Ultra-Processed Food Intake in Children with Inflammatory Bowel Disease: A Pilot Case–Control Study
Highlights
- Children and adolescents with inflammatory bowel disease (IBD), particularly those with ulcerative colitis (UC), had a significantly higher intake of ultra-processed foods (UPFs) than healthy controls.
- In patients with Crohn’s disease, the observed difference in UPF intake was largely influenced by the inclusion of oral nutritional supplements (ONS).
- No association was found between UPF intake and disease activity, possibly due to the small number of patients with active disease.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Assessment of Energy and UPF Intake
2.4. Statistical Methods
2.5. Artificial Intelligence
3. Results
3.1. Study Population
3.2. Energy and UPF Intake
3.3. Disease Activity and UPF Intake
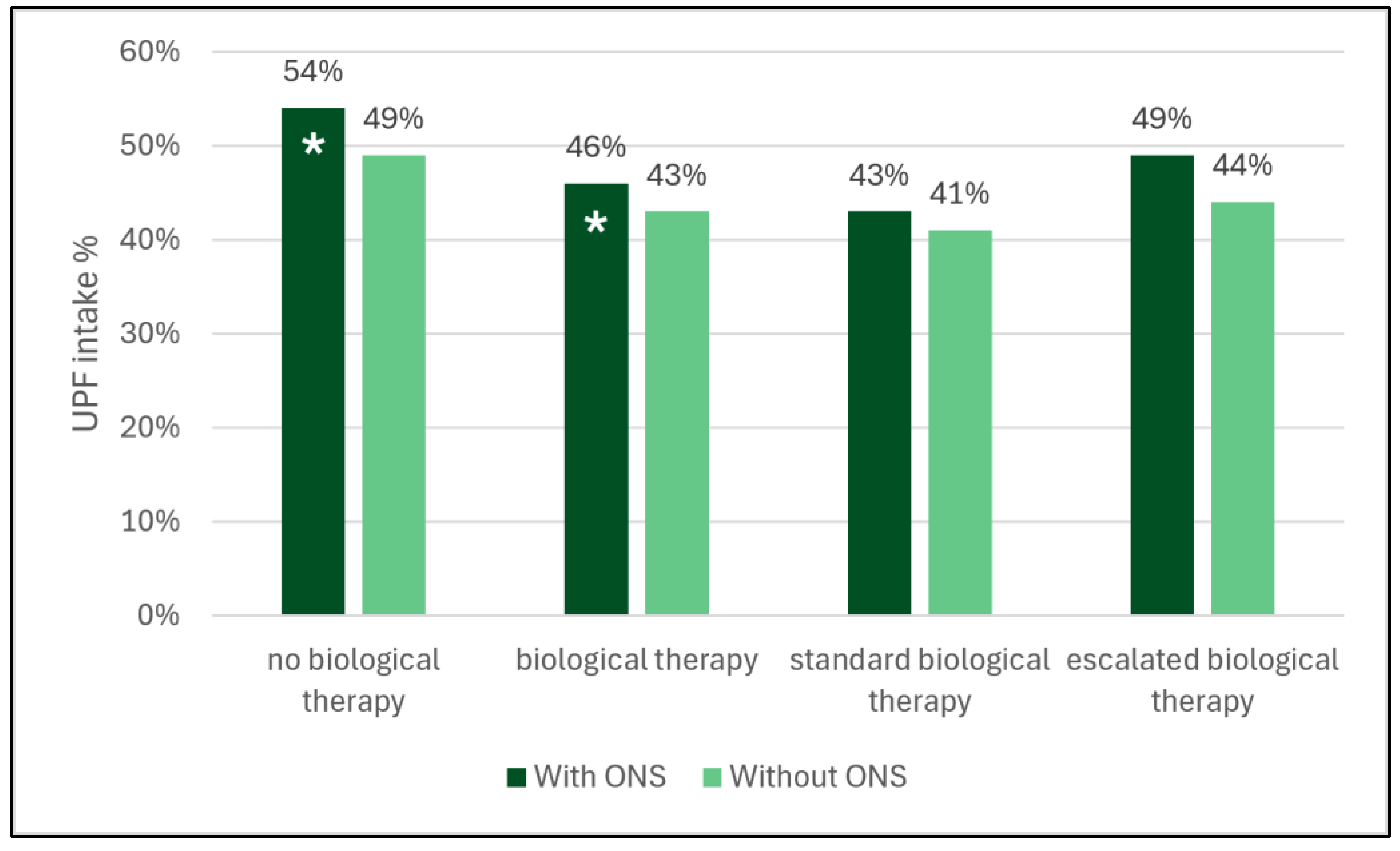
3.4. Need for Biologicals and UPF Intake
3.5. Energy and UPF Intake in IBD Patients Based on BMI Z-Score
3.6. Association of UPF Intake and Age, Sex, and Energy Intake
4. Discussion
4.1. Strengths and Limitations
4.2. Clinical and Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CD | Crohn’s disease |
| CDED | Crohn’s Disease Exclusion Diet |
| HC | healthy control |
| IBD | inflammatory bowel disease |
| MD | mean difference |
| ONS | oral nutritional support |
| PCDAI | pediatric Crohn’s disease activity index |
| PUCAI | pediatric ulcerative colitis activity index |
| SD | standard deviation |
| TNF | tumor necrosis factor |
| UPF | ultra-processed food |
| UC | ulcerative colitis |
References
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Chang, N.H.; Mohammad, D.; Wong, E.C.L.; Ananthakrishnan, A.N.; Chan, S.S.M.; Carbonnel, F.; Meyer, A. Food Processing and Risk of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2483–2495 e2481. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef]
- Borowitz, S.M. The epidemiology of inflammatory bowel disease: Clues to pathogenesis? Front. Pediatr. 2022, 10, 1103713. [Google Scholar] [CrossRef]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Babaei, A.; Pourmotabbed, A.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghoreishy, S.M.; Amirian, P.; Zarpoosh, M.; Mohammadi, H.; Kermani, M.A.H.; et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: A systematic review and dose-response meta-analysis of 4 035 694 participants. Nutr. Rev. 2024, 82, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Lopez, R.N. Exclusive enteral nutrition in children with Crohn’s disease. World J. Gastroenterol. 2015, 21, 6809–6816. [Google Scholar] [CrossRef]
- Lamers, C.R.; de Roos, N.M.; Witteman, B.J.M. The association between inflammatory potential of diet and disease activity: Results from a cross-sectional study in patients with inflammatory bowel disease. BMC Gastroenterol. 2020, 20, 316. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef]
- Hyams, J.S.; Ferry, G.D.; Mandel, F.S.; Gryboski, J.D.; Kibort, P.M.; Kirschner, B.S.; Griffiths, A.M.; Katz, A.J.; Grand, R.J.; Boyle, J.T.; et al. Development and validation of a pediatric Crohn’s disease activity index. J. Pediatr. Gastroenterol. Nutr. 1991, 12, 439–447. [Google Scholar] [CrossRef]
- Turner, D.; Otley, A.R.; Mack, D.; Hyams, J.; de Bruijne, J.; Uusoue, K.; Walters, T.D.; Zachos, M.; Mamula, P.; Beaton, D.E.; et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology 2007, 133, 423–432. [Google Scholar] [CrossRef]
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://www.microsoft.com/excel (accessed on 30 November 2023).
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-based Consensus Guideline From the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2021, 15, jjaa161. [Google Scholar] [CrossRef]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Corp I: IBM SPSS Statistics, 25.0 edition; 2017. Available online: https://www.ibm.com/spss (accessed on 1 May 2025).
- Lauria, F.; Dello Russo, M.; Formisano, A.; De Henauw, S.; Hebestreit, A.; Hunsberger, M.; Krogh, V.; Intemann, T.; Lissner, L.; Molnar, D.; et al. Ultra-processed foods consumption and diet quality of European children, adolescents and adults: Results from the I.Family study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 3031–3043. [Google Scholar] [CrossRef] [PubMed]
- Petridi, E.; Magriplis, E.; Karatzi, K.; Charidemou, E.; Philippou, E.; Zampelas, A. Ultra Processed Food Consumption in Children and Adolescents: Main Food Group Contributors and Associations with Weight Status. Nutr. Bull. 2025, 50, 278–289. [Google Scholar] [CrossRef]
- Mete, B.; Sadikoglu, H.M.; Demirhindi, H.; Melekoglu, E.; Barutcu, A.; Makca, T.; Atun Utuk, F. The association between ultra-processed food consumption and low-grade inflammation in childhood: A cross-sectional study. Nutr. Bull. 2024, 49, 538–549. [Google Scholar] [CrossRef]
- Buytaert, M.; Declercq, D.; Depoorter, F.; Cosijn, Z.; Devisscher, L.; Raevens, S.; Verhelst, X.; Van Vlierberghe, H.; Geerts, A.; De Bruyne, R.; et al. The association of ultra-processed food intake with adolescent metabolic dysfunction-associated steatotic liver disease in the NHANES. Pediatr. Obes. 2025, 20, e13174. [Google Scholar] [CrossRef]
- Polsky, J.Y.; Moubarac, J.C.; Garriguet, D. Consumption of ultra-processed foods in Canada. Health Rep. 2020, 31, 3–15. [Google Scholar]
- Gold, S.L.; Kohler, D.; Freid, H.; Haskey, N.; Raman, M. Food Insecurity Is Common in Patients with Inflammatory Bowel Disease and Is Associated with Increased Ultra-Processed Food Intake. Nutrients 2024, 16, 3736. [Google Scholar] [CrossRef]
- Sarbagili-Shabat, C.; Zelber-Sagi, S.; Isakov, N.F.; Hirsch, A.; Ron, Y.; Grinshpan, L.S.; Anbar, R.; Bromberg, A.; Thurm, T.; Maharshak, N. Ultra-Processed Foods Consumption Is Positively Associated with the Clinical Activity of Inflammatory Bowel Diseases: A Cross-Sectional Single-Center Study. Inflamm. Intest. Dis. 2024, 9, 241–251. [Google Scholar] [CrossRef]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; Yuan, S.; Wang, X.; Theodoratou, E.; Li, X.; et al. Intake of Ultra-processed Foods Is Associated with an Increased Risk of Crohn’s Disease: A Cross-sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J. Crohns Colitis 2023, 17, 535–552. [Google Scholar] [CrossRef] [PubMed]
- Severo, J.S.; da Silva Barros, V.J.; Moraes Mendes, P.H.; Dos Santos, B.L.B.; da Silva, A.C.A.; de Oliveira, K.B.V.; de Moura, M.S.B.; de Almeida Fonseca Viola, P.C.; do Nascimento Nogueira, N.; Luz Parente, J.M.; et al. Phase angle values and ultra-processed food consumption are associated with changes in oxidative stress in inflammatory bowel disease patients. Clin. Nutr. ESPEN 2023, 57, 10–20. [Google Scholar] [CrossRef]
- Vagianos, K.; Dolovich, C.; Witges, K.; Graff, L.A.; Bernstein, C.N. Ultra-Processed Food, Disease Activity, and Inflammation in Ulcerative Colitis: The Manitoba Living with IBD Study. Am. J. Gastroenterol. 2024, 119, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Godny, L.; Vanderstappen, J.; Sarbagili-Shabat, C.; Svolos, V. Role of diet in prevention versus treatment of Crohn’s disease and ulcerative colitis. Frontline Gastroenterol. 2024, 15, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Vissers, E.; Wellens, J.; Sabino, J. Ultra-processed foods as a possible culprit for the rising prevalence of inflammatory bowel diseases. Front. Med. 2022, 9, 1058373. [Google Scholar] [CrossRef]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.; Taylor, L.M.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; Hotte, N.; Shommu, N.; Kaur, S.; Reimer, R.A.; Madsen, K.L.; et al. Dietary patterns, food groups and nutrients in Crohn’s disease: Associations with gut and systemic inflammation. Sci. Rep. 2021, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Limketkai, B.N.; Hamideh, M.; Shah, R.; Sauk, J.S.; Jaffe, N. Dietary Patterns and Their Association with Symptoms Activity in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2022, 28, 1627–1636. [Google Scholar] [CrossRef] [PubMed]

| IBD (n = 47) | CD (n = 24) | UC (n = 23) | HC (n = 47) | |
|---|---|---|---|---|
| Age, mean (SD) | 15.71 (1.91) | 16.11 (1.81) | 15.33 (1.96) | 15.69 (1.97) |
| Female, n (%) | 20 (42.55) | 9 (37.50) | 11 (47.83) | 20 (42.55) |
| Age at diagnosis, mean (SD) | 12.80 (2.39) | 13.39 (1.69) | 12.92 (2.69) | - |
| Disease duration, mean (SD) | 2.50 (1.94) | 2.50 (1.97) | 2.43 (1.91) | - |
| BMI z-score, mean (SD) | 0.12 (1.75) | 0.07 (1.96) | 0.17 (1.54) | −0.05 (0.82) |
| PCDAI/PUCAI, mean (SD) | - | 3.31 (5.48) | 4.78 (9.35) | - |
| Disease location * for CD or UC (n) | ||||
| L1/E1 | - | 10 | 5 | - |
| L2/E2 | - | 5 | 5 | - |
| L3/E3 | - | 11 | 4 | - |
| L4/E4 | - | 14 | 7 | - |
| Disease activity | ||||
| remission (n) | 40 | 22 | 18 | - |
| mild (n) | 5 | 2 | 3 | - |
| moderate (n) | 1 | 0 | 1 | - |
| severe (n) | 0 | 0 | 0 | - |
| 5-ASA (n) | 22 | 4 | 18 | - |
| Azathioprine | 24 | 12 | 12 | - |
| Biologics | ||||
| infliximab (n) | 7 | 3 | 4 | - |
| vedolizumab (n) | 2 | 0 | 2 | - |
| ustekinumab (n) | 1 | 1 | 0 | - |
| adalimumab (n) | 13 | 13 | 0 | - |
| Underweight (n = 12) | Normal Weight (n = 24) | Overweight (n = 11) | p Value | |
|---|---|---|---|---|
| energy intake, mean (SD), kcal | 2524 (454) | 3073 (1236) | 2354 (600) | 0.086 |
| UPF, mean (SD), % | 48 (15) | 50 (15) | 53 (13) | 0.762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasznár, E.; Bajzát, D.; Karoliny, A.; Szentannay, J.; Szabó, A.; Gombos, E.; Regián, V.; Havasi, A.; Pálfi, E.; Müller, K.E. Ultra-Processed Food Intake in Children with Inflammatory Bowel Disease: A Pilot Case–Control Study. Nutrients 2025, 17, 3532. https://doi.org/10.3390/nu17223532
Kasznár E, Bajzát D, Karoliny A, Szentannay J, Szabó A, Gombos E, Regián V, Havasi A, Pálfi E, Müller KE. Ultra-Processed Food Intake in Children with Inflammatory Bowel Disease: A Pilot Case–Control Study. Nutrients. 2025; 17(22):3532. https://doi.org/10.3390/nu17223532
Chicago/Turabian StyleKasznár, Emese, Dorina Bajzát, Anna Karoliny, Judit Szentannay, András Szabó, Eszter Gombos, Vivien Regián, Anikó Havasi, Erzsébet Pálfi, and Katalin Eszter Müller. 2025. "Ultra-Processed Food Intake in Children with Inflammatory Bowel Disease: A Pilot Case–Control Study" Nutrients 17, no. 22: 3532. https://doi.org/10.3390/nu17223532
APA StyleKasznár, E., Bajzát, D., Karoliny, A., Szentannay, J., Szabó, A., Gombos, E., Regián, V., Havasi, A., Pálfi, E., & Müller, K. E. (2025). Ultra-Processed Food Intake in Children with Inflammatory Bowel Disease: A Pilot Case–Control Study. Nutrients, 17(22), 3532. https://doi.org/10.3390/nu17223532







