Effects of Acute Fish Oil Supplementation on Muscle Function and Soreness After Eccentric Contraction-Induced Muscle Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Experimental Design
2.2. Muscle Damaging Exercise Protocol
2.3. Fish Oil Supplementation
2.4. Physical Function Assessment
2.4.1. Vertical Jump Test
2.4.2. Maximal Voluntary Contraction Measurements
2.4.3. Perceived Muscle Soreness Measurements
2.4.4. Blood Collection and Analysis of Systemic Biomarkers
2.4.5. Statistical Analysis
3. Results
3.1. Muscle Performance Recovery
3.2. Perceived Muscle Soreness
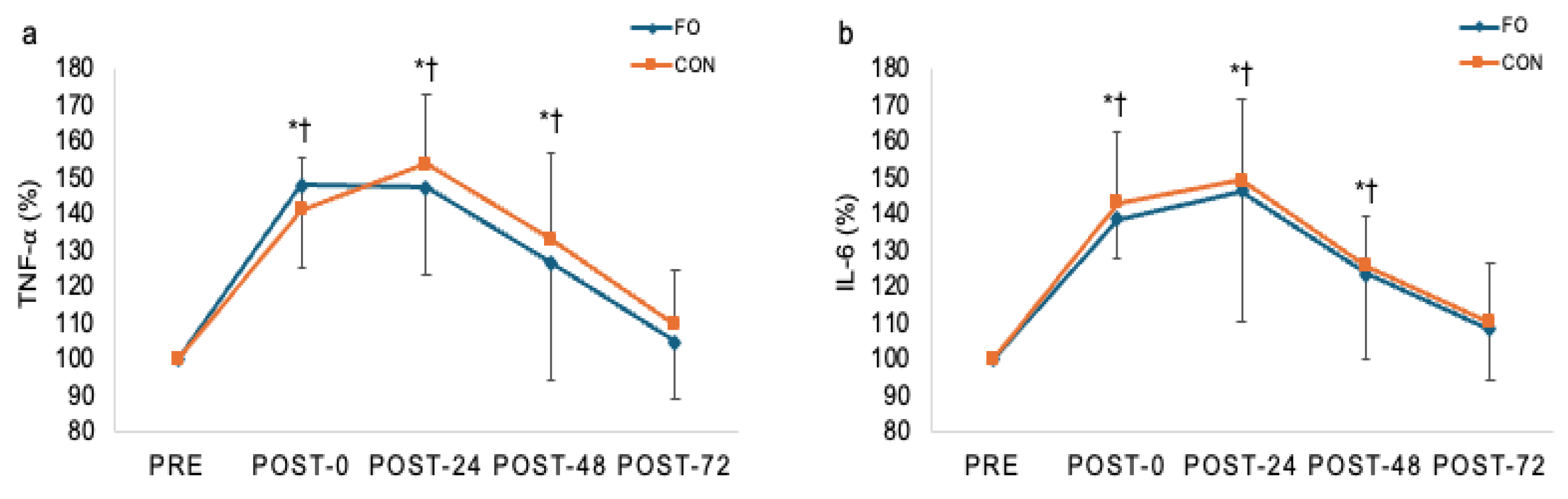
3.3. Biochemical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarkson, P.M.; Sayers, S.P. Etiology of exercise-induced muscle damage. Can. J. Appl. Physiol. 1999, 24, 234–248. [Google Scholar] [CrossRef]
- Ochi, E.; Tsuchiya, Y.; Nosaka, K. Differences in post-exercise T2 relaxation time changes between eccentric and concentric contractions of the elbow flexors. Eur. J. Appl. Physiol. 2016, 116, 2145–2154. [Google Scholar] [CrossRef]
- Armstrong, R.B.; Warren, G.L.; Warren, J.A. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991, 12, 184–207. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar] [PubMed]
- Jamurtas, A.Z. Exercise-Induced Muscle Damage and Oxidative Stress. Antioxidants 2018, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Paoloni, J.A.; Milne, C.; Orchard, J.; Hamilton, B. Non-steroidal anti-inflammatory drugs in sports medicine: Guidelines for practical but sensible use. Br. J. Sports Med. 2009, 43, 863–865. [Google Scholar] [CrossRef]
- Morelli, K.M.; Brown, L.B.; Warren, G.L. Effect of NSAIDs on Recovery From Acute Skeletal Muscle Injury: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2018, 46, 224–233. [Google Scholar] [CrossRef]
- Fetterman, J.W., Jr.; Zdanowicz, M.M. Therapeutic potential of n-3 polyunsaturated fatty acids in disease. Am. J. Health Syst. Pharm. 2009, 66, 1169–1179. [Google Scholar] [CrossRef]
- Hajianfar, H.; Paknahad, Z.; Bahonar, A. The effect of omega-3 supplements on antioxidant capacity in patients with type 2 diabetes. Int. J. Prev. Med. 2013, 4 (Suppl. S2), S234–S238. [Google Scholar]
- Zebrowska, A.; Mizia-Stec, K.; Mizia, M.; Gasior, Z.; Poprzecki, S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur. J. Sport Sci. 2015, 15, 305–314. [Google Scholar] [CrossRef]
- Macartney, M.J.; Hingley, L.; Brown, M.A.; Peoples, G.E.; McLennan, P.L. Intrinsic heart rate recovery after dynamic exercise is improved with an increased omega-3 index in healthy males. Br. J. Nutr. 2014, 112, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. JPEN J. Parenter. Enter. Nutr. 2015, 39 (Suppl. S1), 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lazaro, D.; Arribalzaga, S.; Gutierrez-Abejon, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults-A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Wood, C.; Ferrier, C.; Dolci, A.; Elliott, B. The effect of Omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2021, 18, 9. [Google Scholar] [CrossRef]
- Loss, L.C.; Benini, D.; de Lima-E-Silva, F.X.; Möller, G.B.; Friedrich, L.R.; Meyer, E.; Baroni, B.M.; Schneider, C.D. Effects of omega-3 supplementation on muscle damage after resistance exercise in young women: A randomized placebo-controlled trial. Nutr. Health 2022, 28, 425–432. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: A randomized, double-blind, placebo-controlled, parallel-group trial. Eur. J. Appl. Physiol. 2016, 116, 1179–1188. [Google Scholar] [CrossRef]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Kerksick, C.M.; Mangine, G.T.; Holmes, A.J.; Lee, M.; Endito, M.R.; et al. Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients 2020, 12, 2246. [Google Scholar] [CrossRef]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J. Int. Soc. Sports Nutr. 2017, 14, 23. [Google Scholar] [CrossRef]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537 Pt 2, 333–345. [Google Scholar] [CrossRef]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef]
- Gravina, L.; Brown, F.F.; Alexander, L.; Dick, J.; Bell, G.; Witard, O.C.; Galloway, S.D.R. n-3 Fatty Acid Supplementation During 4 Weeks of Training Leads to Improved Anaerobic Endurance Capacity, but not Maximal Strength, Speed, or Power in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 305–313. [Google Scholar] [CrossRef][Green Version]
- Twist, C.; Eston, R. The effects of exercise-induced muscle damage on maximal intensity intermittent exercise performance. Eur. J. Appl. Physiol. 2005, 94, 652–658. [Google Scholar] [CrossRef]
- Burt, D.G.; Twist, C. The effects of exercise-induced muscle damage on cycling time-trial performance. J. Strength Cond. Res. 2011, 25, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, E.; Veit, S.; Flenker, U.; Lesch, A.; Lachenmeier, D.W.; Diel, P. Influence of short-term chronic oral cannabidiol application on muscle recovery and performance after an intensive training protocol—A randomized double-blind crossover study. J. Int. Soc. Sports Nutr. 2024, 21, 2337252. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Eston, R. The effect of exercise-induced muscle damage on isometric and dynamic knee extensor strength and vertical jump performance. J. Sports Sci. 2002, 20, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Stozer, A.; Vodopivc, P.; Krizancic Bombek, L. Pathophysiology of exercise-induced muscle damage and its structural, functional, metabolic, and clinical consequences. Physiol. Res. 2020, 69, 565–598. [Google Scholar] [CrossRef]
- Helge, J.W.; Therkildsen, K.J.; Jorgensen, T.B.; Wu, B.J.; Storlien, L.H.; Asp, S. Eccentric contractions affect muscle membrane phospholipid fatty acid composition in rats. Exp. Physiol. 2001, 86, 599–604. [Google Scholar] [CrossRef]
- Peoples, G.E.; McLennan, P.L. Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. Br. J. Nutr. 2010, 104, 1771–1779. [Google Scholar] [CrossRef]
- Gorjao, R.; Azevedo-Martins, A.K.; Rodrigues, H.G.; Abdulkader, F.; Arcisio-Miranda, M.; Procopio, J.; Curi, R. Comparative effects of DHA and EPA on cell function. Pharmacol. Ther. 2009, 122, 56–64. [Google Scholar] [CrossRef]
- Therdyothin, A.; Phiphopthatsanee, N. The Effect of Omega-3 on Mitigating Exercise-Induced Muscle Damage. Cureus 2025, 17, e81559. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Yoo, S.; Yang, T.; Cho, H.; Kim, Y.; Hwang, S. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br. J. Pharmacol. 2010, 161, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.-R. Specialized pro-resolving mediators as resolution pharmacology for the control of pain and itch. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 273–293. [Google Scholar] [CrossRef]
- Ji, R.-R.; Gereau IV, R.W.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Turczyn, P.; Frasuńska, J.; Paradowska-Gorycka, A.; Tarnacka, B. Significance of Omega-3 Fatty Acids in the Prophylaxis and Treatment after Spinal Cord Injury in Rodent Models. Mediat. Inflamm. 2020, 2020, 3164260. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Liu, X.J.; Berta, T.; Park, C.K.; Lü, N.; Serhan, C.N.; Ji, R.R. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann. Neurol. 2013, 74, 490–495. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Berta, T.; Ji, R.R. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J. Neuroimmune Pharmacol. 2013, 8, 37–41. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. Omega-3 fatty acids supplementation attenuates inflammatory markers after eccentric exercise in untrained men. Clin. J. Sport. Med. 2011, 21, 131–137. [Google Scholar] [CrossRef]
- Massaro, M.; Habib, A.; Lubrano, L.; Turco, S.D.; Lazzerini, G.; Bourcier, T.; Weksler, B.B.; De Caterina, R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP (H) oxidase and PKCε inhibition. Proc. Natl. Acad. Sci. USA 2006, 103, 15184–15189. [Google Scholar] [CrossRef]
- Paoli, A.; Cerullo, G.; Bianco, A.; Neri, M.; Gennaro, F.; Charrier, D.; Moro, T. Not Only Protein: Dietary Supplements to Optimize the Skeletal Muscle Growth Response to Resistance Training: The Current State of Knowledge. J. Hum. Kinet. 2024, 91, 225–244. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef]

| FO | CON | |
|---|---|---|
| Age (years) | 23.3 ± 4.5 | 20.6 ± 1.1 |
| Height (cm) | 170.3 ± 6.3 | 171.0 ± 10.4 |
| Weight (kg) | 71.2 ± 11.8 | 69.9 ± 12.1 |
| Body mass index (kg/m2) | 24.6 ± 4.5 | 23.8 ± 2.1 |
| Outcome Measure | Group | Baseline | Post-0 h | Post-24 h | Post-48 h | Post-72 h | Time Effect | Group Effect | Time × Group Interaction |
|---|---|---|---|---|---|---|---|---|---|
| Vertical Jump (cm) | FO | 49.42 ± 8.7 | 43.1 ± 7.4 | 43.9 ± 8.8 | 46.7 ± 9.3 | 48.4 ± 10.1 | p < 0.001 | p = 0.933 | p = 0.002 |
| CON | 50.1 ± 13.1 | 42.4 ± 10.4 | 40.3 ± 13.2 | 44.3 ± 12.4 | 48.9 ± 14.4 | ||||
| Quadriceps Peak Torque (Nm) | FO | 177.7 ± 39.1 | 150.2 ± 37.3 | 157.2 ± 42.9 | 168.4 ± 37.3 | 173.0 ± 41.5 | p < 0.001 | p = 0.507 | p = 0.001 |
| CON | 172.3 ± 50.1 | 146.5 ± 51.4 | 139.9 ± 50.9 | 153.7 ± 52.8 | 167.8 ± 57.0 | ||||
| Hamstring Peak Torque (Nm) | FO | 72.8 ± 16.0 | 61.6 ± 15.6 | 65.8 ± 15.5 | 72.0 ± 15.6 | 72.0 ± 15.6 | p < 0.001 | p = 0.465 | p = 0.019 |
| CON | 71.8 ± 17.4 | 57.8 ± 15.7 | 56.3 ± 16.2 | 64.0 ± 18.0 | 66.4 ± 18.7 | ||||
| Muscle Soreness (Nm) | FO | 0.9 ± 0.9 | 3.6 ± 1.4 | 4.0 ± 1.3 | 1.3 ± 1.3 | 1.1 ± 1.6 | p < 0.001 | p = 0.038 | p < 0.001 |
| CON | 0.7 ± 0.7 | 3.8 ± 1.5 | 4.8 ± 1.2 | 3.7 ± 1.4 | 1.3 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-R.; Directo, D.; Kang, Y.; Stein, J.; Calvert, M.; An, Y.W.; Kim, D.-H. Effects of Acute Fish Oil Supplementation on Muscle Function and Soreness After Eccentric Contraction-Induced Muscle Damage. Nutrients 2025, 17, 3408. https://doi.org/10.3390/nu17213408
Lee S-R, Directo D, Kang Y, Stein J, Calvert M, An YW, Kim D-H. Effects of Acute Fish Oil Supplementation on Muscle Function and Soreness After Eccentric Contraction-Induced Muscle Damage. Nutrients. 2025; 17(21):3408. https://doi.org/10.3390/nu17213408
Chicago/Turabian StyleLee, Sang-Rok, Dean Directo, Yangmi Kang, Joshua Stein, Mason Calvert, Yong Woo An, and Do-Houn Kim. 2025. "Effects of Acute Fish Oil Supplementation on Muscle Function and Soreness After Eccentric Contraction-Induced Muscle Damage" Nutrients 17, no. 21: 3408. https://doi.org/10.3390/nu17213408
APA StyleLee, S.-R., Directo, D., Kang, Y., Stein, J., Calvert, M., An, Y. W., & Kim, D.-H. (2025). Effects of Acute Fish Oil Supplementation on Muscle Function and Soreness After Eccentric Contraction-Induced Muscle Damage. Nutrients, 17(21), 3408. https://doi.org/10.3390/nu17213408







