Biomarkers Linked to Malnutrition Identified According to GLIM Criteria Among Older Community-Dwelling Adults: Results from the ilSIRENTE Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
2.3. Malnutrition
2.4. Blood Markers
2.5. Covariates and Adjustment Variables
2.6. Statistical Analysis
3. Results
3.1. Main Characteristics of Study Participants
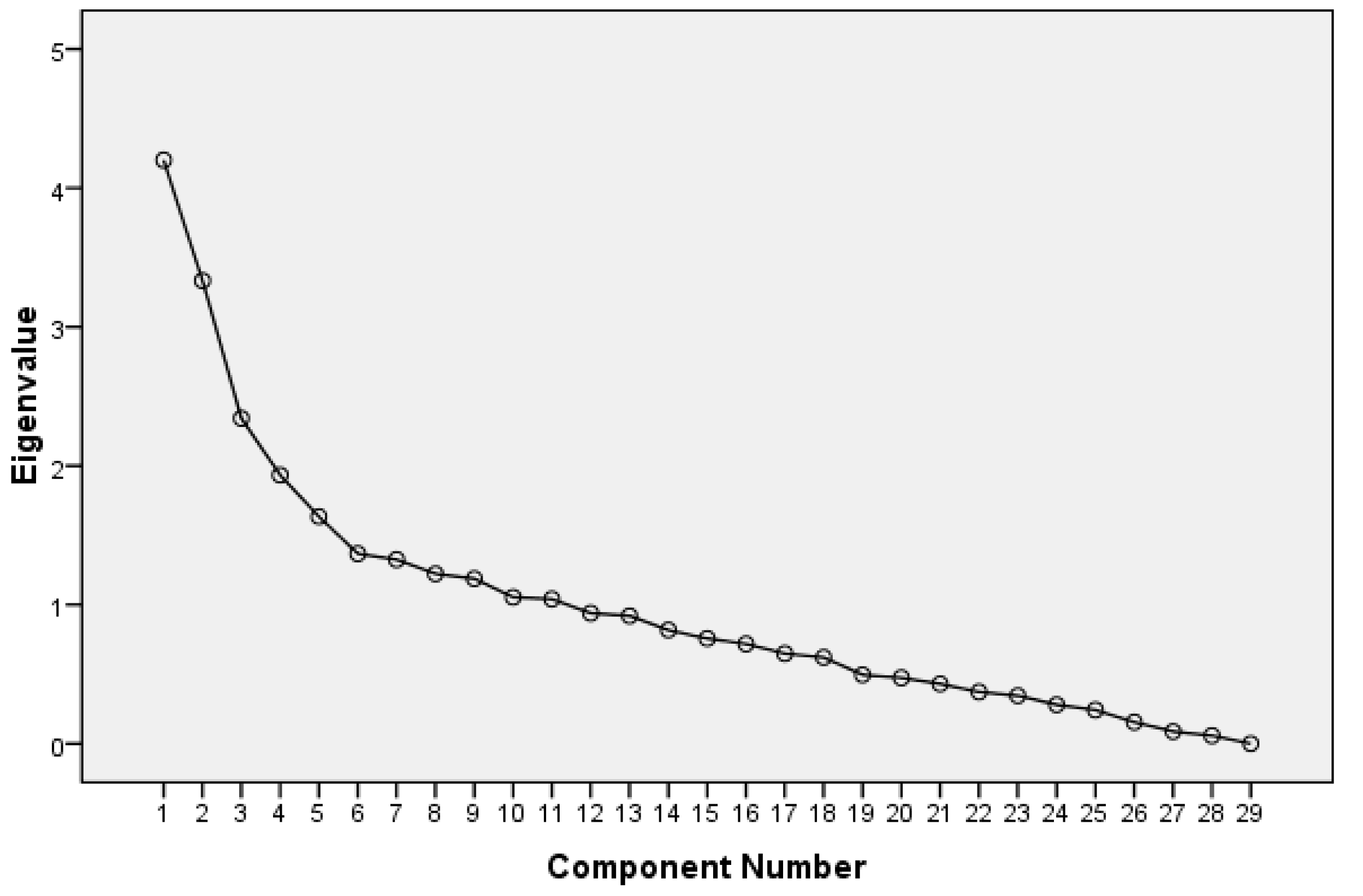
3.2. Identification of the Main Principal Components
3.3. Associations Between PCAs and Malnutrition
3.4. Molecule Load
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leij-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of Protein-Energy Malnutrition Risk in European Older Adults in Community, Residential and Hospital Settings, According to 22 Malnutrition Screening Tools Validated for Use in Adults ≥65 Years: A Systematic Review and Meta-Analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Kupisz-Urbanska, M.; Marcinowska-Suchowierska, E. Malnutrition in Older Adults-Effect on Falls and Fractures: A Narrative Review. Nutrients 2022, 14, 3132. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Chan, R.S.M.; Kwok, T.; Lee, J.S.W.; Woo, J. Malnutrition According to GLIM Criteria and Adverse Outcomes in Community-Dwelling Chinese Older Adults: A Prospective Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1953–1959.e4. [Google Scholar] [CrossRef] [PubMed]
- Söderström, L.; Rosenblad, A.; Thors Adolfsson, E.; Bergkvist, L. Malnutrition Is Associated with Increased Mortality in Older Adults Regardless of the Cause of Death. Br. J. Nutr. 2017, 117, 532–540. [Google Scholar] [CrossRef]
- Ulugerger Avci, G.; Suzan, V.; Bektan Kanat, B.; Unal, D.; Emiroglu Gedik, T.; Doventas, A.; Suna Erdincler, D.; Yavuzer, H. Depressive Symptoms Are Associated with Sarcopenia and Malnutrition in Older Adults. Psychogeriatrics 2023, 23, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.N.; Cheng, Y.; Chen, L.K.; Tung, H.H.; Chu, K.H.; Liang, S.Y. Cognition and Social-Physiological Factors Associated with Malnutrition in Hospitalized Older Adults in Taiwan. J. Nurs. Res. 2015, 23, 1–5. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Landi, F.; Russo, A.; Cesari, M.; Barillaro, C.; Onder, G.; Zamboni, V.; De Santis, A.; Pahor, M.; Ferrucci, L.; Bernabei, R. The IlSIRENTE Study: A Prospective Cohort Study on Persons Aged 80 Years and Older Living in a Mountain Community of Central Italy. Aging Clin. Exp. Res. 2005, 17, 486–493. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Santos, L.P.; Gonzalez, M.C.; Orlandi, S.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Heymsfield, S.B.; COCONUT Study Group. New Prediction Equations to Estimate Appendicular Skeletal Muscle Mass Using Calf Circumference: Results From NHANES 1999–2006. J. Paren. Enter. Nutr. 2019, 43, 998–1007. [Google Scholar] [CrossRef]
- Coelho-Junior, H.J.; Marzetti, E.; Picca, A.; Tosato, M.; Calvani, R.; Landi, F. Sex- and Age-Specific Normative Values of Lower Extremity Muscle Power in Italian Community-Dwellers. J. Cachexia Sarcopenia Muscle 2024, 15, 45–54. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Álvarez-Bustos, A.; Tosato, M.; Russo, A.; Landi, F.; Picca, A.; Marzetti, E. Physical Performance and Negative Events in Very Old Adults: A Longitudinal Study Examining the IlSIRENTE Cohort. Aging Clin. Exp. Res. 2024, 36, 33. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.M.; Bertschi, D.; Beerli, N.; Berres, M.; Kressig, R.W.; Fischer, A.M. Calf Circumference as a Surrogate Indicator for Detecting Low Muscle Mass in Hospitalized Geriatric Patients. Aging Clin. Exp. Res. 2024, 36, 25. [Google Scholar] [CrossRef] [PubMed]
- Bachettini, N.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Menezes, A.M.B.; Tomasi, E.; Gonzalez, M.C. Sarcopenia as a Mortality Predictor in Community-Dwelling Older Adults: A Comparison of the Diagnostic Criteria of the European Working Group on Sarcopenia in Older People. Eur. J. Clin. Nutr. 2020, 74, 573–580. [Google Scholar] [CrossRef]
- C-Reactive Protein Test—Mayo Clinic. Available online: https://www.mayoclinic.org/tests-procedures/c-reactive-protein-test/about/pac-20385228 (accessed on 17 December 2024).
- Vetrano, D.L.; Palmer, K.; Marengoni, A.; Marzetti, E.; Lattanzio, F.; Roller-Wirnsberger, R.; Samaniego, L.L.; Rodríguez-Mañas, L.; Bernabei, R.; Onder, G. Frailty and Multimorbidity: A Systematic Review and Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 659–666. [Google Scholar] [CrossRef]
- Landi, F.; Tua, E.; Onder, G.; Carrara, B.; Sgadari, A.; Rinaldi, C.; Gambassi, G.; Lattanzio, F.; Bernabei, R. Minimum Data Set for Home Care: A Valid Instrument to Assess Frail Older People Living in the Community. Med. Care 2000, 38, 1184–1190. [Google Scholar] [CrossRef]
- Felder, S.; Braun, N.; Stanga, Z.; Kulkarni, P.; Faessler, L.; Kutz, A.; Steiner, D.; Laukemann, S.; Haubitz, S.; Huber, A.; et al. Unraveling the Link between Malnutrition and Adverse Clinical Outcomes: Association of Acute and Chronic Malnutrition Measures with Blood Biomarkers from Different Pathophysiological States. Ann. Nutr. Metab. 2016, 68, 164–172. [Google Scholar] [CrossRef]
- Benabe, J.E.; Martinez-Maldonado, M. The Impact of Malnutrition on Kidney Function. Min. Electrolyte Metab. 1998, 24, 20–26. [Google Scholar] [CrossRef]
- Hunter, R.W.; Bailey, M.A. Hyperkalemia: Pathophysiology, Risk Factors and Consequences. Nephrol. Dial. Transpl. 2019, 34, III2–III11. [Google Scholar] [CrossRef]
- Turgutalp, K.; Bardak, S.; Helvacı, I.; İşgüzar, G.; Payas, E.; Demir, S.; Kıykım, A. Community-Acquired Hyperkalemia in Elderly Patients: Risk Factors and Clinical Outcomes. Ren. Fail. 2016, 38, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, Y.; Liu, L.; Wang, X.; Wang, D.; Song, Y.; Shen, L.; Liu, Y.; Liu, Y.; Peng, Y.; et al. Dynamic Changes in the Real-Time Glomerular Filtration Rate and Kidney Injury Markers in Different Acute Kidney Injury Models. J. Transl. Med. 2024, 22, 857. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ran, J.; Jiang, T. Urea. Subcell. Biochem. 2014, 73, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Updates in Hyperkalemia: Outcomes and Therapeutic Strategies. Rev. Endocr. Metab. Disord. 2017, 18, 41–47. [Google Scholar] [CrossRef]
- Monti, E.; Sarto, F.; Sartori, R.; Zanchettin, G.; Löfler, S.; Kern, H.; Narici, M.V.; Zampieri, S. C-Terminal Agrin Fragment as a Biomarker of Muscle Wasting and Weakness: A Narrative Review. J. Cachexia Sarcopenia Muscle 2023, 14, 730–744. [Google Scholar] [CrossRef]
- Steubl, D.; Roos, M.; Hettwer, S.; Satanovskij, R.; Tholen, S.; Wen, M.; Schmaderer, C.; Hasenau, A.L.; Luppa, P.; Stecher, L.; et al. Plasma Total C-Terminal Agrin Fragment (TCAF) as a Marker for Kidney Function in Patients with Chronic Kidney Disease. Clin. Chem. Lab. Med. 2016, 54, 1487–1495. [Google Scholar] [CrossRef]
- Drey, M.; Behnes, M.; Kob, R.; Lepiorz, D.; Hettwer, S.; Bollheimer, C.; Sieber, C.C.; Bertsch, T.; Hoffmann, U. C-Terminal Agrin Fragment (CAF) Reflects Renal Function in Patients Suffering from Severe Sepsis or Septic Shock. Clin. Lab. 2015, 61, 69–76. [Google Scholar] [CrossRef]
- Lorenz, G.; Hettwer, S.; McCallum, W.; Angermann, S.; Wen, M.; Schmaderer, C.; Heemann, U.; Roos, M.; Renders, L.; Steubl, D. Plasma C-Terminal Agrin Fragment and Rapid Kidney Function Decline in Chronic Kidney Disease Patients. Medicine 2019, 98, e15597. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Lorenzi, M.; Martone, A.M.; Tosato, M.; Drey, M.; D’Angelo, E.; Capoluongo, E.; Russo, A.; Bernabei, R.; et al. Serum Levels of C-Terminal Agrin Fragment (CAF) Are Associated with Sarcopenia in Older Multimorbid Community-Dwellers: Results from the IlSIRENTE Study. Exp. Gerontol. 2016, 79, 31–36. [Google Scholar] [CrossRef]
- Nishi, H.; Takemura, K.; Higashihara, T.; Inagi, R. Uremic Sarcopenia: Clinical Evidence and Basic Experimental Approach. Nutrients 2020, 12, 1814. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Covinsky, M.H.; Palmer, R.M.; Sehgal, A.R. Serum Albumin Concentration and Clinical Assessments of Nutritional Status in Hospitalized Older People: Different Sides of Different Coins? J. Am. Geriatr. Soc. 2002, 50, 631–637. [Google Scholar] [CrossRef]
- Bianchi, V.E. Role of Nutrition on Anemia in Elderly. Clin. Nutr. ESPEN 2016, 11, e1–e11. [Google Scholar] [CrossRef]
- Brock, F.; Bettinelli, L.A.; Dobner, T.; Stobbe, J.C.; Pomatti, G.; Telles, C.T. Prevalence of Hypoalbuminemia and Nutritional Issues in Hospitalized Elders. Rev. Lat. Am. Enferm. 2016, 24, e2736. [Google Scholar] [CrossRef]
- Frangos, E.; Trombetti, A.; Graf, C.E.; Lachat, V.; Samaras, N.; Vischer, U.M.; Zekry, D.; Rizzoli, R.; Herrmann, F.R. Malnutrition in Very Old Hospitalized Patients: A New Etiologic Factor of Anemia? J. Nutr. Health Aging 2016, 20, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Chaparro, C.M.; Suchdev, P.S. Anemia Epidemiology, Pathophysiology, and Etiology in Low- and Middle-Income Countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, M.P.; Charney, P.; Mueller, C.M. Hepatic Proteins and Nutrition Assessment. J. Am. Diet. Assoc. 2004, 104, 1258–1264. [Google Scholar] [CrossRef] [PubMed]
- Shlisky, J.; Mandlik, R.; Askari, S.; Abrams, S.; Belizan, J.M.; Bourassa, M.W.; Cormick, G.; Driller-Colangelo, A.; Gomes, F.; Khadilkar, A.; et al. Calcium Deficiency Worldwide: Prevalence of Inadequate Intakes and Associated Health Outcomes. Ann. N. Y. Acad. Sci. 2022, 1512, 10–28. [Google Scholar] [CrossRef]
- Nieves, J.W. Calcium, Vitamin D, and Nutrition in Elderly Adults. Clin. Geriatr. Med. 2003, 19, 321–335. [Google Scholar] [CrossRef]
- Taylor, G.O.; Agbedana, E.O.; Johnson, A.O.K. High-Density-Lipoprotein-Cholesterol in Protein-Energy Malnutrition. Br. J. Nutr. 1982, 47, 489–494. [Google Scholar] [CrossRef]
- Rolland, Y.; Lauwers-Cances, V.; Cournot, M.; Nourhashémi, F.; Reynish, W.; Rivière, D.; Vellas, B.; Grandjean, H. Sarcopenia, Calf Circumference, and Physical Function of Elderly Women: A Cross-Sectional Study. J. Am. Geriatr. Soc. 2003, 51, 1120–1124. [Google Scholar] [CrossRef]
- Kawakami, R.; Murakami, H.; Sanada, K.; Tanaka, N.; Sawada, S.S.; Tabata, I.; Higuchi, M.; Miyachi, M. Calf Circumference as a Surrogate Marker of Muscle Mass for Diagnosing Sarcopenia in Japanese Men and Women. Geriatr. Gerontol. Int. 2015, 15, 969–976. [Google Scholar] [CrossRef]
- Kawakami, R.; Miyachi, M.; Sawada, S.S.; Torii, S.; Midorikawa, T.; Tanisawa, K.; Ito, T.; Usui, C.; Ishii, K.; Suzuki, K.; et al. Cut-Offs for Calf Circumference as a Screening Tool for Low Muscle Mass: WASEDA’S Health Study. Geriatr. Gerontol. Int. 2020, 20, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Beghé, C.; Wilson, A.; Ershler, W.B. Prevalence and Outcomes of Anemia in Geriatrics: A Systematic Review of the Literature. Am. J. Med. 2004, 116, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Landi, F.; Coelho-Júnior, H.J.; Picca, A. Mitochondrial Quality Control Processes at the Crossroads of Cell Death and Survival: Mechanisms and Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 7305. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Coelho-Junior, H.J.; Picca, A. Mitochondrial Pathways and Sarcopenia in the Geroscience Era. J. Nutr. Health Aging 2024, 28, 100397. [Google Scholar] [CrossRef]
- Ferrucci, L.; Guerra, F.; Bucci, C.; Marzetti, E.; Picca, A. Mitochondria Break Free: Mitochondria-Derived Vesicles in Aging and Associated Conditions. Ageing Res. Rev. 2024, 102, 102549. [Google Scholar] [CrossRef]
| Variable | Non-Malnourished (n = 151) | Malnourished (n = 45) | p-Value |
|---|---|---|---|
| Age (years) | 84.9 ± 4.5 | 87.5 ± 4.9 | 0.001 |
| Body Mass Index (kg/m2) | 26.0 ± 4.5 | 23.8 ± 4.7 | 0.006 |
| Men (%) | 37.7 | 11.1 | 0.001 |
| 4 m walking test (m/s) | 0.54 ± 0.29 | 0.25 ± 0.23 | 0.001 |
| Comorbidities (%) | 73.2 | 26.8 | 0.340 |
| CAF (ng/mL) | 76.65 ± 39.40 | 91.27 ± 44.46 | 0.035 |
| Glucose (mg/dL) | 116.6 ± 40.9 | 120.8 ± 43.4 | 0.550 |
| Urea (mg/dL) | 46.7 ± 18.6 | 50.6 ± 24.3 | 0.256 |
| Cholesterol (mg/dL) | 197.6 ± 43.6 | 180.2 ± 40.4 | 0.018 |
| Triglycerides (mg/dL) | 149.1 ± 65.5 | 138.5 ± 65.4 | 0.344 |
| HDL (mg/dL) | 47.3 ± 14.2 | 43.7 ± 17.5 | 0.166 |
| LDL (mg/dL) | 132.7 ± 38.8 | 116.8 ± 33.6 | 0.014 |
| Amylase (U/L) | 74.9 ± 35.7 | 69.5 ± 39.9 | 0.388 |
| Creatine kinase (U/L) | 83.2 ± 49.9 | 69.7 ± 60.6 | 0.131 |
| Lactate dehydrogenase (U/L) | 318.5 ± 103.6 | 318.4 ± 98.9 | 0.999 |
| Total Proteins (g/dL) | 7.05 ± 0.52 | 6.83 ± 0.56 | 0.016 |
| Albumin (g/dL) | 4.23 ± 0.31 | 4.10 ± 0.33 | 0.016 |
| Calcium (mg/dL) | 8.99 ± 0.51 | 8.68 ± 0.65 | 0.001 |
| Phosphorus (mg/dL) | 3.32 ± 0.57 | 3.44 ± 0.80 | 0.252 |
| Iron (µg/dL) | 74.9 ± 34.0 | 50.2 ± 30.4 | 0.001 |
| Sodium (mEq/L) | 138.8 ± 4.7 | 139.2 ± 9.2 | 0.695 |
| Potassium (mEq/L) | 4.38 ± 0.48 | 4.32 ± 0.47 | 0.445 |
| Magnesium (mEq/L) | 1.91 ± 0.18 | 1.92 ± 0.22 | 0.870 |
| Hemoglobin (g/dL) | 13.64 ± 1.43 | 12.38 ± 1.49 | 0.001 |
| Hematocrit (%) | 40.66 ± 4.46 | 37.94 ± 5.37 | 0.001 |
| Platelets (×103/µL) | 241,088 ± 131,361 | 256,294 ± 90,205 | 0.468 |
| White blood cells (×103/µL) | 6923 ± 5265 | 6631 ± 1973 | 0.716 |
| IGF-1 (ng/mL) | 0.85 ± 0.73 | 0.88 ± 0.74 | 0.781 |
| IGFBP-3 (ng/mL) | 4506.8 ± 1417.6 | 4060.3 ± 1238.9 | 0.058 |
| IL-6 (pg/mL) | 2.38 ± 2.20 | 4.42 ± 2.95 | 0.001 |
| TNF (pg/mL) | 1.90 ± 2.58 | 2.56 ± 2.64 | 0.137 |
| Component | Initial Eigenvalues | ||
|---|---|---|---|
| Total | % of Variance | Cumulative % | |
| 1 | 4.202 | 14.488 | 14.488 |
| 2 | 3.333 | 11.493 | 25.981 |
| 3 | 2.342 | 8.076 | 34.057 |
| 4 | 1.934 | 6.668 | 40.725 |
| 5 | 1.635 | 5.637 | 46.362 |
| 6 | 1.367 | 4.715 | 51.077 |
| 7 | 1.325 | 4.568 | 55.646 |
| 8 | 1.223 | 4.217 | 59.862 |
| 9 | 1.188 | 4.095 | 63.957 |
| 10 | 1.054 | 3.634 | 67.591 |
| 11 | 1.040 | 3.588 | 71.179 |
| 12 | 0.939 | 3.238 | 74.417 |
| 13 | 0.920 | 3.173 | 77.591 |
| 14 | 0.817 | 2.819 | 80.409 |
| 15 | 0.757 | 2.609 | 83.018 |
| 16 | 0.717 | 2.473 | 85.492 |
| 17 | 0.648 | 2.236 | 87.727 |
| 18 | 0.621 | 2.140 | 89.867 |
| 19 | 0.495 | 1.708 | 91.575 |
| 20 | 0.474 | 1.633 | 93.209 |
| 21 | 0.430 | 1.483 | 94.692 |
| 22 | 0.373 | 1.285 | 95.976 |
| 23 | 0.345 | 1.190 | 97.167 |
| 24 | 0.280 | 0.965 | 98.132 |
| 25 | 0.242 | 0.835 | 98.967 |
| 26 | 0.155 | 0.534 | 99.501 |
| 27 | 0.087 | 0.301 | 99.802 |
| 28 | 0.058 | 0.198 | 100.000 |
| 29 | 0.000 | 0.000 | 100.000 |
| OR | 95% C.I. for EXP(B) | p-Value | Exp(B) | 95% C.I. for EXP(B) | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| PC1 | 0.565 | 0.389 | 0.821 | 0.003 | 0.764 | 0.491 | 1.190 | 0.234 |
| PC2 | 1.649 | 1.178 | 2.308 | 0.004 | 1.647 | 1.024 | 2.649 | 0.039 |
| PC3 | 0.747 | 0.521 | 1.071 | 0.113 | 0.567 | 0.349 | 0.921 | 0.022 |
| PC4 | 0.483 | 0.335 | 0.697 | 0.000 | 0.607 | 0.381 | 0.966 | 0.035 |
| PC5 | 0.614 | 0.426 | 0.884 | 0.009 | 0.812 | 0.550 | 1.201 | 0.298 |
| PC6 | 1.041 | 0.749 | 1.447 | 0.810 | 0.970 | 0.630 | 1.492 | 0.889 |
| PC2 | PC3 | PC4 | |
|---|---|---|---|
| Calcium | 0.291 | −0.157 | −0.223 |
| Cholesterol | −0.252 | 0.587 | 0.005 |
| Albumin | 0.369 | −0.262 | −0.436 |
| Proteins | 0.369 | −0.262 | −0.436 |
| Interleukin-6 | 0.263 | 0.059 | −0.018 |
| Low-Density Lipoprotein | −0.105 | 0.408 | −0.087 |
| Insulin-Like Growth Factor Binding Protein 3 | 0.109 | 0.223 | 0.309 |
| Osmolality | 0.825 | −0.147 | 0.022 |
| Urea | 0.820 | 0.023 | 0.301 |
| C-terminal agrin fragment | 0.675 | 0.052 | 0.362 |
| Potassium | 0.494 | −0.044 | 0.074 |
| Hemoglobin | −0.374 | −0.515 | 0.336 |
| Hematocrit | −0.314 | −0.514 | 0.349 |
| Red Blood Cells Count | −0.144 | −0.416 | 0.591 |
| Lactate Dehydrogenase | 0.133 | 0.444 | 0.154 |
| Iron | −0.350 | −0.134 | −0.042 |
| Creatine Kinase | 0.105 | 0.319 | 0.034 |
| Glucose | 0.101 | −0.313 | 0.181 |
| Triglycerides | 0.020 | 0.167 | 0.280 |
| Platelets | 0.042 | 0.036 | 0.054 |
| High-Density Lipoprotein | −0.206 | 0.204 | −0.289 |
| Magnesium | −0.057 | 0.323 | 0.243 |
| Sodium | 0.319 | −0.178 | −0.233 |
| Insulin-Like Growth Factor 1 | 0.006 | 0.319 | 0.273 |
| Amylase | 0.201 | −0.019 | 0.094 |
| Mean Corpuscular Volume | −0.103 | 0.028 | −0.356 |
| White Blood Cells Count | −0.012 | 0.041 | 0.129 |
| Phosphorus | 0.315 | 0.359 | 0.145 |
| Tumor Necrosis Factor | 0.216 | −0.181 | −0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho-Júnior, H.J.; Calvani, R.; Picca, A.; Tosato, M.; Russo, A.; Landi, F.; Marzetti, E. Biomarkers Linked to Malnutrition Identified According to GLIM Criteria Among Older Community-Dwelling Adults: Results from the ilSIRENTE Study. Nutrients 2025, 17, 3543. https://doi.org/10.3390/nu17223543
Coelho-Júnior HJ, Calvani R, Picca A, Tosato M, Russo A, Landi F, Marzetti E. Biomarkers Linked to Malnutrition Identified According to GLIM Criteria Among Older Community-Dwelling Adults: Results from the ilSIRENTE Study. Nutrients. 2025; 17(22):3543. https://doi.org/10.3390/nu17223543
Chicago/Turabian StyleCoelho-Júnior, Hélio José, Riccardo Calvani, Anna Picca, Matteo Tosato, Andrea Russo, Francesco Landi, and Emanuele Marzetti. 2025. "Biomarkers Linked to Malnutrition Identified According to GLIM Criteria Among Older Community-Dwelling Adults: Results from the ilSIRENTE Study" Nutrients 17, no. 22: 3543. https://doi.org/10.3390/nu17223543
APA StyleCoelho-Júnior, H. J., Calvani, R., Picca, A., Tosato, M., Russo, A., Landi, F., & Marzetti, E. (2025). Biomarkers Linked to Malnutrition Identified According to GLIM Criteria Among Older Community-Dwelling Adults: Results from the ilSIRENTE Study. Nutrients, 17(22), 3543. https://doi.org/10.3390/nu17223543










