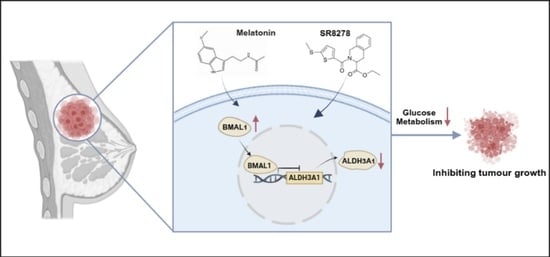
Natural Compound Melatonin Suppresses Breast Cancer Development by Regulating Circadian Rhythm
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Data Collection and Analysis
2.3. Nucleic Acid Transfection
2.4. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
2.5. Western Blot and Immunoprecipitation (IP) Assays
2.6. Cell Viability and Colony Formation Assays
2.7. 5-Ethynyl-2′-Deoxyuridine (EdU) Incorporation Assay
2.8. Chromatin Immunoprecipitation (ChIP)-qPCR
2.9. Immunohistochemical Staining
2.10. Sphere-Formation Assay
2.11. Flow Cytometry
2.12. Metabolic Assay
2.13. Dual Luciferase Reporter Gene Assay
2.14. Animal Experiment
2.15. Statistical Analysis
3. Results
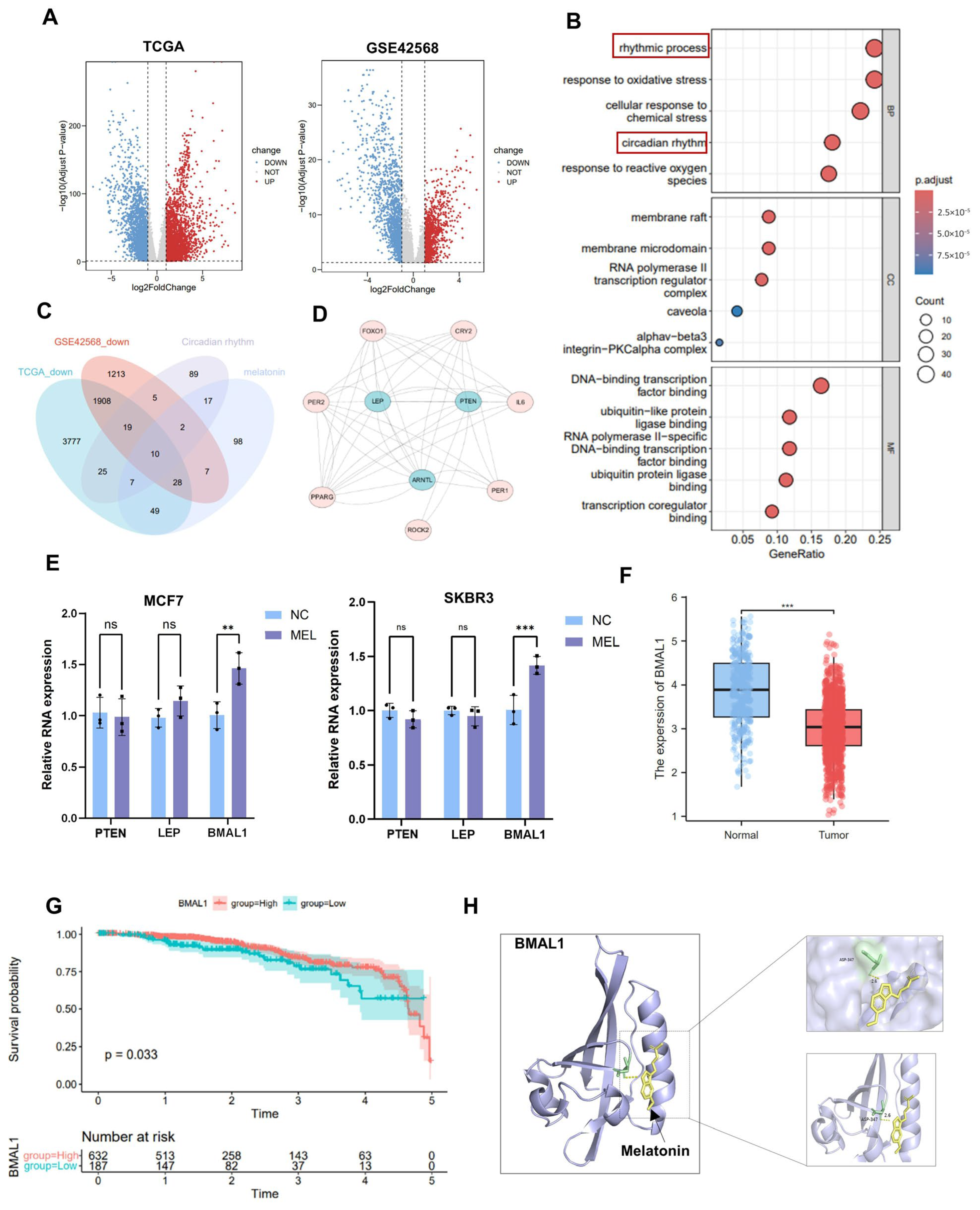
3.1. Melatonin Inhibits Cell Proliferation
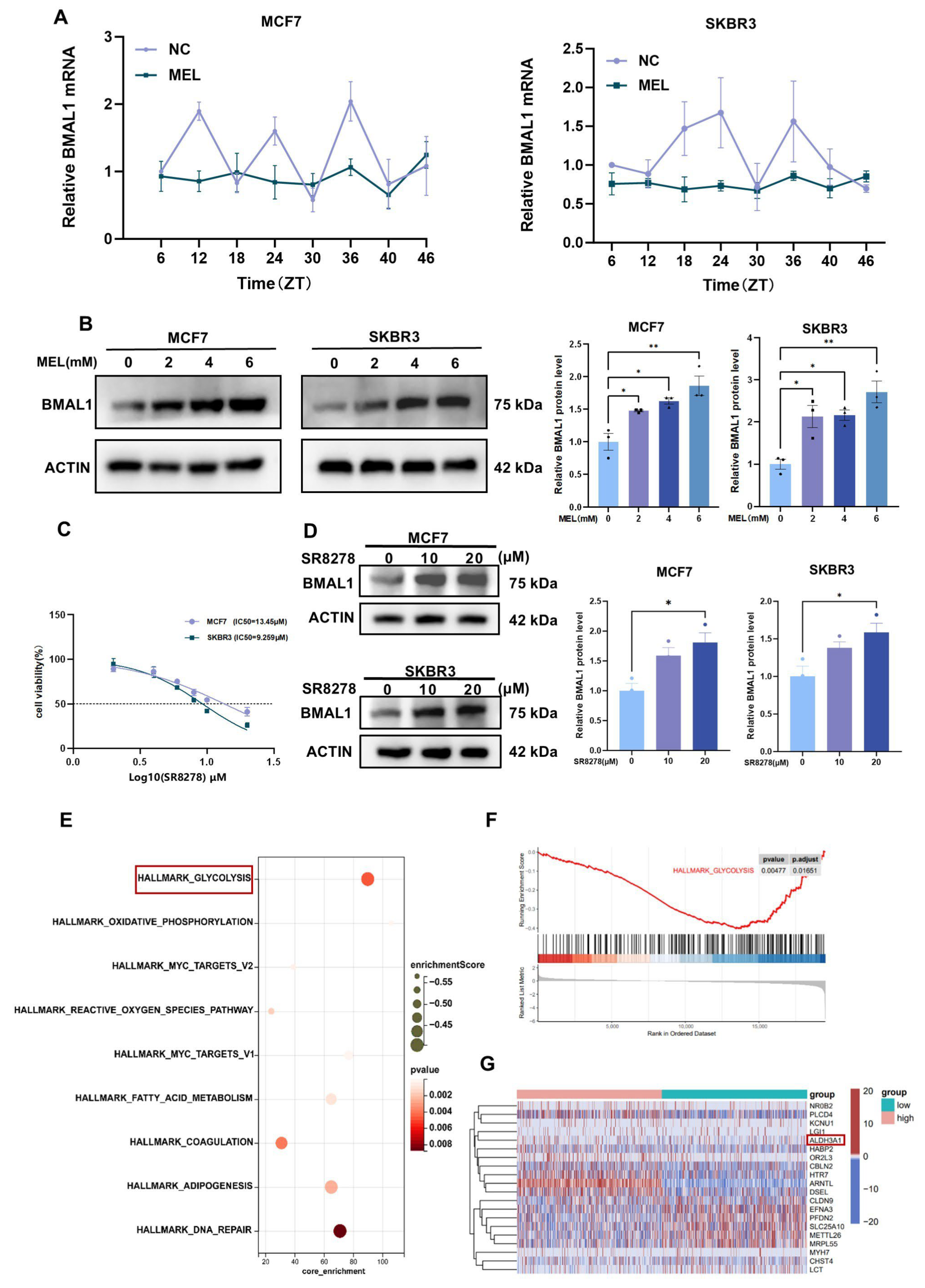
3.2. Melatonin Inhibits Tumor Cell Growth by Regulating BMAL1
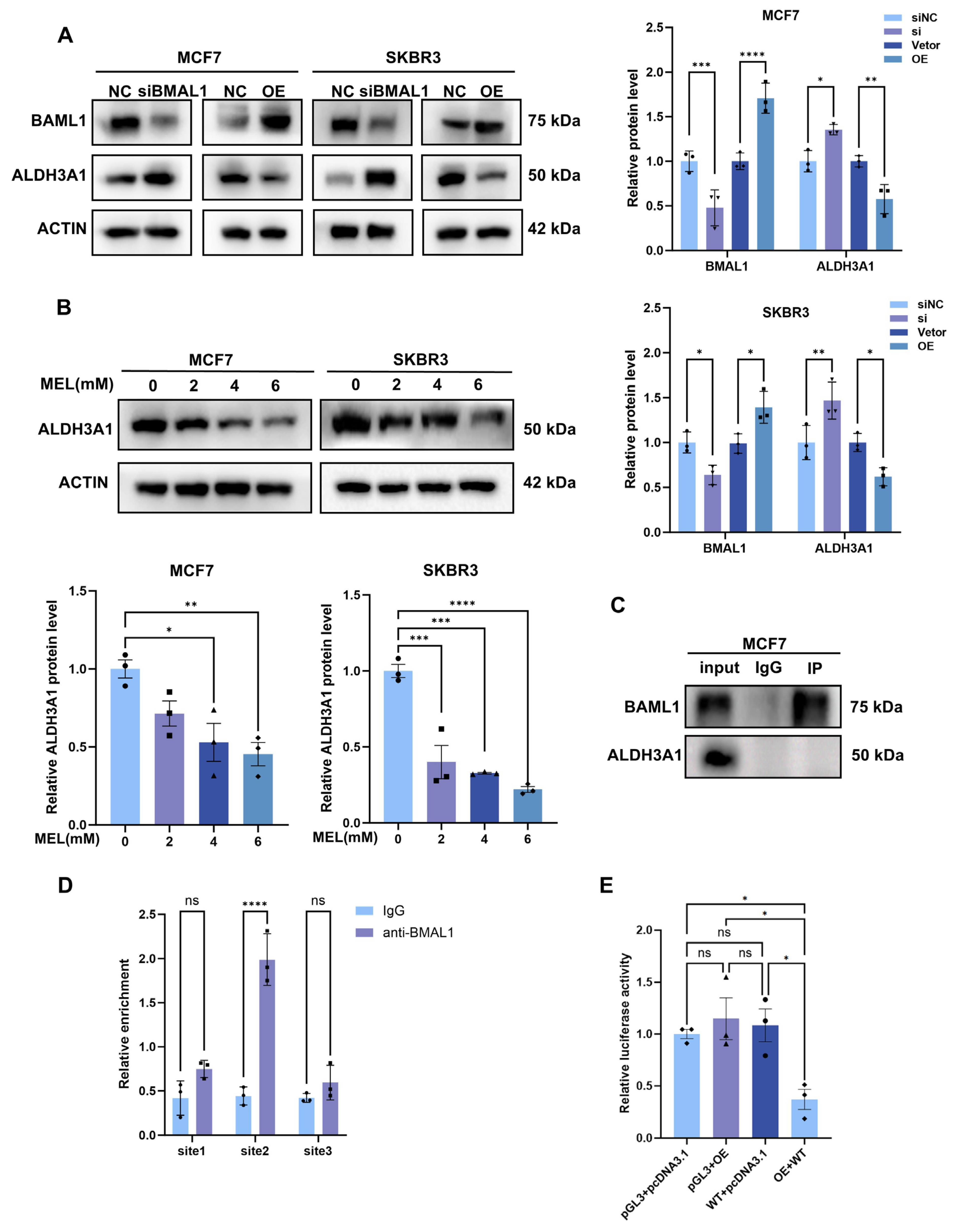
3.3. Melatonin Regulates BMAL1 to Affect Glycolysis via ALDH3A1 in BC Cells
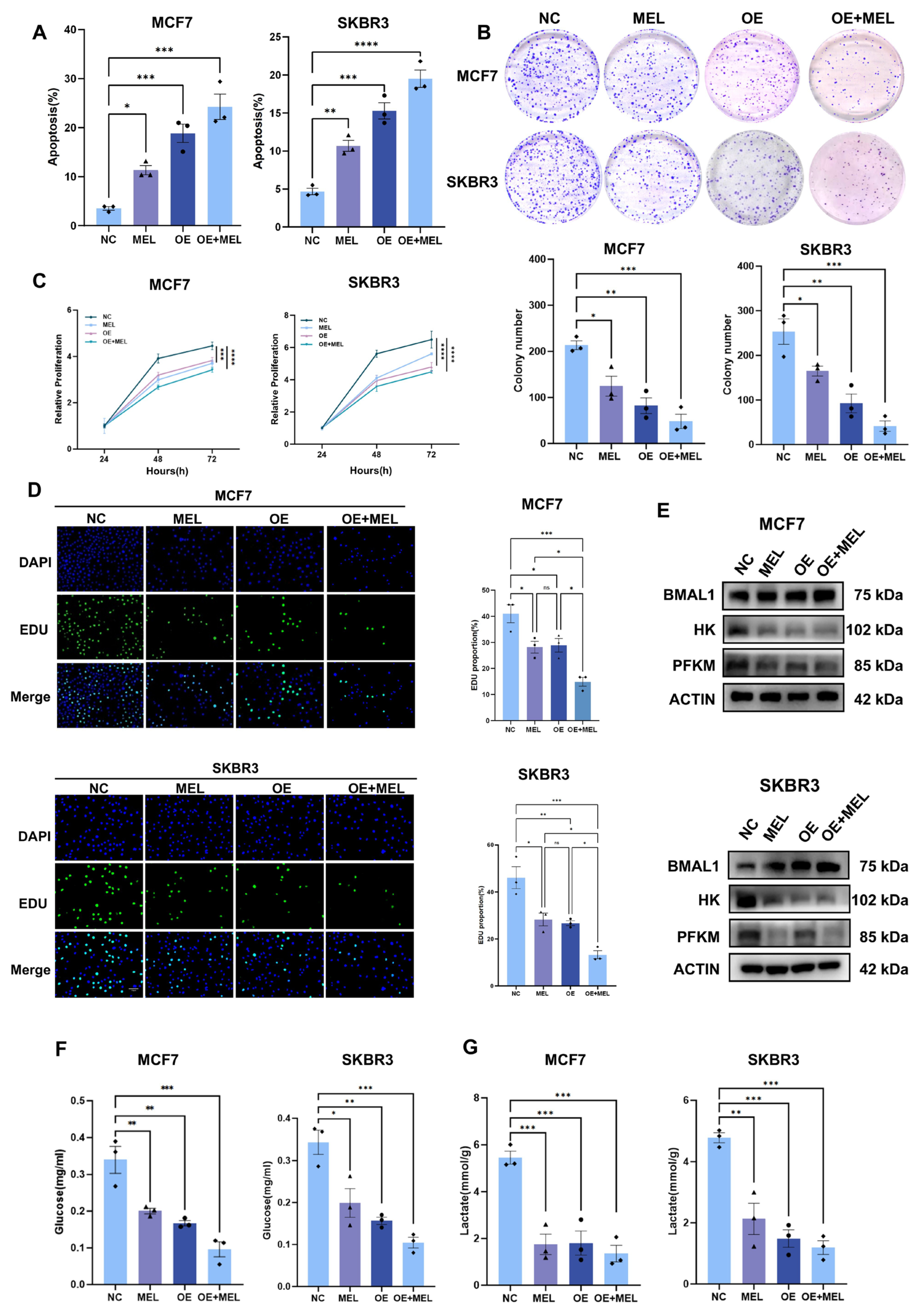
3.4. BMAL1 Inhibits Tumor Growth with Melatonin Potentiating the Effect in BC Cells
3.5. Silencing of BMAL1 Partially Rescues Melatonin-Mediated Attenuation of BC Cell Proliferation and Glycolysis
3.6. BMAL1 Transcriptionally Inhibits ALDH3A1 Expression in BC Cells
3.7. Melatonin and SR8278 Collaboratively Suppress Tumor Growth In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| MEL | Melatonin |
| BMAL1 | Brain and Muscle ARNT-Like 1 |
| CLOCK | Circadian Locomotor Output Cycles Kaput |
| PER | Period |
| CRY | Cryptochrome |
| RORA | RAR-Related Orphan Receptor A |
| NR1D1/2 | Nuclear Receptor Subfamily 1 Group D Member 1/2 |
| SIRT1 | Sirtuin 1 |
| GPAM | Glycerol-3-Phosphate Acyltransferase Mitochondrial |
| EZH2 | Enhancer of Zeste Homolog 2 |
| ALDH3A1 | Aldehyde Dehydrogenase 3 Family Member A1 |
References
- Yan, Q.; Deng, Y.; Zhang, Q. A comprehensive overview of metaplastic breast cancer: Features and treatments. Cancer Sci. 2024, 115, 2506–2514. [Google Scholar] [CrossRef]
- García-Sancha, N.; Corchado-Cobos, R.; Pérez-Losada, J. Understanding Susceptibility to Breast Cancer: From Risk Factors to Prevention Strategies. Int. J. Mol. Sci. 2025, 26, 2993. [Google Scholar] [CrossRef]
- Moura, T.; Laranjeira, P.; Caramelo, O.; Gil, A.M.; Paiva, A. Breast Cancer and Tumor Microenvironment: The Crucial Role of Immune Cells. Curr. Oncol. 2025, 32, 143. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Wichert, K.; Hoppe, R.; Ickstadt, K.; Behrens, T.; Winter, S.; Herold, R.; Terschüren, C.; Lo, W.Y.; Guénel, P.; Truong, T.; et al. Polymorphisms in genes of melatonin biosynthesis and signaling support the light-at-night hypothesis for breast cancer. Eur. J. Epidemiol. 2023, 38, 1053–1068. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N.; et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, A.I.; Ionuţ, R.; Socaciu, M.A.; Ungur, A.P.; Bârsan, M.; Chiorean, A.; Socaciu, C.; Râjnoveanu, A.G. Melatonin, an ubiquitous metabolic regulator: Functions, mechanisms and effects on circadian disruption and degenerative diseases. Rev. Endocr. Metab. Disord. 2020, 21, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Zhou, N.; Wei, Z.X.; Qi, Z.X. Inhibition of autophagy triggers melatonin-induced apoptosis in glioblastoma cells. BMC Neurosci. 2019, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Xiong, Z.; Xiong, W.; Yuan, C.; Xiao, H.; Ruan, H.; Song, Z.; Wang, C.; Bao, L.; Cao, Q.; et al. Melatonin/PGC1A/UCP1 promotes tumor slimming and represses tumor progression by initiating autophagy and lipid browning. J. Pineal Res. 2019, 67, e12607. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, X.; Jin, Z.; Wang, H.; Yang, S.F.; Hu, S. Melatonin modulates metabolic remodeling in HNSCC by suppressing MTHFD1L-formate axis. J. Pineal Res. 2021, 71, e12767. [Google Scholar] [CrossRef]
- Shafi, A.A.; Knudsen, K.E. Cancer and the Circadian Clock. Cancer Res. 2019, 79, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Liang, L.; Deng, W.; Xie, M.; Zhao, M.; Wang, S.; Liu, J.; Yang, M. BMAL1 plays a crucial role in immune homeostasis during sepsis-induced acute lung injury. Biochem. Pharmacol. 2024, 226, 116379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Q.; Liu, R.; Zhang, D.; Hu, G.; Chen, X. Circadian disruption in cancer and regulation of cancer stem cells by circadian clock genes: An updated review. Cancer Lett. 2024, 611, 217391. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, Z.; Ye, L.; Wu, R.; Wang, J.; Yu, Q.X.; Wusiman, D.; Tuo, Z.; Yoo, K.H.; Shu, Z.; et al. Basic helix-loop-helix ARNT like 1 regulates the function of immune cells and participates in the development of immune-related diseases. Burns Trauma 2025, 13, tkae075. [Google Scholar] [CrossRef]
- Albaqami, A. Unravelling the link between circadian clock genes and brain tumors: From pathological disruptions to potential therapeutic interventions. Front. Pharmacol. 2025, 16, 1617713. [Google Scholar] [CrossRef]
- Qu, M.; Zhang, G.; Qu, H.; Vu, A.; Wu, R.; Tsukamoto, H.; Jia, Z.; Huang, W.; Lenz, H.J.; Rich, J.N.; et al. Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc. Natl. Acad. Sci. USA 2023, 120, e2214829120. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, S.; Jiang, X.; Zhang, E.; Hu, G.; Hu, B.; Zheng, P.; Xiao, J.; Lu, Z.; Lu, Y.; et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016, 371, 314–325. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, T.; Zhao, Z.; Zhang, H.; Yuan, P.; Wang, G.; Zhao, Z.; An, J.; Lyu, Z.; Xing, J.; et al. Down-Regulation of BMAL1 by MiR-494-3p Promotes Hepatocellular Carcinoma Growth and Metastasis by Increasing GPAM-Mediated Lipid Biosynthesis. Int. J. Biol. Sci. 2022, 18, 6129–6144. [Google Scholar] [CrossRef]
- Schütze, F.; Röhrig, F.; Vorlová, S.; Gätzner, S.; Kuhn, A.; Ergün, S.; Henke, E. Inhibition of Lysyl Oxidases Improves Drug Diffusion and Increases Efficacy of Cytotoxic Treatment in 3D Tumor Models. Sci. Rep. 2015, 5, 17576. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.E.; Griffin, P.; Sheehan, P.W.; Kim, D.H.; Musiek, E.S.; Yoon, S.Y. Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13078. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.H.; Lee, S.; Kang, J.H.; Lee, S.H.; Cheong, J.H.; Kim, S.Y. Gastric cancer depends on aldehyde dehydrogenase 3A1 for fatty acid oxidation. Sci. Rep. 2019, 9, 16313. [Google Scholar] [CrossRef] [PubMed]
- Counihan, J.L.; Wiggenhorn, A.L.; Anderson, K.E.; Nomura, D.K. Chemoproteomics-Enabled Covalent Ligand Screening Reveals ALDH3A1 as a Lung Cancer Therapy Target. ACS Chem. Biol. 2018, 13, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795–1803. [Google Scholar] [CrossRef]
- Verlande, A.; Masri, S. Circadian Clocks and Cancer: Timekeeping Governs Cellular Metabolism. Trends Endocrinol. Metab. 2019, 30, 445–458. [Google Scholar] [CrossRef]
- Li, D.D.; Zhou, T.; Gao, J.; Wu, G.L.; Yang, G.R. Circadian rhythms and breast cancer: From molecular level to therapeutic advancements. J. Cancer Res. Clin. Oncol. 2024, 150, 419. [Google Scholar] [CrossRef]
- Bevinakoppamath, S.; Ramachandra, S.C.; Yadav, A.K.; Basavaraj, V.; Vishwanath, P.; Prashant, A. Understanding the Emerging Link Between Circadian Rhythm, Nrf2 Pathway, and Breast Cancer to Overcome Drug Resistance. Front. Pharmacol. 2022, 12, 719631. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S. Melatonin: A Potential Antineoplastic Agent in Breast Cancer. J. Environ. Pathol. Toxicol. Oncol. 2022, 41, 55–84. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Xiang, S.; Mao, L.; Brimer, S.; Wren, M.A.; Yuan, L.; Anbalagan, M.; Hauch, A.; Frasch, T.; Rowan, B.G.; et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014, 74, 4099–4110. [Google Scholar] [CrossRef]
- Sabzichi, M.; Samadi, N.; Mohammadian, J.; Hamishehkar, H.; Akbarzadeh, M.; Molavi, O. Sustained release of melatonin: A novel approach in elevating efficacy of tamoxifen in breast cancer treatment. Colloids Surf. B Biointerfaces 2016, 145, 64–71. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, X.; Zhang, Y.; Shi, D.; Chen, W.; Fu, L.; Liu, L.; Xie, F.; Kang, T.; Huang, W.; et al. Simultaneous modulation of COX-2, p300, Akt, and Apaf-1 signaling by melatonin to inhibit proliferation and induce apoptosis in breast cancer cells. J. Pineal Res. 2012, 53, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Peng, F.; Zhu, L.; Li, X.; Ou, S.; Huang, Z.; Wu, S.; Peng, C.; Liu, P.; Kong, Y. Melatonin inhibits triple-negative breast cancer progression through the Lnc049808-FUNDC1 pathway. Cell Death Dis. 2021, 12, 712. [Google Scholar] [CrossRef]
- Li, M.; Hao, B.; Zhang, M.; Reiter, R.J.; Lin, S.; Zheng, T.; Chen, X.; Ren, Y.; Yue, L.; Abay, B.; et al. Melatonin enhances radiofrequency-induced NK antitumor immunity, causing cancer metabolism reprogramming and inhibition of multiple pulmonary tumor development. Signal Transduct. Target. Ther. 2021, 6, 330. [Google Scholar] [CrossRef]
- Innominato, P.F.; Lim, A.S.; Palesh, O.; Clemons, M.; Trudeau, M.; Eisen, A.; Wang, C.; Kiss, A.; Pritchard, K.I.; Bjarnason, G.A. The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Support. Care Cancer 2016, 24, 1097–1105. [Google Scholar] [CrossRef]
- Kim, J.I.; Cheon, H.G. Melatonin ameliorates hepatic fibrosis via the melatonin receptor 2-mediated upregulation of BMAL1 and anti-oxidative enzymes. Eur. J. Pharmacol. 2024, 966, 176337. [Google Scholar] [CrossRef]
- Yu, S.; Tang, Q.; Chen, G.; Lu, X.; Yin, Y.; Xie, M.; Long, Y.; Zheng, W.; Guo, F.; Shao, L.; et al. Circadian rhythm modulates endochondral bone formation via MTR1/AMPKβ1/BMAL1 signaling axis. Cell Death Differ. 2022, 29, 874–887. [Google Scholar] [CrossRef]
- Kojetin, D.; Wang, Y.; Kamenecka, T.M.; Burris, T.P. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem. Biol. 2011, 6, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Terzuoli, E.; Bellan, C.; Aversa, S.; Ciccone, V.; Morbidelli, L.; Giachetti, A.; Donnini, S.; Ziche, M. ALDH3A1 Overexpression in Melanoma and Lung Tumors Drives Cancer Stem Cell Expansion, Impairing Immune Surveillance through Enhanced PD-L1 Output. Cancers 2019, 11, 1963. [Google Scholar] [CrossRef]
- Qu, Y.; He, Y.; Yang, Y.; Li, S.; An, W.; Li, Z.; Wang, X.; Han, Z.; Qin, L. ALDH3A1 acts as a prognostic biomarker and inhibits the epithelial mesenchymal transition of oral squamous cell carcinoma through IL-6/STAT3 signaling pathway. J. Cancer 2020, 11, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Sun, L.; Zhou, B.; Wang, A. Identification and validation of a metabolism-related model and associated with tumor-infiltrating lymphocytes in p53 mutant lung adenocarcinoma patients. Ann. Transl. Med. 2021, 9, 1312. [Google Scholar] [CrossRef]
- Lai, Y.W.; Liu, Z.W.; Lin, M.H.; Yang, C.C.; Chu, C.Y.; Chung, C.H.; Lin, C.W. Melatonin increases Olaparib sensitivity and suppresses cancer-associated fibroblast infiltration via suppressing the LAMB3-CXCL2 axis in TNBC. Pharmacol. Res. 2024, 209, 107429. [Google Scholar] [CrossRef] [PubMed]
- Jardin, I.; Diez-Bello, R.; Falcon, D.; Alvarado, S.; Regodon, S.; Salido, G.M.; Smani, T.; Rosado, J.A. Melatonin downregulates TRPC6, impairing store-operated calcium entry in triple-negative breast cancer cells. J. Biol. Chem. 2021, 296, 100254. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Hu, C.; Hu, F.; Sun, Y.; Guo, L.; Ren, J.; Han, C.; Li, Y.; Zhang, X.; Sun, Y.; et al. Natural Compound Melatonin Suppresses Breast Cancer Development by Regulating Circadian Rhythm. Nutrients 2025, 17, 3386. https://doi.org/10.3390/nu17213386
He Y, Hu C, Hu F, Sun Y, Guo L, Ren J, Han C, Li Y, Zhang X, Sun Y, et al. Natural Compound Melatonin Suppresses Breast Cancer Development by Regulating Circadian Rhythm. Nutrients. 2025; 17(21):3386. https://doi.org/10.3390/nu17213386
Chicago/Turabian StyleHe, Yuanli, Chenchen Hu, Feiming Hu, Yuanjie Sun, Lin Guo, Junyi Ren, Chenying Han, Yuhui Li, Xiyang Zhang, Yubo Sun, and et al. 2025. "Natural Compound Melatonin Suppresses Breast Cancer Development by Regulating Circadian Rhythm" Nutrients 17, no. 21: 3386. https://doi.org/10.3390/nu17213386
APA StyleHe, Y., Hu, C., Hu, F., Sun, Y., Guo, L., Ren, J., Han, C., Li, Y., Zhang, X., Sun, Y., Zhang, J., Cai, S., Wang, Y., Jiang, D., Yang, K., & Yang, S. (2025). Natural Compound Melatonin Suppresses Breast Cancer Development by Regulating Circadian Rhythm. Nutrients, 17(21), 3386. https://doi.org/10.3390/nu17213386