Cichon, B. The relationship between wasting and stunting in young children: A systematic review. Matern. Child Nutr. 2022, 18, e13246. [Google Scholar] [CrossRef]
- Bagamian, K.H.; Iv, J.D.A.; Blohm, G.; Scheele, S. Shigella and childhood stunting: Evidence, gaps, and future research directions. PLoS Neglected Trop. Dis. 2023, 17, e0011475. [Google Scholar] [CrossRef]
| Variable | Category | NA | NAONS | p-Value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| About Siblings | Without Sibling | 14 | 22.22 | 15 | 15.00 | 0.240 ns# |
| Siblings | 49 | 77.78 | 85 | 85.00 | ||
| Parity | <3 | 37 | 58.73 | 43 | 43.00 | 0.036 *## |
| ≥3 | 26 | 41.27 | 57 | 57.00 | ||
| Childbirth History | Sectio Caesaria | 15 | 23.81 | 24 | 24.00 | 0.978 ns# |
| Spontan | 48 | 76.19 | 76 | 76.00 | ||
| Gender | Male | 31 | 49.21 | 43 | 43.00 | 0.270 ns## |
| Female | 32 | 50.79 | 57 | 57.00 | ||
| Ethnic | Bugis | 10 | 15.87 | 16 | 16.00 | 0.701 ns# |
| Makassar | 47 | 74.60 | 78 | 78.00 | ||
| Other | 6 | 9.52 | 6 | 6.00 | ||
| Smoking Status | Non-Smoking | 24 | 38.10 | 32 | 32.00 | 0.264 ns## |
| Smoking | 39 | 61.90 | 68 | 68.00 | ||
| Income | <IDR 3,000,000 | 39 | 61.90 | 59 | 59.00 | 0.420 ns## |
| ≥IDR 3,000,000 | 24 | 38.10 | 41 | 41.00 | ||
| Mother Education | No Education At All | 0.747 ns# | ||||
| Elementary School | ||||||
| Junior High School | ||||||
| Senior High School | ||||||
| Diploma 1 | ||||||
| Diploma 2 | ||||||
| Bachelor | ||||||
| Post-Graduate | ||||||
| Father Education | No Education At All | 0.758 ns# | ||||
| Elementary School | ||||||
| Junior High School | ||||||
| Senior High School | ||||||
| Diploma 1 | ||||||
| Diploma 2 | ||||||
| Bachelor | ||||||
| Post-Graduate | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanjung, C.; Shibata, R.; Fikri, B.; Prawitasari, T.; Zainuddin, A.A.; Juliaty, A.; Yullyana, D.S.; Sundjaya, T.; Kuswanto, H.; Clarensia, J.; et al. Longitudinal Microbiome and Metabolome Shifts After Successful Intervention in Impending Stunting in Indonesian Infants. Nutrients 2025, 17, 3570. https://doi.org/10.3390/nu17223570
Tanjung C, Shibata R, Fikri B, Prawitasari T, Zainuddin AA, Juliaty A, Yullyana DS, Sundjaya T, Kuswanto H, Clarensia J, et al. Longitudinal Microbiome and Metabolome Shifts After Successful Intervention in Impending Stunting in Indonesian Infants. Nutrients. 2025; 17(22):3570. https://doi.org/10.3390/nu17223570
Chicago/Turabian StyleTanjung, Conny, Ryohei Shibata, Bahrul Fikri, Titis Prawitasari, Andi Alfian Zainuddin, Aidah Juliaty, Dwi Sora Yullyana, Tonny Sundjaya, Hedi Kuswanto, Jessica Clarensia, and et al. 2025. "Longitudinal Microbiome and Metabolome Shifts After Successful Intervention in Impending Stunting in Indonesian Infants" Nutrients 17, no. 22: 3570. https://doi.org/10.3390/nu17223570
APA StyleTanjung, C., Shibata, R., Fikri, B., Prawitasari, T., Zainuddin, A. A., Juliaty, A., Yullyana, D. S., Sundjaya, T., Kuswanto, H., Clarensia, J., Shimojo, N., Koletzko, B., Ohno, H., & Massi, N. (2025). Longitudinal Microbiome and Metabolome Shifts After Successful Intervention in Impending Stunting in Indonesian Infants. Nutrients, 17(22), 3570. https://doi.org/10.3390/nu17223570






