Postbiotics from Lacticaseibacillus rhamnosus IOB820 Combat Obesity in HFD Mice by Modulating Gut Microbiota and Enhancing SCFA Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Pretreatment
2.2. Experimental Animals and Groups
2.3. Determination of Fasting Blood Glucose
2.4. ELISA
2.5. HE Staining
2.6. RT-qPCR
2.7. Determination of SCFAs
2.8. 16s rDNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. L. rhamnosus IOB820 and Its Postbiotics Attenuate Weight Gain, Improve Fasting Blood Glucose, and Modulate Organ Indices in HFD Mice
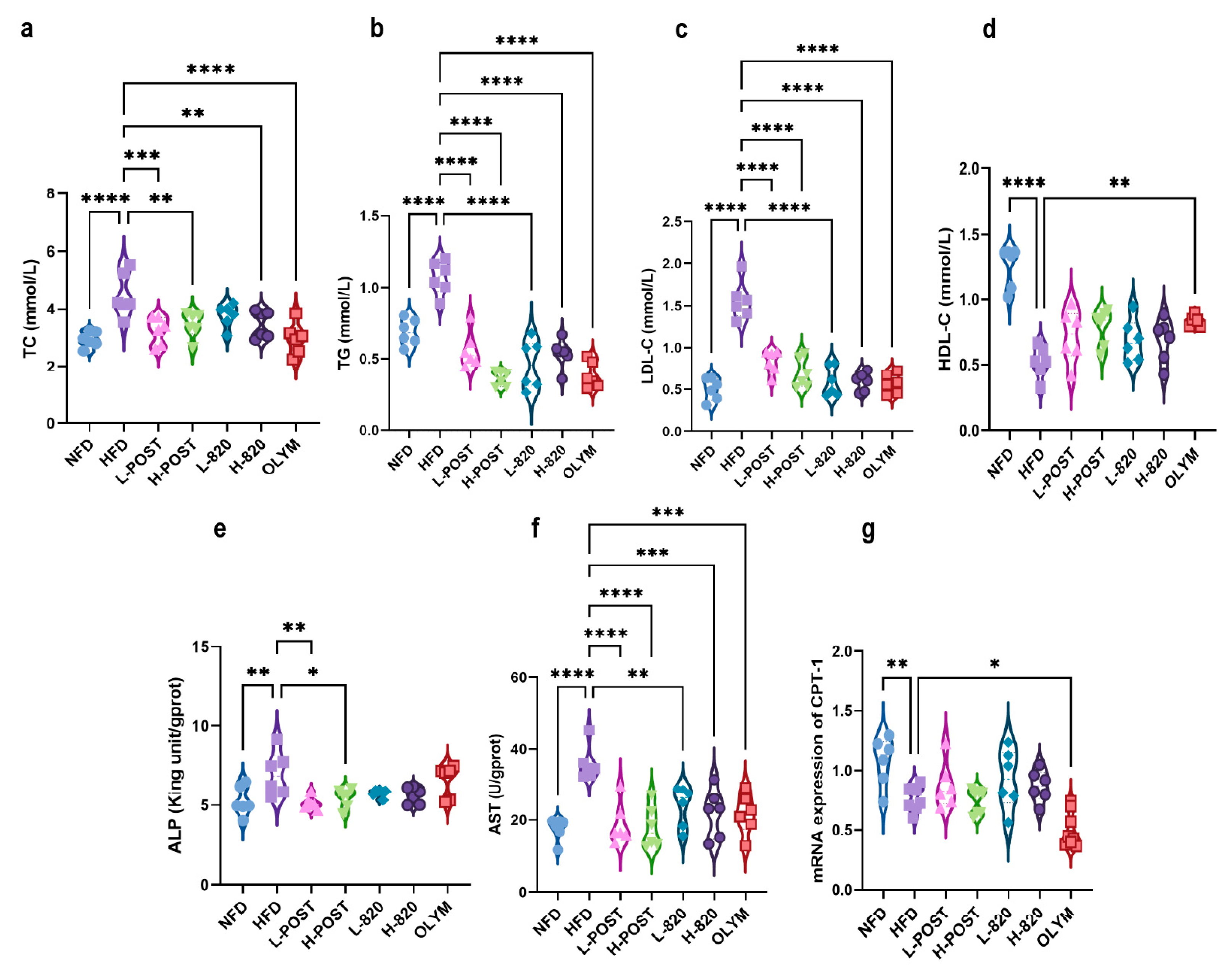
3.2. L. rhamnosus IOB820 and Its Postbiotics Ameliorate Dyslipidemia and Hepatic Metabolic Dysfunction in HFD Mice
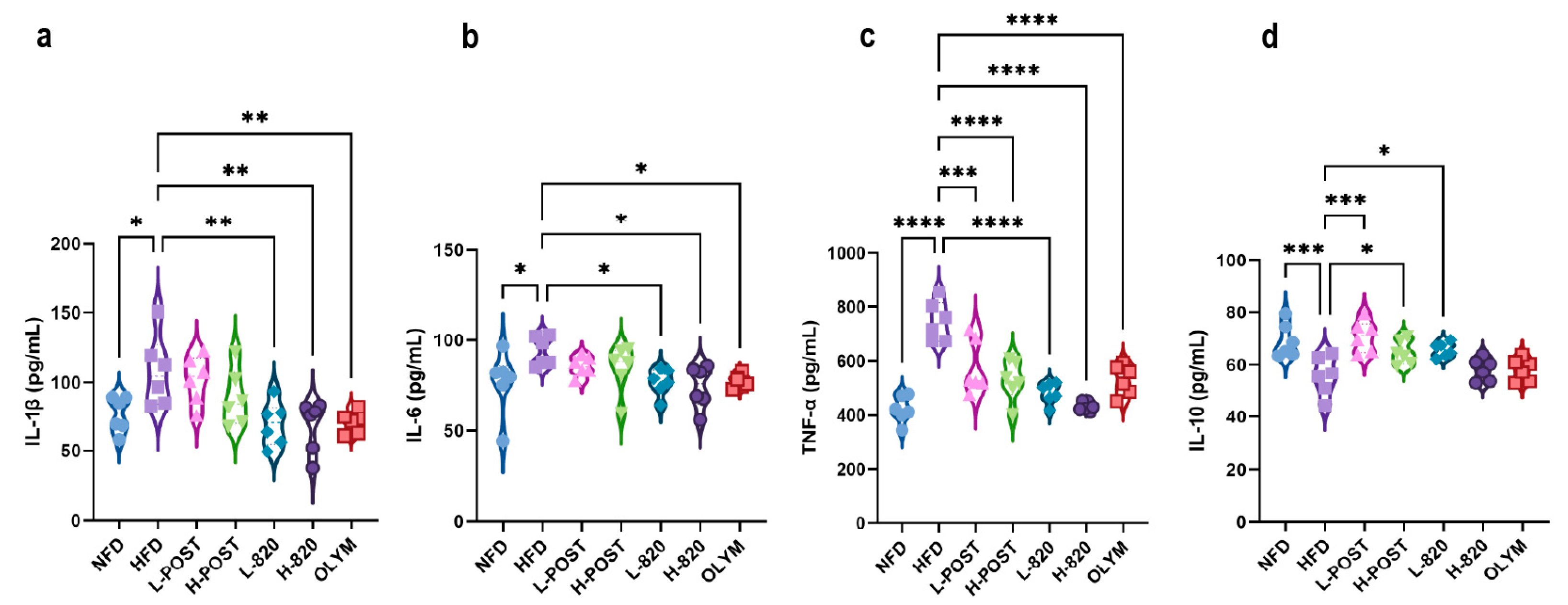
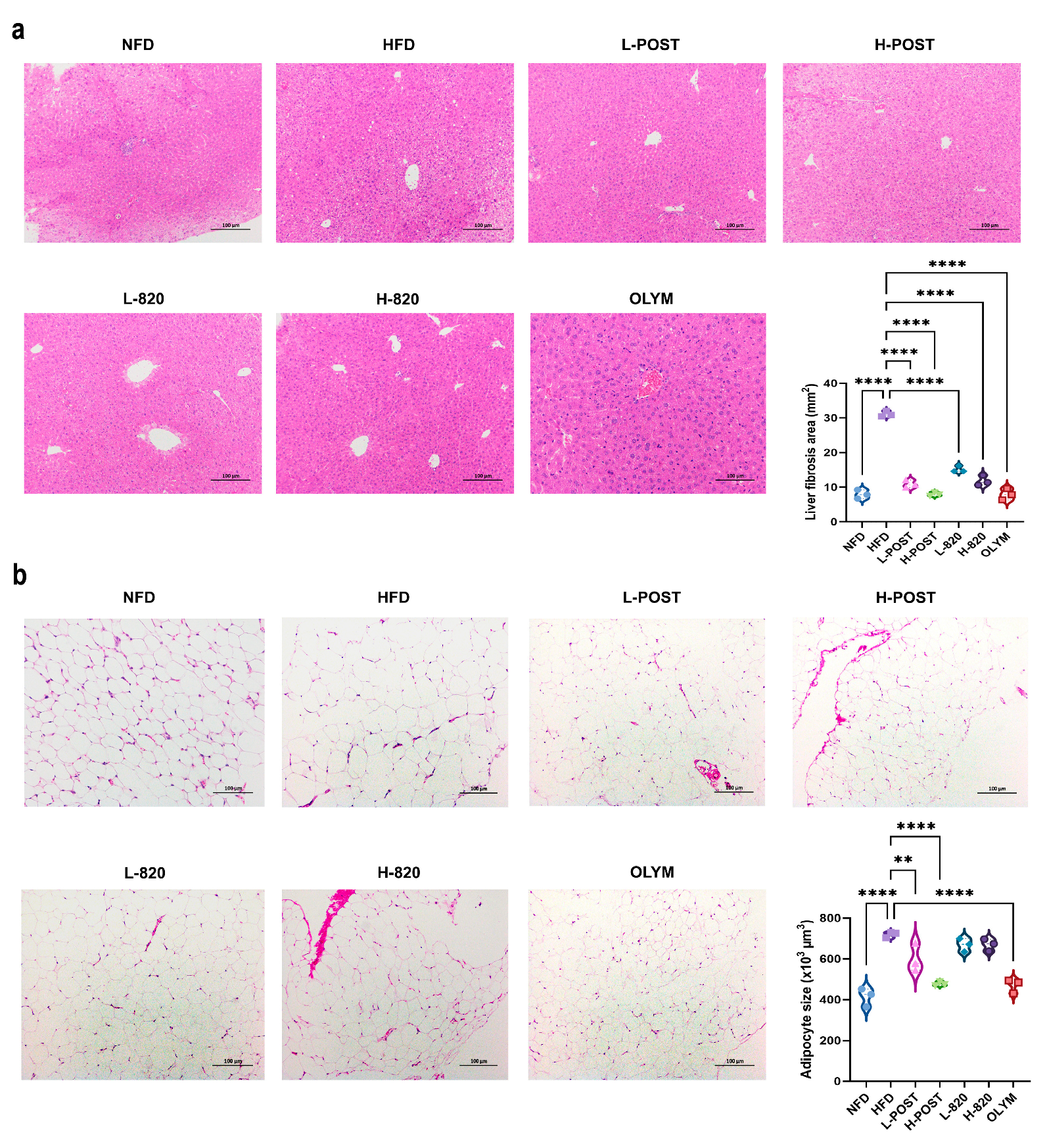
3.3. L. rhamnosus IOB820 and Its Postbiotics Attenuate Obesity-Associated Inflammation and Tissue Damage in HFD Mice
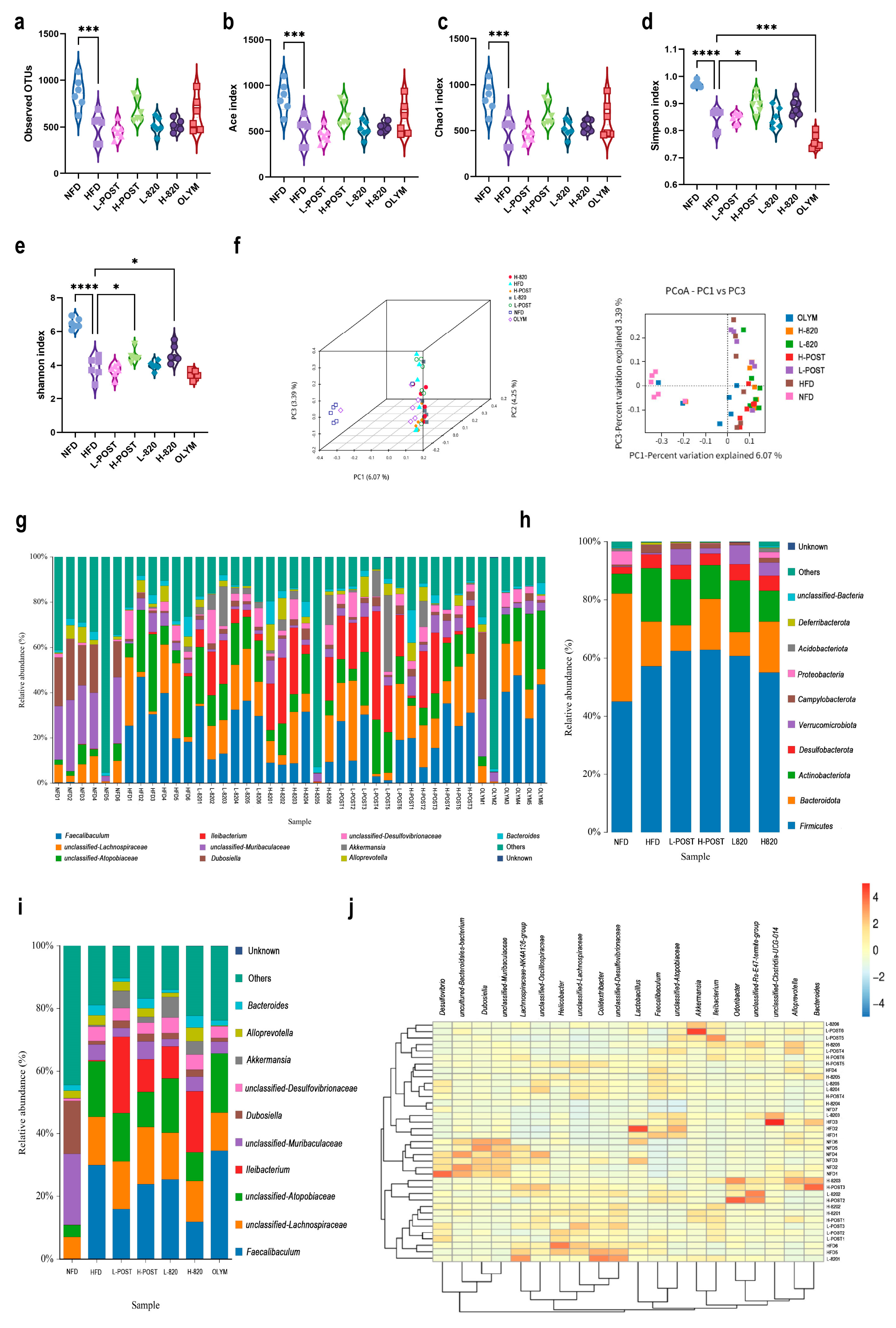
3.4. L. rhamnosus IOB820 and Its Postbiotics Restore Gut Microbiota Homeostasis in HFD Mice
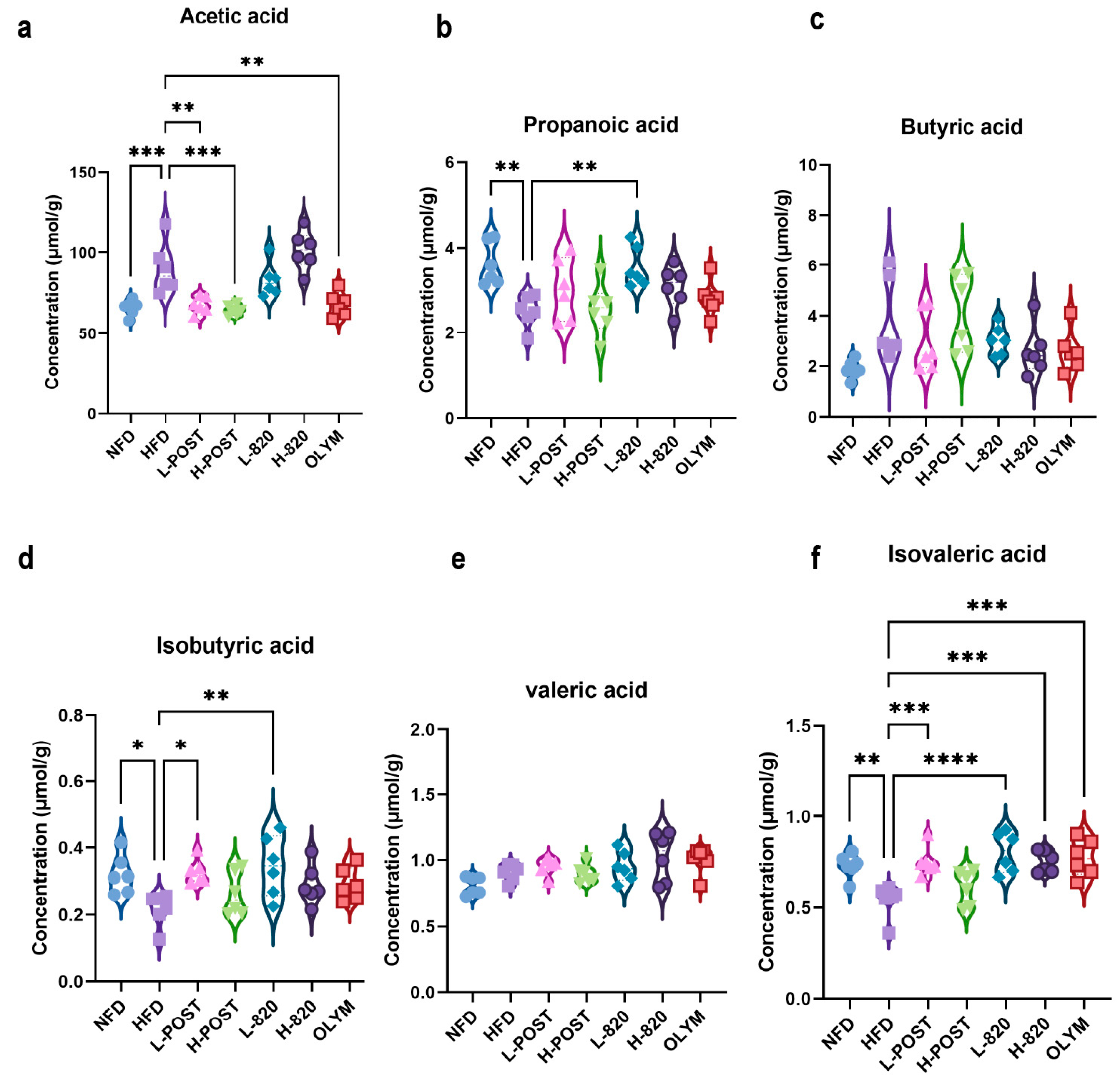
3.5. L. rhamnosus IOB820 and Its Postbiotics Modulate SCFA Production in HFD Mice
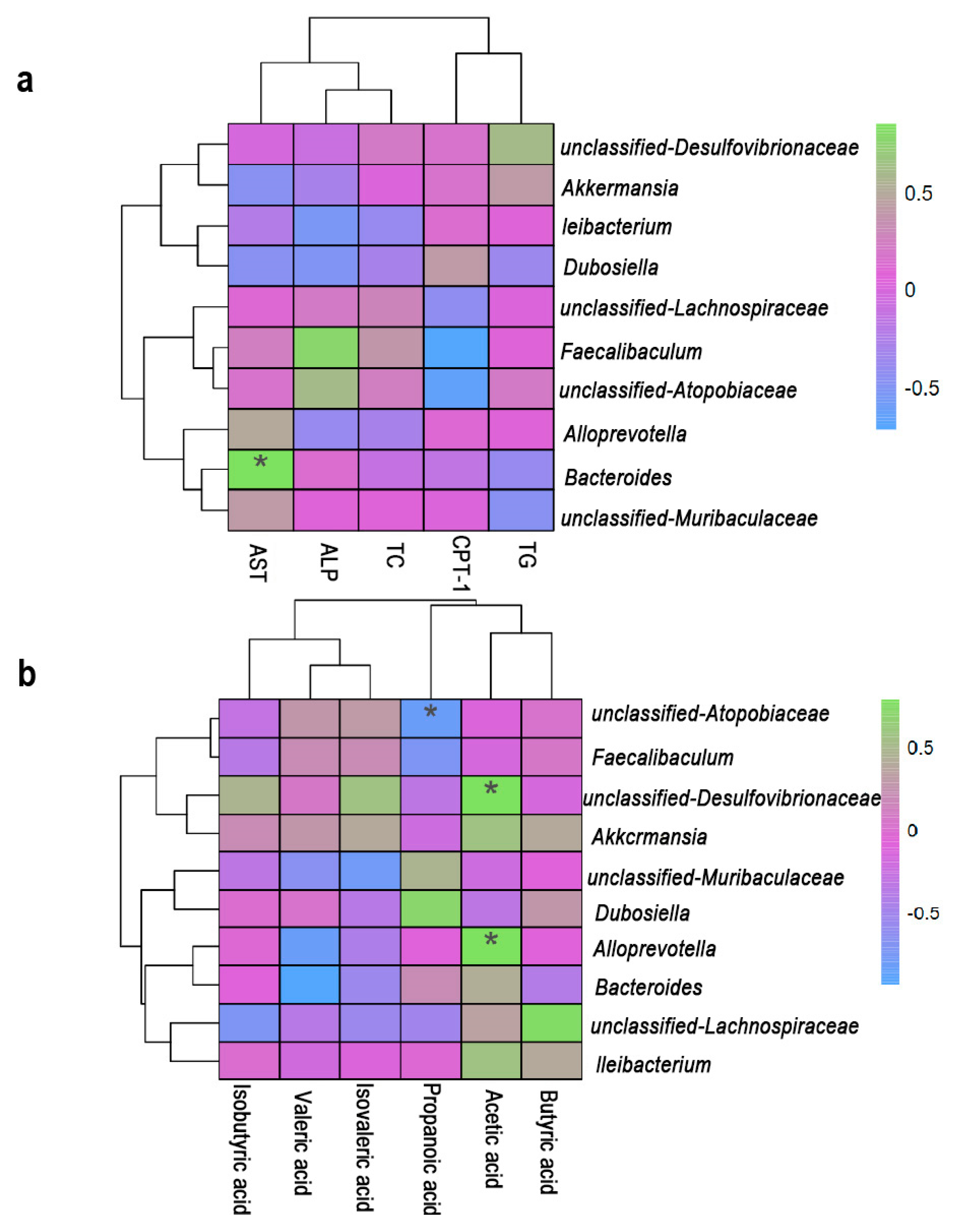
3.6. Correlation Analysis of Lipid Metabolism, Gut Microbiota and SCFAs
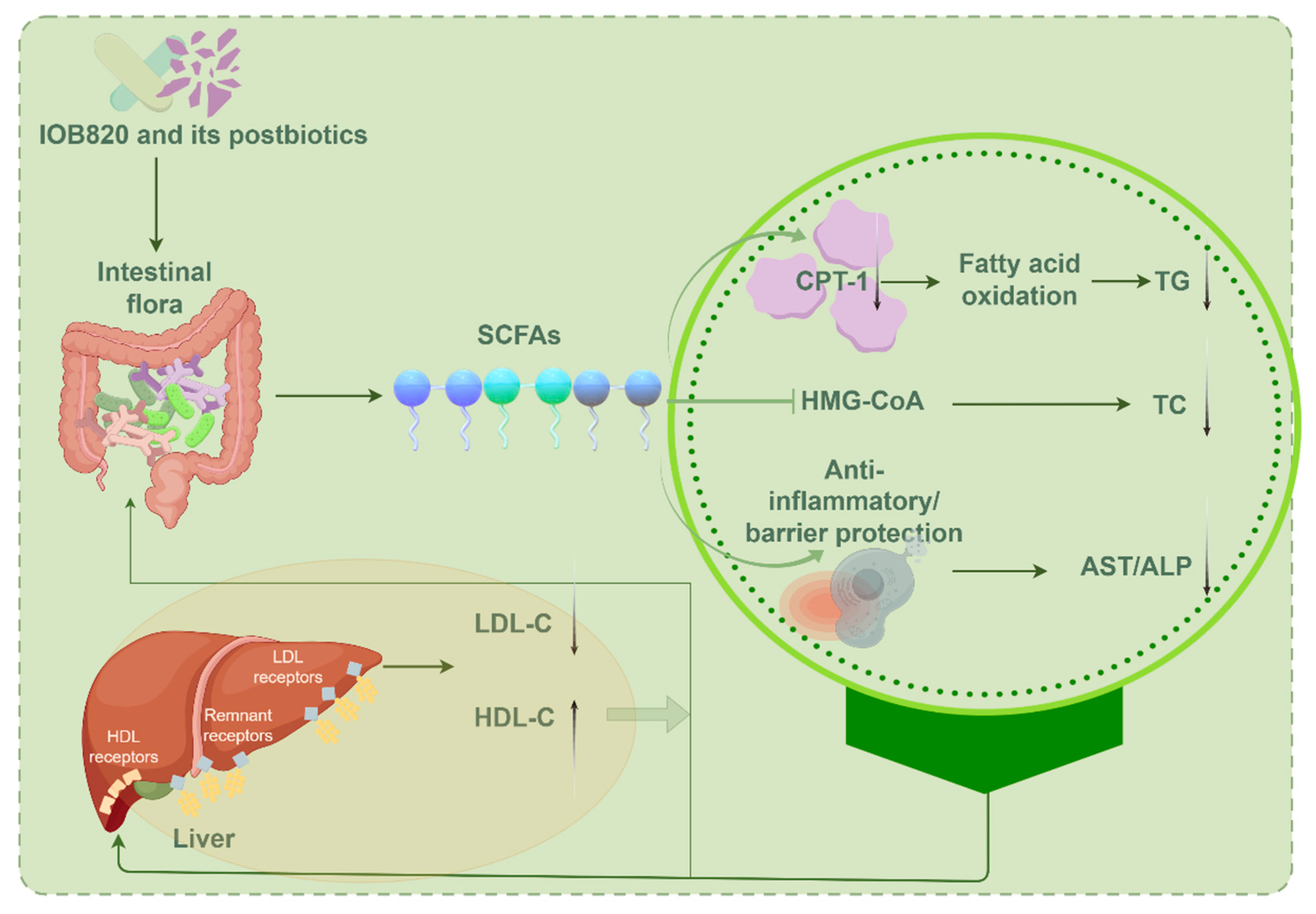
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HFD | High-fat diet |
| SCFA | Short-chain fatty acid |
| NFD | Normal diet |
| L-POST | Low-dose postbiotics |
| H-POST | High-dose postbiotics |
| OLYM | Orlistat-treated positive control |
| HDACs | Histone deacetylases |
| SPF | Specific pathogen-free |
| LDL-C | Low-density lipoprotein cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| TC | Total cholesterol |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-α |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| CPT-1 | Carnitine acyl transferase I |
| PCoA | Principal Coordinates Analysis |
| NAFLD | Nonalcoholic fatty liver disease |
| CVD | Cardiovascular disease |
| IBD | Inflammatory bowel disease |
References
- Nicklas, T.A.; Baranowski, T.; Cullen, K.W.; Berenson, G. Eating patterns, dietary quality and obesity. J. Am. Coll. Nutr. 2001, 20, 599–608. [Google Scholar] [CrossRef]
- Goettler, A.; Grosse, A.; Sonntag, D. Productivity loss due to overweight and obesity: A systematic review of indirect costs. BMJ Open 2017, 7, e014632. [Google Scholar] [CrossRef]
- Chen, K.; Shen, Z.; Gu, W.; Lyu, Z.; Qi, X.; Mu, Y.; Ning, Y.; Meinian Investigator Group. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 2023, 25, 3390–3399. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.L.; Carrera-Bastos, P.; Castillo-García, A.; Lieberman, D.E.; Santos-Lozano, A.; Lucia, A. Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 2023, 20, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Blueher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef]
- Agius, R.; Pace, N.P.; Fava, S. Phenotyping obesity: A focus on metabolically healthy obesity and metabolically unhealthy normal weight. Diabetes-Metab. Res. Rev. 2024, 40, e3725. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–487. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology—Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Reigstad, C.S.; Baeckhed, F. Intestinal Microbiota During Infancy and Its Implications for Obesity. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 249–256. [Google Scholar] [CrossRef]
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109. [Google Scholar] [CrossRef]
- Son, J.W.; Kim, S. Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef]
- Pereira, M.; Menezes, S.; Franco, A.J.; Marcolin, P.; Tomera, M. Role of GLP1-RA in Optimizing Weight Loss Post-Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2024, 34, 3888–3896. [Google Scholar] [CrossRef]
- Caesar, R. The impact of novel probiotics isolated from the human gut on the gut microbiota and health. Diabetes Obes. Metab. 2025, 27, 3–14. [Google Scholar] [CrossRef]
- Stachelska, M.A.; Karpinski, P.; Kruszewski, B. Health-Promoting and Functional Properties of Fermented Milk Beverages with Probiotic Bacteria in the Prevention of Civilization Diseases. Nutrients 2025, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, Y.; Lee, C.; Jung, E.S.; Kang, H.; Holzapfel, W.H. Comprehensive Amelioration of Metabolic Dysfunction through Administration of Lactiplantibacillus plantarum APsulloc 331261 (GTB1™) in High-Fat-Diet-Fed Mice. Foods 2024, 13, 2227. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, X.; Wang, R.; Liu, R.; Lv, X.; Ma, Y.; Li, Q. Lacticaseibacillus rhamnosus HF01 fermented yogurt alleviated high-fat diet-induced obesity and hepatic steatosis via the gut microbiota-butyric acid-hepatic lipid metabolism axis. Food Funct. 2024, 15, 4475–4489. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zheng, J.; Zong, X.; Yang, X.; Zhang, Y.; Man, C.; Jiang, Y. Preventive Effect and Molecular Mechanism of Lacticaseibacillus rhamnosus JL1 on Food-Borne Obesity in Mice. Nutrients 2021, 13, 3989. [Google Scholar] [CrossRef]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Alvarez, B.; Chenoll, E.; Ramon, D.; Tortajada, M.; Martorell, P. Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1: A novel postbiotic that reduces fat deposition via IGF-1 pathway. Microb. Biotechnol. 2022, 15, 805–816. [Google Scholar] [CrossRef]
- Lim, J.-J.; Jung, A.H.; Suh, H.J.; Choi, H.-S.; Kim, H. Lactiplantibacillus plantarum K8-based paraprobiotics prevents obesity and obesity-induced inflammatory responses in high fat diet-fed mice. Food Res. Int. 2022, 155, 111066. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.J.A.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Avellaneda Guimaraes, R.d.C.; Hiane, P.A.; Bogo, D.; Zorgetto Pinheiro, V.A.; Silva de Oliveira, L.C.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Kopczynska, J.; Kowalczyk, M. The potential of short-chain fatty acid epigenetic regulation in chronic low-grade inflammation and obesity. Front. Immunol. 2024, 15, 1380476. [Google Scholar] [CrossRef]
- Overby, H.B.; Ferguson, J.F. Gut Microbiota-Derived Short-Chain Fatty Acids Facilitate Microbiota:Host Cross talk and Modulate Obesity and Hypertension. Curr. Hypertens. Rep. 2021, 23, 8. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Prentki, M.; Corkey, B.E.; Madiraju, S.R.M. Lipid-associated metabolic signalling networks in pancreatic beta cell function. Diabetologia 2020, 63, 10–20. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, L.; Liao, Y.; Lin, Z.; Guo, C.; Luo, S.; Wang, F.; Zou, Z.; Zeng, Z.; Chen, C.; et al. Semaglutide alleviates gut microbiota dysbiosis induced by a high-fat diet. Eur. J. Pharmacol. 2024, 969, 176440. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Pan, W.; Huang, Y.; Zhang, N.; Zheng, B.; Zhang, X.; Xiao, M.; Yang, Y.; Ye, J. Differences in anti-obesity effects between raw and ripened Pu-erh tea polyphenols: Impact on gut microbiota enterotypes. J. Sci. Food Agric. 2025, 105, 4015–4030. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kong, Q.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. A High-Fat Diet Increases Gut Microbiota Biodiversity and En-ergy Expenditure Due to Nutrient Difference. Nutrients 2020, 12, 3197. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Liu, X.; Yang, J.; Feng, Y.; Wu, Y.; Xu, Y.; Zhu, Y.; Li, W. Fenofibrate Ameliorated Systemic and Retinal Inflammation and Modulated Gut Microbiota in High-Fat Diet-Induced Mice. Front. Cell. Infect. Microbiol. 2022, 12, 839592. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Yin, J.; Wei, L.; Li, R.; Peng, W.; Yuan, Y.; Huang, X.; Yin, Y. Lactobacillus delbrueckii might lower serum triglyceride levels via colonic microbiota modulation and SCFA-mediated fat metabolism in parenteral tissues of growing-finishing pigs. Front. Vet. Sci. 2022, 9, 982349. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Y.; Ma, K.Y.; Chen, F.; Chen, Z.Y. Microalga decreases plasma cholesterol by down-regulation of intestinal NPC1L1, hepatic LDL receptor, and HMG-CoA reductase. J. Agric. Food Chem. 2011, 59, 6790–6797. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, P.; Zhou, X.; Yuan, J.; Chen, Q. Probiotics therapy show significant improvement in obesity and neurobehavioral disorders symptoms. Front. Cell. Infect. Microbiol. 2023, 13, 1178399. [Google Scholar] [CrossRef]
- Tang, C.; Kong, L.; Shan, M.; Lu, Z.; Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res. Int. 2021, 147, 110490. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Wu, P.; Yang, S.; Xue, W.; Xu, B.; Zhang, S.; Tang, B.; Xu, D. Akkermansia muciniphila: A promising probiotic against inflammation and metabolic disorders. Virulence 2024, 15, 2375555. [Google Scholar] [CrossRef]
- Ahmed, E.T.; Dukessa, A.; Mateos, T.; Meka, W.; Seyoum Moti, M.; Zawdie, B. Evaluation of the antidyslipidemic and nephroprotective effect of methanolic seed extract of Lepidium sativum on male Swiss albino mice fed on deep fried palm oil. Front. Nutr. 2025, 12, 1468704. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Boby, N.; Lee, E.-B.; Hong, J.-H.; Park, S.-C. Anti-Obesity Effects of Ecklonia cava Extract in High-Fat Diet-Induced Obese Rats. Antioxidants 2022, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fang, R.; Lu, X.; Zhang, Y.; Yang, M.; Su, Y.; Jiang, Y.; Man, C. Lactobacillus reuteri J1 prevents obesity by altering the gut microbiota and regulating bile acid metabolism in obese mice. Food Funct. 2022, 13, 6688–6701. [Google Scholar] [CrossRef] [PubMed]
- Eslick, S.; Thompson, C.; Berthon, B.; Wood, L. Short-chain fatty acids as anti-inflammatory agents in overweight and obesity: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 838–856. [Google Scholar] [CrossRef]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- He, L.; Miao, M.; Li, Q.; Cheng, J.; Li, R. Evaluation of the Effects of High Uric Acid on Glucolipid Metabolism, Renal Injury and the Gut Microbiota in Diabetic Male Hamsters with Dyslipidemia. Toxics 2025, 13, 751. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, K.; Leng, Y.; Zhang, Z.; Li, X.; Li, X.; Ou, H.; Wen, M.; Qiu, F.; Yu, H. Alleviating effect of Lactobacillus fermentum E15 on hyperlipidemia and hepatic lipid metabolism in zebrafish fed by a high-fat diet through the production of short-chain fatty acids. Front. Nutr. 2025, 12, 1522982. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Tian, J.-X.; Lian, F.-M.; Li, M.; Liu, W.-K.; Zhen, Z.; Liao, J.-Q.; Tong, X.-L. Therapeutic mechanisms of traditional Chinese medicine to improve metabolic diseases via the gut microbiota. Biomed. Pharmacother. 2021, 133, 110857. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, H.; Qi, X.; Sun, Y.; Ma, Y.; Li, Q. Lactobacillus plantarum HF02 alleviates lipid accumulation and intestinal microbiota dysbiosis in high-fat diet-induced obese mice. J. Sci. Food Agric. 2023, 103, 4625–4637. [Google Scholar] [CrossRef] [PubMed]
- Nehmi-Filho, V.; Santamarina, A.B.; de Freitas, J.A.; Trarbach, E.B.; de Oliveira, D.R.; Palace-Berl, F.; de Souza, E.; de Miranda, D.A.; Escamilla-Garcia, A.; Otoch, J.P.; et al. Novel nutraceutical supplements with yeast β-glucan, prebiotics, minerals, and Silybum marianum (silymarin) ameliorate obesity-related metabolic and clinical parameters: A double-blind randomized trial. Front. Endocrinol. 2023, 13, 1089938. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Sharma, A.; Lee, H.J. Postbiotics against Obesity: Perception and Overview Based on Pre-Clinical and Clinical Studies. Int. J. Mol. Sci. 2023, 24, 6414. [Google Scholar] [CrossRef]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef]

| Target Gene | Forward Primer | Reveres Primer |
|---|---|---|
| GAPDH | ATGGTGAAGGTCGGTGTGAACGG | TGGAACATGTAGACCATGTAGTGAGG |
| CPT-1 | CAAGAACAGCAACGAGTACCG | GTCACTGGTCAACTCCAGCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Li, H.; Tian, J.; Han, X.; Liang, W.; Zhong, F.; Luo, X. Postbiotics from Lacticaseibacillus rhamnosus IOB820 Combat Obesity in HFD Mice by Modulating Gut Microbiota and Enhancing SCFA Production. Nutrients 2025, 17, 3525. https://doi.org/10.3390/nu17223525
Feng X, Li H, Tian J, Han X, Liang W, Zhong F, Luo X. Postbiotics from Lacticaseibacillus rhamnosus IOB820 Combat Obesity in HFD Mice by Modulating Gut Microbiota and Enhancing SCFA Production. Nutrients. 2025; 17(22):3525. https://doi.org/10.3390/nu17223525
Chicago/Turabian StyleFeng, Xiaomin, Hanlu Li, Jianxia Tian, Xuemei Han, Wu Liang, Feiliang Zhong, and Xuegang Luo. 2025. "Postbiotics from Lacticaseibacillus rhamnosus IOB820 Combat Obesity in HFD Mice by Modulating Gut Microbiota and Enhancing SCFA Production" Nutrients 17, no. 22: 3525. https://doi.org/10.3390/nu17223525
APA StyleFeng, X., Li, H., Tian, J., Han, X., Liang, W., Zhong, F., & Luo, X. (2025). Postbiotics from Lacticaseibacillus rhamnosus IOB820 Combat Obesity in HFD Mice by Modulating Gut Microbiota and Enhancing SCFA Production. Nutrients, 17(22), 3525. https://doi.org/10.3390/nu17223525







