Iron Deficiency Prevention, Screening, and Treatment: A Quality Improvement Initiative Introducing Reticulocyte Hemoglobin in a Level III Neonatal Intensive Care Unit
Abstract
1. Introduction
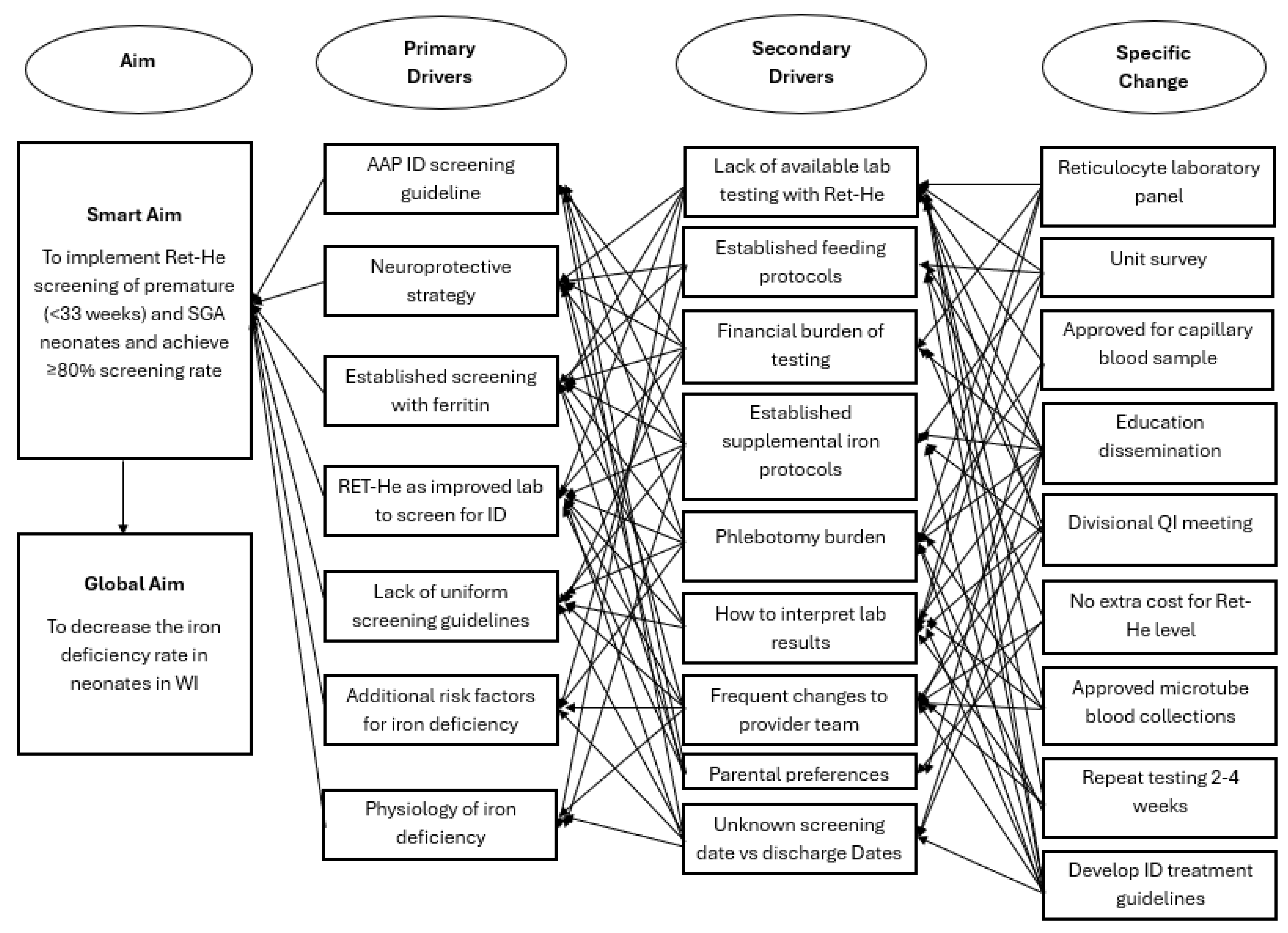
2. Methods
2.1. Context
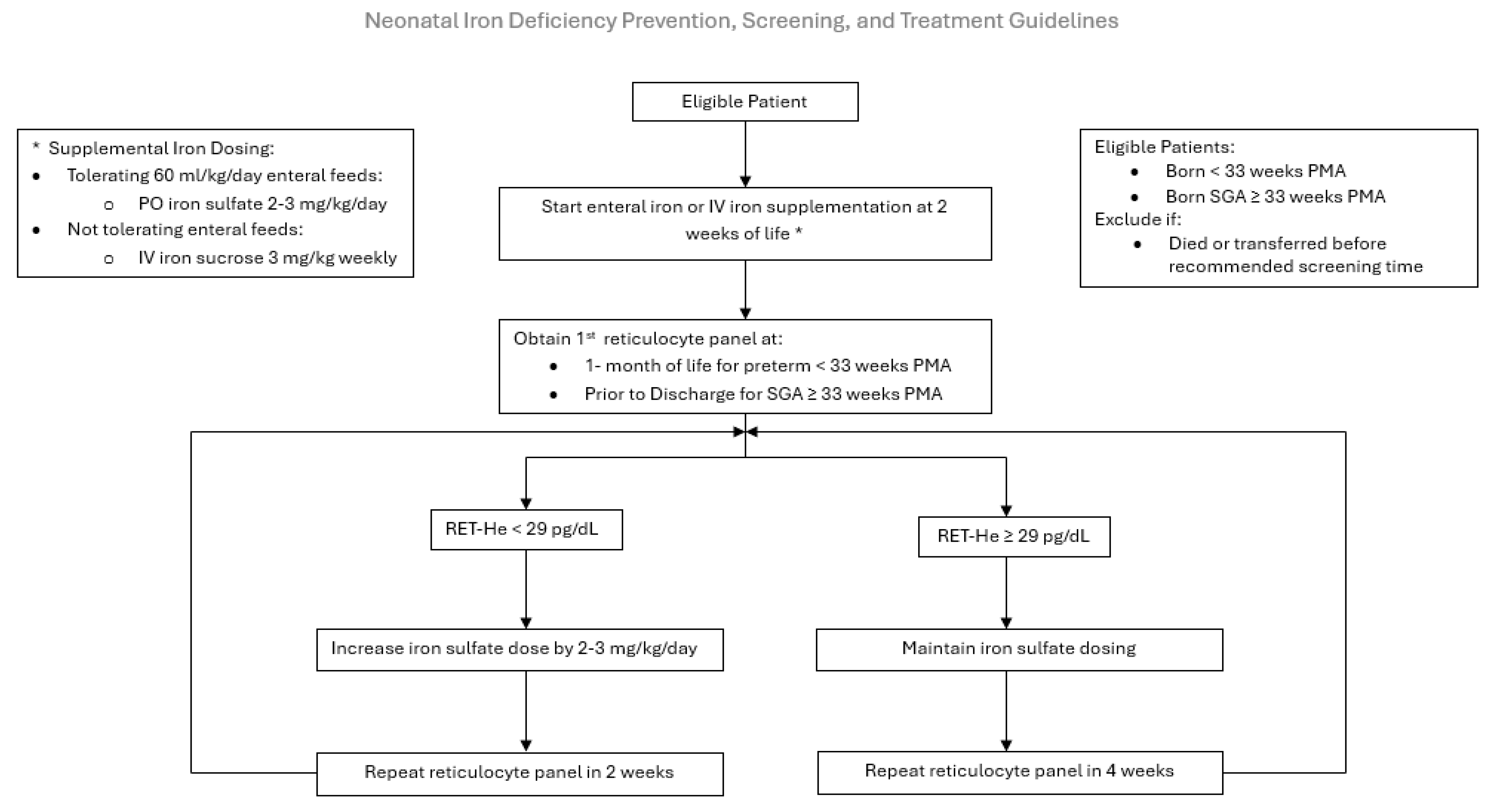
2.2. Inclusion and Exclusion Criteria
2.3. Intervention
2.4. Measures
2.5. Studying the Intervention
2.6. Analysis
3. Results
3.1. Demographics
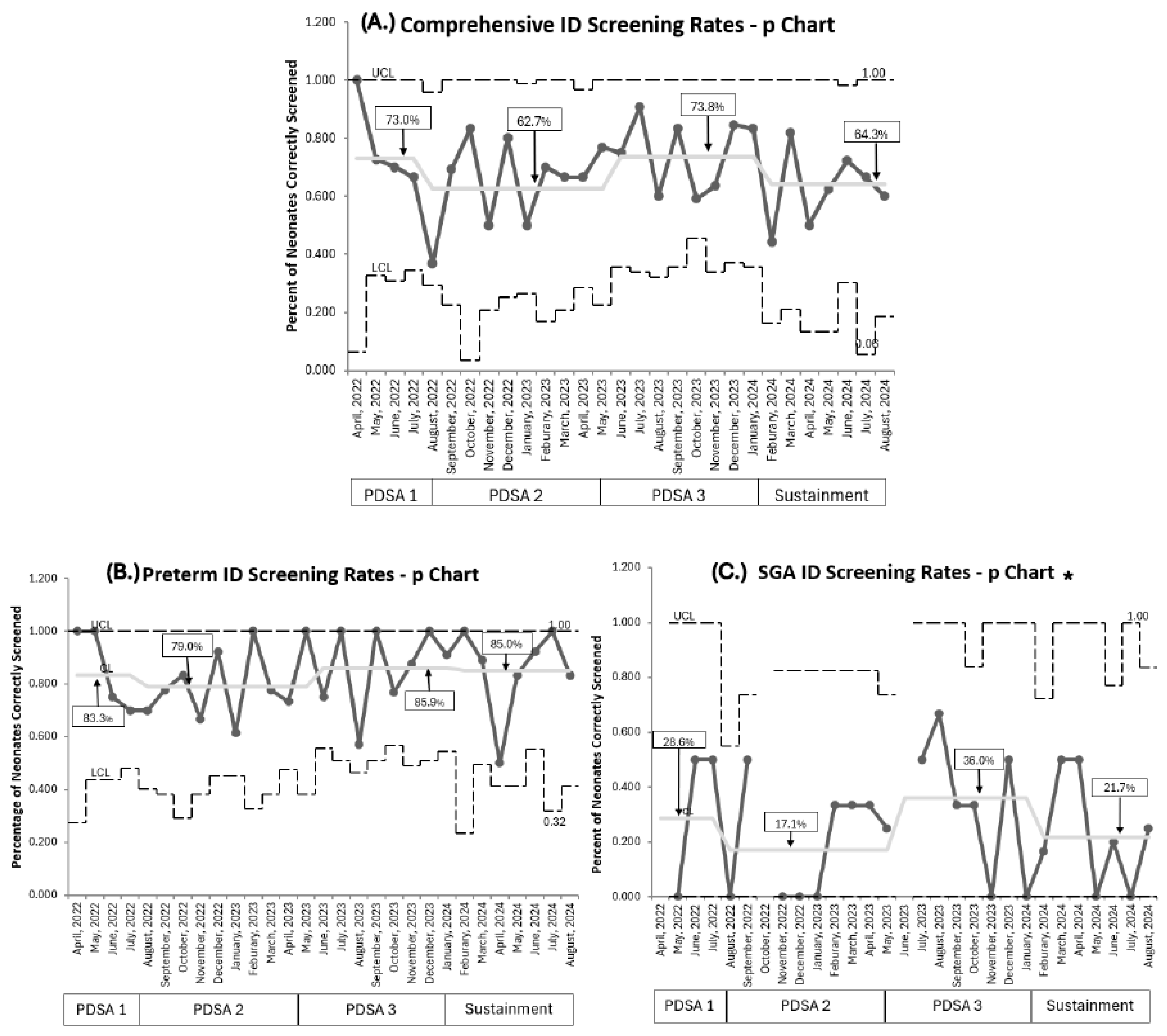
3.2. Primary Outcomes:
3.3. Process Measures
3.4. Balancing Measures
4. Discussion
4.1. Primary Outcome
4.2. Clinical Implications
4.3. High- Risk Populations
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgieff, M.K. Long-term Brain and Behavioral Consequences of Early Iron Deficiency. Nutr. Rev. 2011, 69 (Suppl. S1), S43–S48. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, T.; Wang, X.; Zhu, C. Iron Metabolism and Brain Development in Premature Infants. Front. Physiol. 2019, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; Zurakowski, D.; DiCanzio, J.; Boyd, T.; Platt, O. Reticulocyte Hemoglobin Content to Diagnose Iron Deficiency in Children. JAMA 1999, 281, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Karagülle, M.; Gündüz, E.; Şahin Mutlu, F.; Olga Akay, M. Clinical Significance of Reticulocyte Hemoglobin Content in the Diagnosis of Iron Deficiency Anemia. Turk J. Hematol. 2013, 30, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.B.; Lubach, G.R.; Ennis-Czerniak, K.M.; Lock, E.F.; Kling, P.J.; Georgieff, M.K.; Coe, C.L. Reticulocyte Hemoglobin Equivalent has Comparable Predictive Accuracy as Conventional Serum Iron Indices for Predicting Iron Deficiency and Anemia in a Nonhuman Primate model of Infantile Iron Deficiency. J. Nutr. 2023, 153, 148–157. [Google Scholar]
- Kling, P.J. Iron Nutrition, Erythrocytes, and Erythropoietin in the NICU: Erythropoietic and Neuroprotective Effects. NeoReviews 2020, 21, e80–e88. [Google Scholar] [CrossRef]
- Ennis, K.M.; Dahl, L.V.; Rao, R.B.; Georgieff, M.K. Reticulocyte hemoglobin content as an early predictive biomarker of brain iron deficiency. Pediatr. Res. 2018, 84, 765–769. [Google Scholar] [CrossRef]
- German, K.R.; Vu, P.T.; Neches, S.; Juul, S.E. Comparison of two markers of iron sufficiency and neurodevelopmental outcomes. Early Hum. Dev. 2021, 158, 105395. [Google Scholar] [CrossRef]
- Luciano, R.; Romeo, D.M.; Mancini, G.; Sivo, S.; Dolci, C.; Velli, C.; Colonna, A.T.; Vento, G.; Romagnoli, C.; Mercuri, E.M. Neurological development and iron supplementation in healthy late-preterm neonates: A randomized double-blind controlled trial. Eur. J. Pediatr. 2022, 181, 295–302. [Google Scholar] [CrossRef]
- Sriranjan, J.; Kalata, C.; Fusch, G.; Thomas, K.; Goswami, I. Prevalence and Implications of Low Reticulocyte–Hemoglobin Levels among Extreme Preterm Neonates: A Single-Center Retrospective Study. Nutrients 2022, 14, 5343. [Google Scholar]
- Lorenz, L.; Arand, J.; Büchner, K.; Wacker-Gussmann, A.; Peter, A.; Poets, C.F.; Franz, A.R. Reticulocyte haemoglobin content as a marker of iron deficiency. Arch. Dis. Child.-Fetal Neonatal Ed. 2014, 100, F198–F202. [Google Scholar] [PubMed]
- Parodi, E.; Giraudo, M.T.; Davitto, M.; Ansaldi, G.; Mondino, A.; Garbarini, L.; Franzil, A.; Mazzone, R.; Russo, G.; Ramenghi, U.; et al. Reticulocyte Parameters: Markers of Early Response to Oral Treatment in Children With Severe Iron-deficiency Anemia. J. Pediatr. Hematol. Oncol. 2012, 34, e249–e252. [Google Scholar] [PubMed]
- Armstrong, C. AAP Reports on Diagnosis and Prevention of Iron Deficiency Anemia. J. Pediatr. Hematol./Oncol. 2012, 34, e249–e252. [Google Scholar]
- Baker, R.D.; Greer, F.R.; Committee on Nutrition. Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics 2010, 126, 1040–1050. [Google Scholar] [CrossRef]
- Kennady, G.; Afridi, F.; Neumann, D.; Amendolia, B.; Kilic, N.; Bhat, V.; Bhandari, V.; Aghai, Z.H. Iron Deficiency Prior to Discharge in Very Low Birth Weight Infants: Screening with Reticulocyte Hemoglobin Content. Am. J. Perinatol. 2023, 41, 1560–1566. [Google Scholar] [CrossRef]
- Bahr, T.M.; Carr, N.R.; Christensen, T.R.; Wilkes, J.; O'BRien, E.A.; German, K.R.; Ohls, R.K.; Ward, D.M.; Christensen, R.D. Early iron supplementation and iron sufficiency at one month of age in NICU patients at-risk for iron deficiency. Blood Cells, Mol. Dis. 2021, 90, 102575. [Google Scholar] [CrossRef]
- Morton, S.U.; Yuen, J.C.M.; Feldman, H.A.; Hashim, E.M.; Rudie, C.R.; Lindamood, K.E.N.; Caughey, D.B.; Moline, M.; Sims, J.K.B.; Sola-Visner, M.C.; et al. Screening With Reticulocyte Hemoglobin Increased Iron Sufficiency Among NICU Patients. Pediatr. Qual. Saf. 2020, 5, e258. [Google Scholar] [CrossRef]
- Pomrop, M.; Manopunya, S.; Tantiprabha, W.; Khuwuthyakorn, V.; Kosarat, S.; Natesirinilkul, R. Reticulocyte hemoglobin concentration for screening iron deficiency in very low birth weight preterm neonates. J. Matern. Neonatal Med. 2022, 35, 3348–3352. [Google Scholar]
- Auerbach, M.; Brugnara, C.; Staffa, S. Measuring Reticulocyte Hemoglobin Content As a Marker for Iron Deficiency and Response to Therapy Represents a Paradigm Shift in Care. Blood 2020, 136 (Suppl. S1), 42–43. [Google Scholar] [CrossRef]
- Sandri, B.J.; Kim, J.; Lubach, G.R.; Lock, E.F.; Ennis-Czerniak, K.; Kling, P.J.; Georgieff, M.K.; Coe, C.L.; Rao, R.B. Prognostic Performance of Hematological and Serum Iron and Metabolite Indices for Detection of Early Iron Deficiency Induced Metabolic Brain Dysfunction in Infant Rhesus Monkeys. J. Nutr. 2024, 154, 875–885. [Google Scholar]
- Brichta, C.E.; Godwin, J.; Norlin, S.; Kling, P.J. Impact and interactions between risk factors on the iron status of at-risk neonates. J. Perinatol. 2022, 42, 1103–1109. [Google Scholar] [PubMed]
- Akkermans, M.D.; Uijterschout, L.; Abbink, M.; Vos, P.; Rövekamp-Abels, L.; Boersma, B.; van Goudoever, J.B.; Brus, F. Predictive factors of iron depletion in late preterm infants at the postnatal age of 6 weeks. Eur. J. Clin. Nutr. 2016, 70, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Langley, G.J.; Moen, R.D.; Nolan, K.M.; Nolan, T.W.; Norman, C.L.; Provost, L.P. The Improvement Guide, 2nd ed.; Jossey Bass Wiley: San Francisco, CA, USA, 2009. [Google Scholar]
- The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement|IHI-Institute for Healthcare Improvement. Available online: https://www.ihi.org:443/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreakthroughImprovement.aspx (accessed on 4 August 2022).
- Brugnara, C.; Schiller, B.; Moran, J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin. Lab. Haematol. 2006, 28, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Widness, J.A. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. NeoReviews 2008, 9, e520. [Google Scholar] [CrossRef]
- Flannery, D.D.; Edwards, E.M.; Coggins, S.A.; Horbar, J.D.; Puopolo, K.M. Late-Onset Sepsis Among Very Preterm Infants. Pediatrics 2022, 150, e2022058813. [Google Scholar] [CrossRef]
- Ginglen, J.G.; Butki, N. Necrotizing Enterocolitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK513357/ (accessed on 25 August 2025).
- Juul, S.; German, K. Iron supplementation for infants in the NICU: What preparation, how much, and how long is optimal? Semin. Fetal Neonatal Med. 2025, 30, 101612. [Google Scholar] [CrossRef]
- Provost, L. The Health Care Data Guide: Learning From Data for Improvement; JosseyBass: San Fransisco, CA, USA, 2011. [Google Scholar]
- Control Charts on Patient Satisfaction Survey Results. Available online: https://www.qimacros.com/free-excel-tips/patient-satisfaction-control-charts/ (accessed on 17 October 2025).
- Zamora, T.G.; Guiang, S.F.; A Widness, J.; Georgieff, M.K. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr. Res. 2016, 79, 922–928. [Google Scholar] [CrossRef]
- Steinmacher, J.; Pohlandt, F.; Bode, H.; Sander, S.; Kron, M.; Franz, A.R. Randomized Trial of Early Versus Late Enteral Iron Supplementation in Infants With a Birth Weight of Less Than 1301 Grams: Neurocognitive Development at 5.3 Years’ Corrected Age. Pediatrics 2007, 120, 538–546. [Google Scholar] [CrossRef]
- Christensen, R.D.; Henry, E.; Bennett, S.T.; Yaish, H.M. Reference intervals for reticulocyte parameters of infants during their first 90 days after birth. J. Perinatol. 2016, 36, 61–66. [Google Scholar]
- McCarthy, P.J.; Zundel, H.R.; Johnson, K.R.; Blohowiak, S.E.; Kling, P.J. Impact of Growth Restriction and Other Prenatal Risk Factors on Cord Blood Iron Status in Prematurity. J. Pediatr. Hematol. 2016, 38, 210. [Google Scholar] [CrossRef]
- Erythrocyte Zinc Protoporphyrin Is Elevated With Prematurity and Fetal Hypoxemia | Pediatrics | American Academy of Pediatrics. Available online: https://publications.aap.org/pediatrics/article-abstract/116/2/414/62897/Erythrocyte-Zinc-Protoporphyrin-Is-Elevated-With?redirectedFrom=fulltext (accessed on 10 September 2025).
| A. Maternal Factors | Baseline Demographics N = 257 | QI Initiative Comprehensive Demographics N = 345 | p Value |
| Maternal Minority | 59 (23.1%) | 108 (31.3) | p = 0.02 |
| Maternal Diabetes | 36 (13.9%) | 55 (15.9%) | 0.51 |
| Hypertension/Preeclampsia | 86 (33.3%) | 138 (40%) | 0.1 |
| B. Fetal Factors | Baseline Demographics N = 257 | QI Initiative comprehensive demographics N = 345 | p value |
| Premature < 33 weeks PMA | 227 (88.3%) | 254 (73.6%) | p < 0.01 |
| Gestational Age at Birth | 31.5 ± 2 | 31.5 ± 3.8 | 1.0 |
| Birthweight | 1501 g ± 515 | ||
| Birth Weight Z-Score (mean) | −0.21 | −0.6 | p < 0.01 |
| Sex Male % | 136 (53.1%) | 185 (53.6%) | 0.87 |
| Small for Gestation ≥ 33 weeks PMA | 30 (11.7%) | 91 (26.4%) | p < 0.01 |
| Multifetal Gestation | 75 (29.2%) | 111 (32.2%) | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javadova, N.; Kling, P.J.; Norlin, S.; Hulse, W.N. Iron Deficiency Prevention, Screening, and Treatment: A Quality Improvement Initiative Introducing Reticulocyte Hemoglobin in a Level III Neonatal Intensive Care Unit. Nutrients 2025, 17, 3391. https://doi.org/10.3390/nu17213391
Javadova N, Kling PJ, Norlin S, Hulse WN. Iron Deficiency Prevention, Screening, and Treatment: A Quality Improvement Initiative Introducing Reticulocyte Hemoglobin in a Level III Neonatal Intensive Care Unit. Nutrients. 2025; 17(21):3391. https://doi.org/10.3390/nu17213391
Chicago/Turabian StyleJavadova, Narmin, Pamela J. Kling, Sally Norlin, and Whitley N. Hulse. 2025. "Iron Deficiency Prevention, Screening, and Treatment: A Quality Improvement Initiative Introducing Reticulocyte Hemoglobin in a Level III Neonatal Intensive Care Unit" Nutrients 17, no. 21: 3391. https://doi.org/10.3390/nu17213391
APA StyleJavadova, N., Kling, P. J., Norlin, S., & Hulse, W. N. (2025). Iron Deficiency Prevention, Screening, and Treatment: A Quality Improvement Initiative Introducing Reticulocyte Hemoglobin in a Level III Neonatal Intensive Care Unit. Nutrients, 17(21), 3391. https://doi.org/10.3390/nu17213391






