High-Quality Nutritional and Medical Care in Celiac Disease Follow-Up
Abstract
1. Introduction
2. Methods
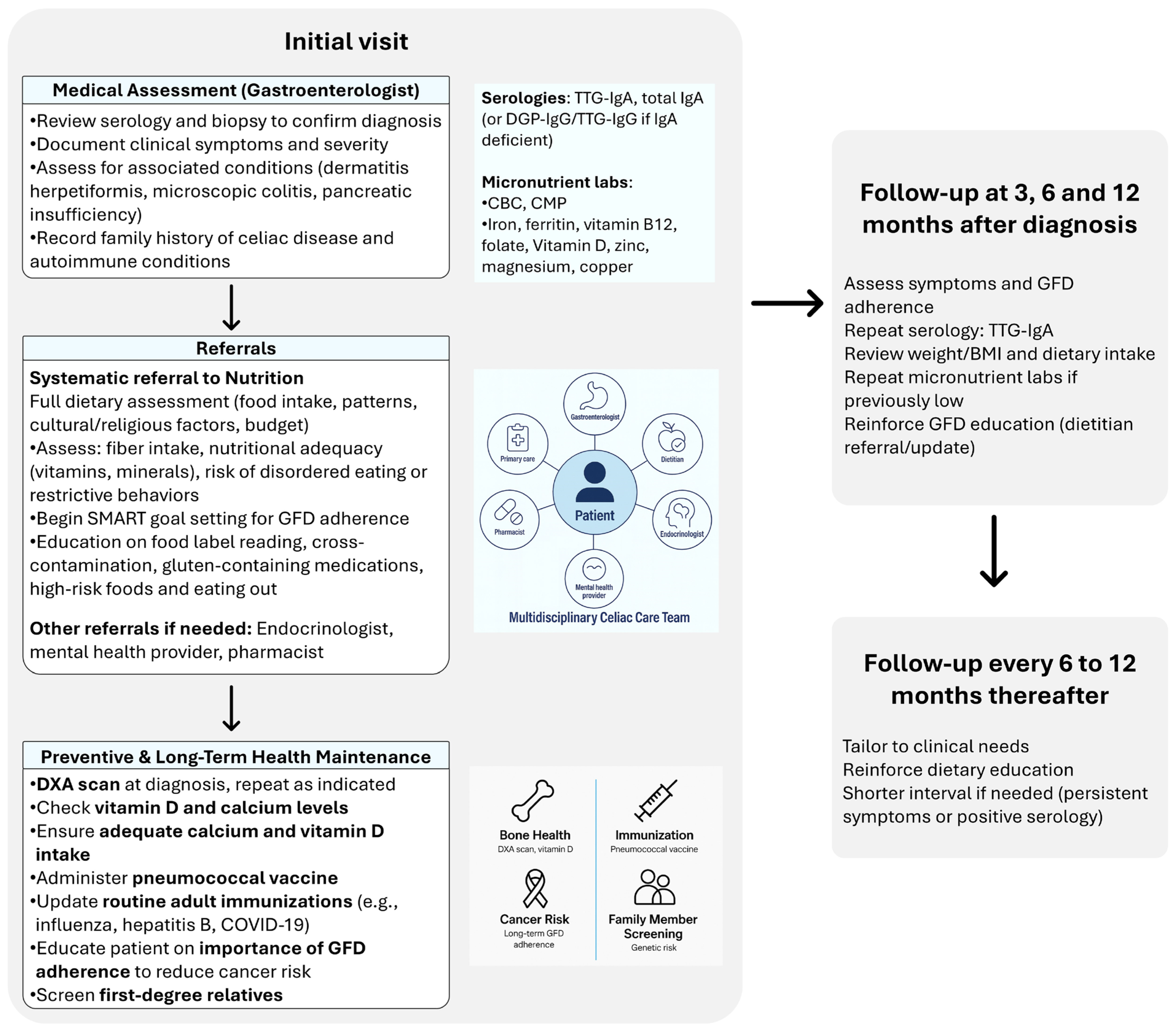
3. Multidisciplinary Approach to Celiac Disease Follow-Up
4. Nutritional Management and Challenges in Celiac Disease
5. Medical Management
5.1. Clinical and Serologic Monitoring
5.2. Preventive and Long-Term Health Maintenance
6. Future Directions
6.1. Nondietary and Adjunctive Pharmacologic Therapies
6.2. Digital Health, Remote Monitoring, and AI
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Choung, R.S.; Unalp-Arida, A.; Ruhl, C.E.; Brantner, T.L.; Everhart, J.E.; Murray, J.A. Less Hidden Celiac Disease But Increased Gluten Avoidance Without a Diagnosis in the United States: Findings from the National Health and Nutrition Examination Surveys from 2009 to 2014. Mayo Clin. Proc. 2016, 92, 30–38. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef]
- Elli, L.; Leffler, D.; Cellier, C.; Lebwohl, B.; Ciacci, C.; Schumann, M.; Lundin, K.E.A.; Chetcuti Zammit, S.; Sidhu, R.; Roncoroni, L.; et al. Guidelines for best practices in monitoring established coeliac disease in adult patients. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 198–215. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.; Rubio-Tapia, A. Quality Care in Celiac Disease. In Quality in Gastroenterology: A Concise Guide to Establishing High Value Clinical Care; Feuerstein, J.D., Stein, D.J., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 115–132. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Blom, J.J.; Gibson, P.R.; Armstrong, D. Nutrition Assessment and Management in Celiac Disease. Gastroenterology 2024, 167, 116–131.e1. [Google Scholar] [CrossRef]
- Spahn, J.M.; Reeves, R.S.; Keim, K.S.; Laquatra, I.; Kellogg, M.; Jortberg, B.; Clark, N.A. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J. Am. Diet. Assoc. 2010, 110, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Barkmeijer, A.; Molder, H.T.; Janssen, M.; Jager-Wittenaar, H. Towards effective dietary counseling: A scoping review. Patient Educ. Couns. 2022, 105, 1801–1817. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.; Payne, K.; Jansson-Knodell, C.; Cavagnaro, S.; Weekley, K.; Gardinier, D.; Rubio-Tapia, A. A Dedicated Celiac Disease Program Improves Celiac Quality Care Metrics and Short-Term Outcomes in Real Life. Dig. Dis. Sci. 2024, 69, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Estévez, V.; Rodríguez, J.M.; Schlack, P.; Navarrete, P.; Bascuñán, K.A.; Núñez, V.; Oyarce, C.; Flores, C.; Ayala, J.; Araya, M. Persistent Barriers of the Gluten-Free Basic Food Basket: Availability, Cost, and Nutritional Composition Assessment. Nutrients 2024, 16, 885. [Google Scholar] [CrossRef]
- Lee, A.R.; Wolf, R.L.; Lebwohl, B.; Ciaccio, E.J.; Green, P.H.R. Persistent Economic Burden of the Gluten Free Diet. Nutrients 2019, 11, 399. [Google Scholar] [CrossRef]
- Fry, L.; Madden, A.M.; Fallaize, R. An investigation into the nutritional composition and cost of gluten-free versus regular food products in the UK. J. Hum. Nutr. Diet. 2018, 31, 108–120. [Google Scholar] [CrossRef]
- Burden, M.; Mooney, P.D.; Blanshard, R.J.; White, W.L.; Cambray-Deakin, D.R.; Sanders, D.S. Cost and availability of gluten-free food in the UK: In store and online. Postgrad. Med. J. 2015, 91, 622–626. [Google Scholar] [CrossRef]
- Panagiotou, S.; Kontogianni, M.D. The economic burden of gluten-free products and gluten-free diet: A cost estimation analysis in Greece. J. Hum. Nutr. Diet. 2017, 30, 746–752. [Google Scholar] [CrossRef]
- Chrysostomou, S.; Andreou, S.N.; Andreou, C. The development of the gluten free healthy food basket in Cyprus. Is it affordable among low-income adults diagnosed with celiac disease? J. Public Health 2020, 42, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Kılınç, G.E. Gluten-free foods: Healthy choice or expensive alternative? What evidence is there in Turkey? J. Sci. Food Agric. 2025, 105, 8798–8805. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.; Ng, D.L.; Diamond, B.; Ciaccio, E.J.; Green, P.H. Living with coeliac disease: Survey results from the USA. J. Hum. Nutr. Diet. 2012, 25, 233–238. [Google Scholar] [CrossRef]
- Shah, S.; Akbari, M.; Vanga, R.; Kelly, C.P.; Hansen, J.; Theethira, T.; Tariq, S.; Dennis, M.; Leffler, D.A. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am. J. Gastroenterol. 2014, 109, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Posterick, A.; Ayars, C.L. Celiac Disease Dietary Adherence on the Rural-Urban Continuum. Nutrients 2023, 15, 4535. [Google Scholar] [CrossRef]
- Ribeiro, C.D.S.; Pratesi, C.B.; Zandonadi, R.P. Celiac Disease and Gluten-Free Diets: A Path or Barrier to Food (In)Security? Nutrients 2025, 17, 1956. [Google Scholar] [CrossRef]
- McDermid, J.M.; Almond, M.A.; Roberts, K.M.; Germer, E.M.; Geller, M.G.; Taylor, T.A.; Sinley, R.C.; Handu, D. Celiac Disease: An Academy of Nutrition and Dietetics Evidence-Based Nutrition Practice Guideline. J. Acad. Nutr. Diet. 2023, 123, 1793–1807.e4. [Google Scholar] [CrossRef]
- Allen, B.; Orfila, C. The Availability and Nutritional Adequacy of Gluten-Free Bread and Pasta. Nutrients 2018, 10, 1370. [Google Scholar] [CrossRef]
- Maleki, F.; Hosseinpour, M.; Delpisheh, A.; Bahardoust, M.; Hajizadeh-Sharafabad, F.; Pashaei, M.R. The prevalence of obesity and underweight in celiac patients at the time of diagnosis: A systematic review and meta-analysis. BMC Gastroenterol. 2024, 24, 357. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; Iannone, A.; Cristofori, F.; Dargenio, V.N.; Indrio, F.; Verduci, E.; Di Leo, A.; Francavilla, R. Risk of obesity during a gluten-free diet in pediatric and adult patients with celiac disease: A systematic review with meta-analysis. Nutr. Rev. 2023, 81, 252–266. [Google Scholar] [CrossRef]
- Lebwohl, B.; Cao, Y.; Zong, G.; Hu, F.B.; Green, P.H.R.; Neugut, A.I.; Rimm, E.B.; Sampson, L.; Dougherty, L.W.; Giovannucci, E.; et al. Long term gluten consumption in adults without celiac disease and risk of coronary heart disease: Prospective cohort study. BMJ 2017, 357, j1892. [Google Scholar] [CrossRef]
- Ciccone, A.; Gabrieli, D.; Cardinale, R.; Di Ruscio, M.; Vernia, F.; Stefanelli, G.; Necozione, S.; Melideo, D.; Viscido, A.; Frieri, G.; et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion 2019, 100, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Rispo, A.; Imperatore, N.; Guarino, M.; Tortora, R.; Alisi, A.; Cossiga, V.; Testa, A.; Ricciolino, S.; Fiorentino, A.; Morisco, F. Metabolic-associated fatty liver disease (MAFLD) in coeliac disease. Liver Int. 2021, 41, 788–798. [Google Scholar] [CrossRef]
- Aggarwal, N.; Agarwal, A.; Alarouri, H.; Dwarakanathan, V.; Dang, S.; Ahuja, V.; Makharia, G.K. Patients with Celiac Disease Have High Prevalence of Fatty Liver and Metabolic Syndrome. Dig. Dis. Sci. 2024, 69, 3029–3042. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536.e2. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Bianchi, P.I.; Marchese, A.; Trotta, L.; Vattiato, C.; Balduzzi, D.; Brusco, G.; Andrealli, A.; Cisarò, F.; Astegiano, M.; et al. A score that verifies adherence to a gluten-free diet: A cross-sectional, multicentre validation in real clinical life. Br. J. Nutr. 2012, 108, 1884–1888. [Google Scholar] [CrossRef]
- Bledsoe, A.C.; King, K.S.; Larson, J.J.; Snyder, M.; Absah, I.; Choung, R.S.; Murray, J.A. Micronutrient Deficiencies Are Common in Contemporary Celiac Disease Despite Lack of Overt Malabsorption Symptoms. Mayo Clin. Proc. 2019, 94, 1253–1260. [Google Scholar] [CrossRef]
- Lee, A.R.; Lebwohl, B.; Lebovits, J.; Wolf, R.L.; Ciaccio, E.J.; Green, P.H.R. Factors Associated with Maladaptive Eating Behaviors, Social Anxiety, and Quality of Life in Adults with Celiac Disease. Nutrients 2021, 13, 4494. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Eating Disorders; American Psychiatric Publishing: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Mårild, K.; Størdal, K.; Bulik, C.M.; Rewers, M.; Ekbom, A.; Liu, E.; Ludvigsson, J.F. Celiac Disease and Anorexia Nervosa: A Nationwide Study. Pediatrics 2017, 139, e20164367. [Google Scholar] [CrossRef]
- Nikniaz, Z.; Beheshti, S.; Abbasalizad Farhangi, M.; Nikniaz, L. A systematic review and meta-analysis of the prevalence and odds of eating disorders in patients with celiac disease and vice-versa. Int. J. Eat. Disord. 2021, 54, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Jansson-Knodell, C.; Simons, M.L.; Weekley, K.; Gardinier, D.; Rubio-Tapia, A. Avoidant/Restrictive Food Intake Disorder in Celiac Disease. Nutrients 2025, 17, 3197. [Google Scholar] [CrossRef]
- Lebovits, J.; Lee, A.R.; Ciaccio, E.J.; Wolf, R.L.; Davies, R.H.; Cerino, C.; Lebwohl, B.; Green, P.H.R. Impact of Celiac Disease on Dating. Dig. Dis. Sci. 2022, 67, 5158–5167. [Google Scholar] [CrossRef]
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef]
- Ruiz-Carnicer, Á.; Coronel-Rodríguez, C.; Guisado-Rasco, M.C.; Comino, I.; Sousa, C.; Segura, V. Uncovering Hidden Gluten Exposure in Celiac Patients: A Case Study in Family-Based Management and the Role of Point-of-Care Urine Testing and Psychological Assessment. Int. J. Mol. Sci. 2025, 26, 5135. [Google Scholar] [CrossRef]
- Russell, A.K.; Lucas, E.C.; Henneken, L.M.; Pizzey, C.J.; Clarke, D.; Myleus, A.; Tye-Din, J.A. Stool Gluten Peptide Detection Is Superior to Urinary Analysis, Coeliac Serology, Dietary Adherence Scores and Symptoms in the Detection of Intermittent Gluten Exposure in Coeliac Disease: A Randomised, Placebo-Controlled, Low-Dose Gluten Challenge Study. Nutrients 2024, 16, 279. [Google Scholar] [CrossRef]
- Monachesi, C.; Catassi, G.; Catassi, C. The use of urine peptidomics to define dietary gluten peptides from patients with celiac disease and the clinical relevance. Expert. Rev. Proteom. 2023, 20, 281–290. [Google Scholar] [CrossRef]
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Małgorzewicz, S. Celiac Dietary Adherence Test and Standardized Dietician Evaluation in Assessment of Adherence to a Gluten-Free Diet in Patients with Celiac Disease. Nutrients 2020, 12, 2300. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Scott-Sheldon, L.A.J.; Risech-Neyman, Y.; Moss, S.F.; Ludvigsson, J.F.; Green, P.H.R. Celiac Disease and Increased Risk of Pneumococcal Infection: A Systematic Review and Meta-Analysis. Am. J. Med. 2018, 131, 83–89. [Google Scholar] [CrossRef]
- Emilsson, L.; Semrad, C.; Lebwohl, B.; Green, P.H.R.; Ludvigsson, J.F. Risk of Small Bowel Adenocarcinoma, Adenomas, and Carcinoids in a Nationwide Cohort of Individuals with Celiac Disease. Gastroenterology 2020, 159, 1686–1694.e2. [Google Scholar] [CrossRef]
- Ilus, T.; Kaukinen, K.; Virta, L.J.; Pukkala, E.; Collin, P. Incidence of malignancies in diagnosed celiac patients: A population-based estimate. Am. J. Gastroenterol. 2014, 109, 1471–1477. [Google Scholar] [CrossRef]
- van Gils, T.; Nijeboer, P.; Overbeek, L.I.; Hauptmann, M.; Castelijn, D.A.; Bouma, G.; Mulder, C.J.; van Leeuwen, F.E.; de Jong, D. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up: Celiac disease, lymphoma and GI carcinoma. United Eur. Gastroenterol. J. 2018, 6, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Verdu, E.F.; Bai, J.C.; Lionetti, E. Coeliac disease. Lancet 2022, 399, 2413–2426. [Google Scholar] [CrossRef]
- Elfström, P.; Granath, F.; Ekström Smedby, K.; Montgomery, S.M.; Askling, J.; Ekbom, A.; Ludvigsson, J.F. Risk of lymphoproliferative malignancy in relation to small intestinal histopathology among patients with celiac disease. J. Natl. Cancer Inst. 2011, 103, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Tio, M.; Cox, M.R.; Eslick, G.D. Meta-analysis: Coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment. Pharmacol. Ther. 2012, 35, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Green, P.H.R.; Emilsson, L.; Mårild, K.; Söderling, J.; Roelstraete, B.; Ludvigsson, J.F. Cancer Risk in 47,241 Individuals with Celiac Disease: A Nationwide Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 20, e111–e131. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Montgomery, S.M.; Ekbom, A.; Brandt, L.; Granath, F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA 2009, 302, 1171–1178. [Google Scholar] [CrossRef]
- Silano, M.; Volta, U.; Vincenzi, A.D.; Dessì, M.; Vincenzi, M.D. Effect of a gluten-free diet on the risk of enteropathy-associated T-cell lymphoma in celiac disease. Dig. Dis. Sci. 2008, 53, 972–976. [Google Scholar] [CrossRef]
- Green, P.H.R.; Fleischauer, A.T.; Bhagat, G.; Goyal, R.; Jabri, B.; Neugut, A.I. Risk of malignancy in patients with celiac disease. Am. J. Med. 2003, 115, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, E.; Yadav, A.; Kelly, C.P.; Mukherjee, R. Novel Nondietary Therapies for Celiac Disease. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 335–345. [Google Scholar] [CrossRef]
- Kivelä, L.; Caminero, A.; Leffler, D.A.; Pinto-Sanchez, M.I.; Tye-Din, J.A.; Lindfors, K. Current and emerging therapies for coeliac disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 181–195. [Google Scholar] [CrossRef]
- Klonarakis, M.; Andrews, C.N.; Raman, M.; Panaccione, R.; Ma, C. Review article: Therapeutic targets for the pharmacologic management of coeliac disease-the future beyond a gluten-free diet. Aliment. Pharmacol. Ther. 2022, 55, 1277–1296. [Google Scholar] [CrossRef]
- Kerbage, A.; Loesch, J.; Jansson-Knodell, C.L.; Neil, N.; Rubio-Tapia, A. Racial, Ethnic, and Socioeconomic Disparities in Celiac Disease Trials: A Systematic Review of Participant Diversity and Trial Site Distribution. Am. J. Gastroenterol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Mitea, C.; Havenaar, R.; Drijfhout, J.W.; Edens, L.; Dekking, L.; Koning, F. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: Implications for coeliac disease. Gut 2008, 57, 25–32. [Google Scholar] [CrossRef]
- König, J.; Holster, S.; Bruins, M.J.; Brummer, R.J. Randomized clinical trial: Effective gluten degradation by Aspergillus niger-derived enzyme in a complex meal setting. Sci. Rep. 2017, 7, 13100. [Google Scholar] [CrossRef]
- Slifer, Z.M.; Krishnan, B.R.; Madan, J.; Blikslager, A.T. Larazotide acetate: A pharmacological peptide approach to tight junction regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G983–G989. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; Harris, L.A.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J.A. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 2012, 107, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Green, P.H.; Murray, J.A.; Dimarino, A.; Colatrella, A.; Leffler, D.A.; Alexander, T.; Arsenescu, R.; Leon, F.; Jiang, J.G.; et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: A randomised placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 37, 252–262. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Green, P.H.; Fedorak, R.N.; DiMarino, A.; Perrow, W.; Rasmussen, H.; Wang, C.; Bercik, P.; Bachir, N.M.; et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology 2015, 148, 1311–1319.e6. [Google Scholar] [CrossRef]
- Schuppan, D.; Mäki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, Ø.; et al. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Dotsenko, V.; Tewes, B.; Hils, M.; Pasternack, R.; Isola, J.; Taavela, J.; Popp, A.; Sarin, J.; Huhtala, H.; Hiltunen, P.; et al. Transcriptomic analysis of intestine following administration of a transglutaminase 2 inhibitor to prevent gluten-induced intestinal damage in celiac disease. Nat. Immunol. 2024, 25, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Okura, Y.; Ikawa-Teranishi, Y.; Mizoroki, A.; Takahashi, N.; Tsushima, T.; Irie, M.; Harfuddin, Z.; Miura-Okuda, M.; Ito, S.; Nakamura, G.; et al. Characterizations of a neutralizing antibody broadly reactive to multiple gluten peptide:HLA-DQ2.5 complexes in the context of celiac disease. Nat. Commun. 2023, 14, 8502. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Siegel, M.; Bergseng, E.; Sollid, L.M.; Khosla, C. Inhibition of HLA-DQ2-mediated antigen presentation by analogues of a high affinity 33-residue peptide from alpha2-gliadin. J. Am. Chem. Soc. 2006, 128, 1859–1867. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goel, G.; King, T.; Daveson, A.J.; Andrews, J.M.; Krishnarajah, J.; Krause, R.; Brown, G.J.E.; Fogel, R.; Barish, C.F.; Epstein, R.; et al. Epitope-specific immunotherapy targeting CD4-positive T cells in coeliac disease: Two randomised, double-blind, placebo-controlled phase 1 studies. Lancet Gastroenterol. Hepatol. 2017, 2, 479–493. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Ee, H.C.; Andrews, J.M.; King, T.; Goldstein, K.E.; Dzuris, J.L.; MacDougall, J.A.; Williams, L.J.; Treohan, A.; Cooreman, M.P.; et al. Epitope-Specific Immunotherapy Targeting CD4-Positive T Cells in Celiac Disease: Safety, Pharmacokinetics, and Effects on Intestinal Histology and Plasma Cytokines with Escalating Dose Regimens of Nexvax2 in a Randomized, Double-Blind, Placebo-Controlled Phase 1 Study. EBioMedicine 2017, 26, 78–90. [Google Scholar] [CrossRef]
- Kelly, C.P.; Murray, J.A.; Leffler, D.A.; Getts, D.R.; Bledsoe, A.C.; Smithson, G.; First, M.R.; Morris, A.; Boyne, M.; Elhofy, A.; et al. TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Gastroenterology 2021, 161, 66–80.e8. [Google Scholar] [CrossRef]
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease-A Narrative Review. Nutrients 2023, 15, 2499. [Google Scholar] [CrossRef]
- Seiler, C.L.; Kiflen, M.; Stefanolo, J.P.; Bai, J.C.; Bercik, P.; Kelly, C.P.; Verdu, E.F.; Moayyedi, P.; Pinto-Sanchez, M.I. Probiotics for Celiac Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2020, 115, 1584–1595. [Google Scholar] [CrossRef]
- Scherer, T.C.; De Marco, I.; Ferreira, N.R.C.; Pimentel, T.C.; Magnani, M.; Hassemer, G.S.; Nascimento, A.B.D.; Verruck, S. Bifidobacteria and Celiac Disease: Mechanisms of Probiotic Action in Reducing Gluten-Induced Cytotoxicity and Inflammation. Mol. Nutr. Food Res. 2025, e70222. [Google Scholar] [CrossRef]
- Lasa, A.; Larretxi, I.; Simón, E.; Churruca, I.; Navarro, V.; Martínez, O.; Bustamante, M.; Miranda, J. New Software for Gluten-Free Diet Evaluation and Nutritional Education. Nutrients 2019, 11, 2505. [Google Scholar] [CrossRef]
- Vriezinga, S.; Borghorst, A.; van den Akker-van Marle, E.; Benninga, M.; George, E.; Hendriks, D.; Hopman, E.; de Meij, T.; van der Meulen-de Jong, A.; Putter, H.; et al. E-Healthcare for Celiac Disease-A Multicenter Randomized Controlled Trial. J. Pediatr. 2018, 195, 154–160.e7. [Google Scholar] [CrossRef] [PubMed]
- Nikniaz, Z.; Shirmohammadi, M.; Akbari Namvar, Z. Development and effectiveness assessment of a Persian-language smartphone application for celiac patients: A randomized controlled clinical trial. Patient Educ. Couns. 2021, 104, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Naveh, G. Mobile Application for Promoting Gluten-Free Diet Self-Management in Adolescents with Celiac Disease: Proof-of-Concept Study. Nutrients 2021, 13, 1401. [Google Scholar] [CrossRef]
- Basil, A.; Littlejohn, B.; Perl, J.; Adams, D.W. Use of technology to educate patients with celiac disease. Nutr. Clin. Pract. 2025, 40, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Månsson, A.L.; Meijer-Boekel, C.; Mårild, K. Utilization and Effectiveness of eHealth Technology in the Follow-up of Celiac Disease: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 812–818. [Google Scholar] [CrossRef]
- Jansson-Knodell, C.L.; Gardinier, D.; Weekley, K.; Yang, Q.; Rubio-Tapia, A. Artificial Intelligence Chatbots Not Yet Ready for Celiac Disease Patient Care. Clin. Gastroenterol. Hepatol. 2025, 23, 1065–1067.e1. [Google Scholar] [CrossRef]
| Professional | Key Responsibilities | Timing/Context |
|---|---|---|
| Gastroenterologist | Confirm diagnosis, assess clinical improvement and mucosal healing, manage complications (e.g., refractory celiac disease, malignancy risk), coordinate care | Baseline, 3, 6, and 12 months after diagnosis, then every 6–12 months thereafter or as clinically indicated |
| Dietitian | Provide education on gluten-free diet (GFD), assess nutritional adequacy, monitor for deficiencies, reinforce adherence strategies | At diagnosis, 6–12 months, and ongoing as needed |
| Primary Care Provider | Preventive health (vaccinations, cardiovascular/metabolic risk), screen for comorbidities (osteoporosis, thyroid disease, diabetes), ensure continuity of care | Ongoing, annual wellness |
| Psychologist/Psychiatrist/Mental Health Professional | Screen for anxiety, depression, eating disorders, or maladaptive behaviors (e.g., ARFID, excessive fear of gluten), provide counseling | At diagnosis if risk factors present, or as needed |
| Endocrinologist | Evaluate and manage bone health in patients with osteopenia or osteoporosis, monitor calcium, vitamin D, and parathyroid hormone levels, manage associated endocrine comorbidities (e.g., thyroid disease, diabetes), guide therapy when indicated. | As indicated by abnormal bone density or metabolic findings. |
| Pharmacist | Review medications and supplements for hidden gluten, provide counseling on safe formulations, collaborate with prescribers to ensure gluten-free options | Baseline and when new medications are prescribed. |
| Challenge | Symptoms/Things to Ask About in Clinic | Suggested Management * |
|---|---|---|
| High salt content in gluten-free processed foods | Elevated blood pressure, headaches, patient-reported high salt intake, edema | Check BP regularly Counsel on sodium intake and label literacy Encourage whole foods |
| High sugar and saturated fat content | Weight gain, elevated HbA1c, dyslipidemia | Screen HbA1c and lipid panel annually Advise to limit ultra-processed foods |
| Low fiber content | Constipation, incomplete evacuation of bowel movements, low stool frequency, ask about whole grain intake | Promote naturally gluten-free high-fiber foods (e.g., quinoa, lentils) Consider fiber supplements |
| Low in essential vitamins and minerals | Fatigue, pallor, neuropathy, bone pain, hair loss; ask about supplement use and food variety | Test iron, B12, D, folate, zinc, copper at diagnosis and annually if risk persists; supplement as needed |
| Increased caloric density leading to weight gain/obesity | Weight trends, BMI increase, discuss satiety and portion sizes, lifestyle activity level, changes in fit of clothing | Track weight and waist circumference at every visit Personalize calorie and meal plans If overweight or obese, monitor for metabolic syndrome associated complications (e.g., metabolic-dysfunction-associated liver disease), consider transient elastography of liver Consider weight management strategies including weight-loss medications, metabolic and bariatric endoscopy and surgery |
| Psychological distress and food-related anxiety | Fear of contamination, avoidance of social meals, restrictive eating habits, mood symptoms | Screen for disordered eating and eating disorders Refer to behavioral health, psychology or psychiatry if needed Provide balanced, non-alarmist counseling |
| Higher cost and reduced availability of gluten-free products | Financial stress, skipped meals, reliance on limited food options, ask about food insecurity | Refer to social work Suggest budget-friendly GFD staples Educate on affordable nutrition |
| Social isolation and stigma | Avoidance of travel/restaurants, reluctance to eat outside home, emotional burden of diagnosis | Validate social/emotional challenges Connect with support groups Suggest coping strategies |
| Frequent unintentional gluten exposure | GI symptoms despite reported adherence, inconsistent symptom patterns, unclear label reading | Educate on cross-contamination and reading labels Consider GIP stool/urine testing for awareness and adherence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerbage, A.; Jansson-Knodell, C.; Weekley, K.; Gardinier, D.; Rubio-Tapia, A. High-Quality Nutritional and Medical Care in Celiac Disease Follow-Up. Nutrients 2025, 17, 3530. https://doi.org/10.3390/nu17223530
Kerbage A, Jansson-Knodell C, Weekley K, Gardinier D, Rubio-Tapia A. High-Quality Nutritional and Medical Care in Celiac Disease Follow-Up. Nutrients. 2025; 17(22):3530. https://doi.org/10.3390/nu17223530
Chicago/Turabian StyleKerbage, Anthony, Claire Jansson-Knodell, Kendra Weekley, David Gardinier, and Alberto Rubio-Tapia. 2025. "High-Quality Nutritional and Medical Care in Celiac Disease Follow-Up" Nutrients 17, no. 22: 3530. https://doi.org/10.3390/nu17223530
APA StyleKerbage, A., Jansson-Knodell, C., Weekley, K., Gardinier, D., & Rubio-Tapia, A. (2025). High-Quality Nutritional and Medical Care in Celiac Disease Follow-Up. Nutrients, 17(22), 3530. https://doi.org/10.3390/nu17223530






