Abstract
The oral cavity contains a diverse group of bacteria in the saliva, as well as structured aggregates of bacterial cells on the mucosal surfaces. Oral microbiota (OM) dysbiosis not only induces local inflammation, it can also trigger systemic inflammation leading to metabolic diseases and neuropsychiatric diseases (NPDs). While primary evidence indicates that oral microbiota dysbiosis induces gut microbiota aberrations, which exacerbate inflammation associated with metabolic diseases (obesity, dyslipidemia, diabetes, nonalcoholic fatty liver disease (NAFLD), and insulin resistance), other studies revealed the contribution of the oral microbiota–brain axis in the pathogenesis of NPDs. GM dysbiosis and inflammation also induce epigenetic alterations in cytokine genes, such as IL-1β, IL-6, TNF-α, NF-kB, BTLA, IL-18R1, TGF-β, P13k/Akt1, Ctnnb1, and Hsp90aa1, as well as DNMTs, HDACs, and DAT1 associated with the development and progression of metabolic disorders and/or NPDs. Therefore, the epigenome could serve as a target for preventive or therapeutic interventions. Here, we (i) review emerging evidence of the potential impact of OM dysbiosis in the pathogenesis of metabolic diseases and NPDs, (ii) highlight the relationship between OM-induced inflammation and epigenetic alterations driving NPDs pathogenesis and interlinked metabolic aberrations, (iii) discuss therapeutic approaches capable of treating metabolic diseases and NPDs through reshaping the microbiota and its epigenetic metabolites, and hence mitigating epigenetic aberrations linked to metabolic diseases and NPDs. Finally, we outline challenges and current research gaps related to investigating the relationship between microbiota, epigenetic aberrations, and metabolic abnormalities associated with NPDs.
1. Introduction
Metabolic disorders and neuropsychiatric disorders (NPDs) are wide-ranging classes of diseases, each with a complex nature and high degree of genetic heterogeneity that negatively influence various body organs, the nervous system, and mental health conditions like mood, thinking, cognition, and behavior [1,2,3,4]. Common NPDs include several categories of disorders, such as neurodevelopmental (e.g., autism spectrum disorders (ASDs)), neurodegenerative (e.g., Parkinson’s disease (PD) and Alzheimer’s disease (AD)), and psychiatric disorders (e.g., bipolar disorder (BD) and schizophrenia (SCZ)) [5,6]. Metabolic disorders are conditions that influence any aspect of metabolism and are associated with energy balance dysregulation [7]. Examples of metabolic disorders are obesity, dyslipidemia, diabetes, metabolic syndrome, nonalcoholic fatty liver disease (NAFLD), and insulin resistance [8]. Notably, as will be discussed in this work, most NPDs are associated with metabolic diseases. Therefore, NPDs and metabolic diseases are interlinked and may, at least in part, originate from a common underlying functional aberration [9,10].
Epigenetic alterations have been found to be one of the key players in the pathogenesis of both metabolic diseases and NPDs by defining how environmental factors such as infections, malnutrition, and stress could interplay with genes to affect the function of different organs and contribute to the development of various diseases [11,12,13]. Abnormalities in epigenetic modifications in DNA methylation, histone modifications, and non-coding RNAs (ncRNAs) contribute to disease susceptibility by influencing gene expression without changing the DNA sequence itself [14]. Interestingly, while epigenetic modifications influence cellular metabolism, the metabolic state of cells can also induce epigenetic changes that, in turn, affect cellular functions, including those of brain cells, and thereby impact mental health [15,16]. Multiple lines of experimental evidence have suggested an interesting link between NPDs and metabolic dysregulation, which may cause epigenetic modifications [17,18]. For example, AD is considered type 3 diabetes [19]. Subjects with BD and SCZ also exhibit an elevated risk for metabolic disturbances, OB, and metabolic syndrome, which may be associated with epigenetic aberrations [16,20]. Approximately 37% of subjects with BD demonstrate symptoms of metabolic syndrome, which contribute to worsening of the course of BD, leading to both poor treatment response and disability [21]. Some of the metabolic pathways contribute to one-carbon metabolism (involving folic acid, betaine, choline, methionine, and vitamins B6 and B12), which determines DNA and histone methylation levels, playing key roles in brain function [22]. Derangements in the functionality of responsible metabolic pathways may contribute to the development of metabolic diseases and NPDs due to, or associated with, epigenetic aberrations [23]. For example, Frajerman et al. examined the prevalence of folate and vitamin B12 deficiency and hyperhomocysteinemia in subjects with psychotic disorders ranging in age from 15 to 30 years [24]. Their findings showed deficiencies in folate, vitamin B12 (key products of some bacteria of gastrointestinal (GI) tracts), and hyperhomocysteinemia (one of the consequences of folate and vitamin B12 deficiency) in 38% of subjects with first-episode psychosis, 27% of schizophrenic subjects, and 36% of patients with schizoaffective disorders [24]. Therefore, while extensive epigenetic aberrations are reported in different NPDs [25,26], metabolic dysregulations due to an imbalanced nutritional conformation and/or oral and gut bacteria composition affecting the body reserve of these key vitamins may be linked to the development or worsening of NPDs through epigenetic mechanisms [27].
The mouth is the main artery for the entry of environmental microbiota to the GI tract. The oral cavity is a complex environment, and its microorganisms inhabit several distinctive niches such as the gingival sulcus, the tongue, saliva, teeth, the hard and soft palates, the cheek, the floor of the mouth, and the throat [28,29]. Experimental evidence indicates that while oral microbiota (OM) is affected by metabolic diseases such as type 2 diabetes (T2DM) [30], it can affect the brain and mental health through the oral–brain axis. This axis is bidirectional and mediates a complex interplay between oral microbes, the metabolic, immune, and nervous systems, and influences the function of neuronal cells through the activity of specific microorganisms [31]. In pathological conditions such as periodontitis, imbalance in the OM composition causes dysbiosis, which contributes to systemic inflammation and the release of specific cytokines and neurotoxins, contributing to the development of metabolic diseases and NPDs [32,33,34]. Oral bacteria are capable of producing different metabolites that affect brain function by passing the blood–brain barrier. Moreover, pathogenic microbes in the oral cavity are capable of entering the bloodstream, initiating inflammatory responses, and even translocating to the brain via the trigeminal nerve or olfactory system [27]. Abnormal alterations in the composition and diversity of OM are also associated with an increased risk and severity of metabolic diseases and NPDs. For example, increase in the abundance of oral pathogens, like Porphyromonas gingivalis (P. gingivalis), in combination with virulence factors like lipopolysaccharides (LPS) and gingipains cause neuroinflammation, which in turn leads to cognitive decline [35]. On the other hand, Alex et al. found that women with recent excessive life stresses and symptoms of depression exhibited an increased abundance of Proteobacteria and Spirochaetes, respectively, in saliva samples [36]. Additionally, increased abundance of the members of the phylum Firmicutes was seen in pregnant women with high levels of anxiety and depressive symptoms [36]. Lee et al. also found an increased abundance of pathogenic taxa, such as Veillonella and Prevotella, in the OM of patients with SCZ-related psychosis and psychotic BD [37].
In addition to microbially induced neuroinflammation, NPD pathogenesis (the process by which a disease or disorder develops) may be linked to abnormal metabolic and epigenetic changes involving metabolites such as short-chain fatty acids (SCFAs) synthesized by oral and gut microbes via two other processes, including carbohydrate hydrolysis or amino acid metabolism [38,39].
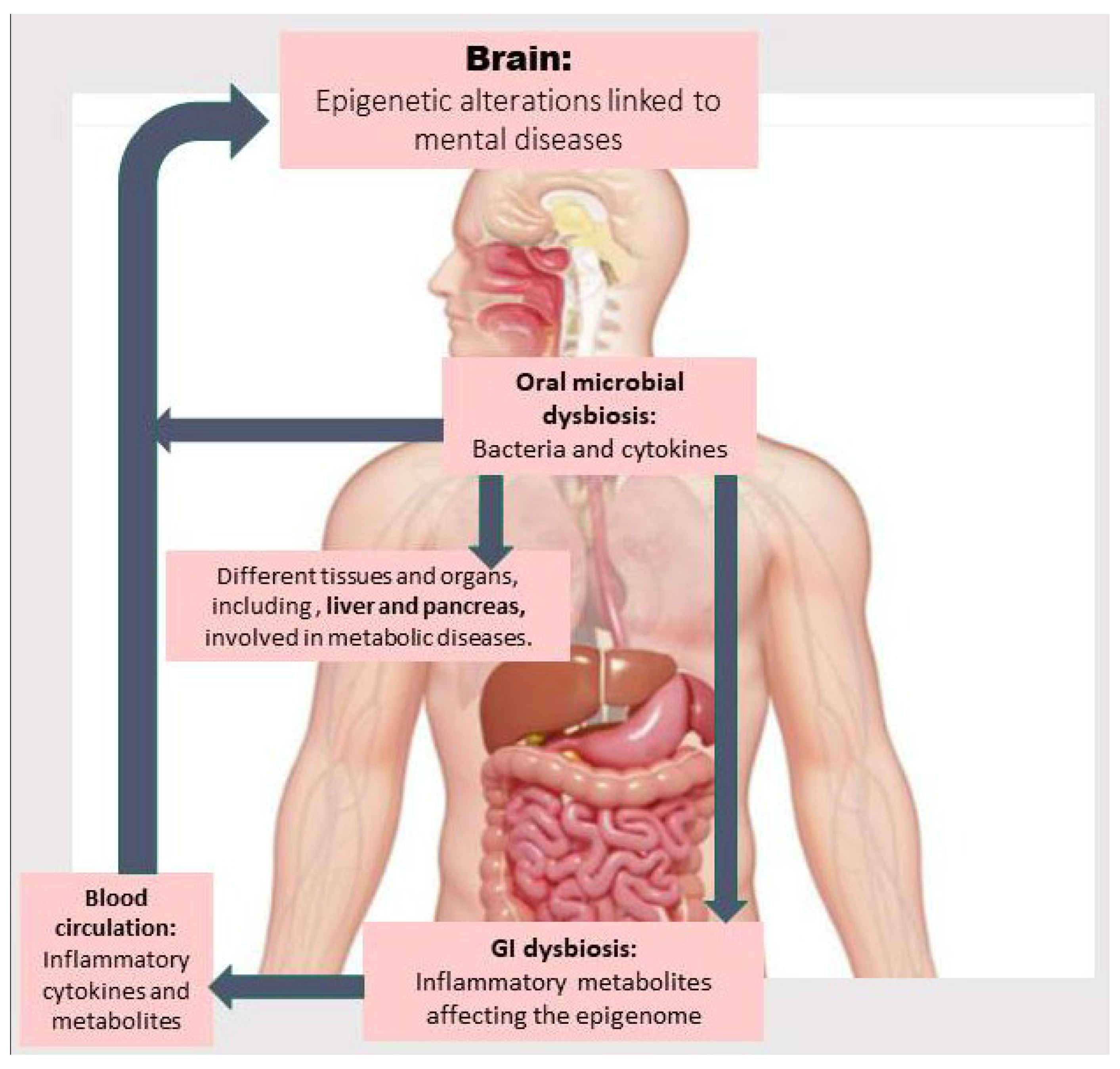
In this article, we first review the existing literature about the relationship between oral dysbiosis and developing metabolic diseases and NPDs. Then, we discuss how abnormalities in metabolic pathways and those related to one-carbon metabolism (involved in methylation reactions) due to oral and gut dysbiosis may contribute to inflammation and the onset and development of metabolic diseases and NPDs via epigenetic aberrations. Next, we explore the main findings about therapeutic approaches capable of treating metabolic diseases and NPDs by modulating the OM, improving metabolic abnormalities through reshaping the gut microbiota (GM) and its epigenetic metabolites, and mitigating epigenetic aberrations. Finally, we discuss challenges and current research gaps related to investigating the correlation between OM, epigenetic aberrations, and metabolic abnormalities associated with NPDs and the GM along with corresponding preventive and therapeutic interventions. Figure 1 provides an overview of how OM dysbiosis may directly introduce bacterial elements and inflammatory cytokines into the bloodstream, as well as induce gut dysbiosis, thereby contributing to epigenetic alterations linked to the pathogenesis of metabolic diseases and related NPDs. These mechanisms are described in detail in the following sections.
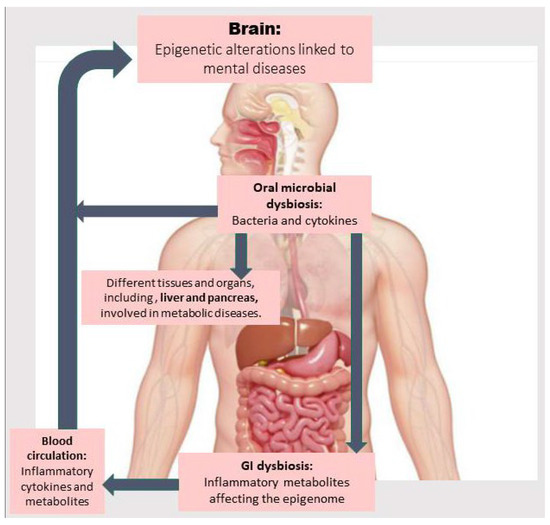
Figure 1.
The oral microbiota, by passing through the oropharynx, the sole gateway to the gastrointestinal (GI) tract, influences the gut microbiome. Oral microbial dysbiosis is associated not only with the invasion of bacteria and inflammatory cytokines into the bloodstream, but it can also induce GI dysbiosis. This leads to the production of toxic and inflammatory metabolites that trigger cytokine production by white blood cells, which induce epigenetic alterations in the brain or in organs related to metabolic diseases (e.g., the liver), which in turn can affect brain function.
2. Oral Microbiota and the Pathogenesis of Metabolic Diseases
It has been shown that oral dysbiosis is linked to the development of metabolic diseases, which in turn contribute to the onset and aggravation of periodontal disease and tooth loss. Several lines of evidence have shown an interesting link between metabolic disease (obesity, dyslipidemia, and metabolic syndrome) and periodontitis [40,41,42]. Obesity is linked to tooth loss five years later, and the periodontal condition of individuals with obesity is inferior to that of subjects with normal weight [43]. Obesity is capable of altering both the OM and GM composition and, hence, contributing to the development of oral diseases via inflammation. For example, leptin-deficient obesity in mice is associated with a decreased abundance of beneficial bacteria, including Akkermansia and Ruminococcaceae_UCG_014, and increased abundance of inflammation-related Flavobacterium in the salivary samples, indicating that leptin-deficient obesity is a risk factor for developing periodontitis [44]. Sato et al. found that obesity elevates the risk of periodontal disease by escalating production of uric acid, mediated by gut dysbiosis [45]. Jia et al. reported that subjects classed as obese exhibited the highest proportion of severe periodontitis (stage III and IV) and higher amounts of the inflammatory mediators in gingival crevicular fluid versus controls [46]. On the other hand, periodontitis and oral dysbiosis may contribute to the development of metabolic diseases. For example, salivary microbiota of subjects with periodontitis are capable of worsening liver function in HFD-induced obese mice, and contribute to the development of NAFLD by disrupting gut barrier function, activating the TLR4 signaling pathway, and causing liver inflammation [47]. Here, first, we will review the emerging evidence of the potential impact of OM dysbiosis in the pathogenesis of metabolic diseases and NPDs (Table 1).

Table 1.
Association between metabolic diseases and alterations in the OM composition.
It is also worth noting that the association between metabolic diseases and periodontitis may be mediated by epigenetic mechanisms. For example, the DNA methylation status of buccal cells may represent markers related to obesity and metabolic disorders [58]. As another example, individuals with obesity and periodontitis exhibited overexpression of miR-200b in gingival tissue [59]. Byun et al. also found that salivary exosomal miR-25-3p plays a key role in developing and aggravating diabetes-related periodontitis [60]. Liu et al. reported that miR-223 and miR-200b in the gingival crevicular fluid are strongly connected to the pathogenesis of the periodontal disease and vulnerability to T2DM [61]. In another study, elevated levels of miR-146a were observed in T2DM compared to non-diabetic, periodontally healthy subjects [62]. A different clinical study also reported that miRNA 146a is a reliable marker of periodontitis among diabetic patients, with an optimum cut-off value of ≥11.04 and an accuracy of 86.1% [63].
3. Oral Microbiome and the Pathogenesis of NPDs
The oral microflora is a key player in oral health and a contributor to systemic health by influencing host physiological mechanisms. People with greater oral microbial α-diversity exhibit better cognitive performance status [64]. In an interesting study, Qiao et al. examined the potential role of mouth–microbial–brain connections in the development of NPDs and found that the OM of ASD children could cause ASD-like behaviors by changing microbial community structures and neural signaling activities in the prefrontal cortex of recipient mice, along with the upregulation of genes related to serotonin and TGF-β signaling pathways [65]. As ASD children exhibit lower oral bacterial diversity versus controls [66], the lower OM α-diversity is linked to a greater risk of depression as well [67]. In PD, aberrant salivary OM releases harmful metabolites that pass through biofilm and trigger iron dysregulation, collectively perturbing the function of the mouth–gut–brain axis and leading to degenerative damage and necrosis of dopamine neurons [68]. Furthermore, while increased intestinal permeability and elevated serum LPS-binding protein levels have been found in subjects newly diagnosed with PD [69], higher levels of specific gut microbial genera, such as Cloacibacterium, Microbacterium, and Isoptericola, have been detected in the blood samples of patients with PD [70]. Periodontal pathogens may also induce or accelerate the progression of AD via the formation of beta-amyloid protein (Aβ) and, subsequently, increasing neuroinflammation and other pathogenic pathways [71]. In addition, as OM dysbiosis is connected to a greater brain Aβ load and the onset or progression of AD, oral-derived microbes, such as P. gingivalis, have been detected in the brains of patients with AD [72]. However, small-molecule inhibitors targeting gingipains, the neurotoxic components of P. gingivalis, can reduce brain bacterial load following P. gingivalis infection, decrease Aβ1–42 production and neuroinflammation, and ultimately rescue neurons in the mouse hippocampus [73]. Mechanistically, P. gingivalis infection in iPSC-derived neurons has been shown to increase autophagic vacuoles and multivesicular bodies, the phospho-tau/tau ratio, synapse loss, and cytoskeletal disruption [74]. A growing body of studies has also shown a correlation between psychosocial factors and periodontitis. Wu et al. found that adolescents with BD exhibited an elevated risk for periodontitis [75]. Moreover, depression is considered a risk factor for periodontal disease [76]. Therefore, not only can OM aberrations predispose individuals to metabolic and mental diseases, but NPDs are also associated with a higher risk of periodontitis, which in turn could exacerbate NPDs, creating a vicious cycle. Other studies addressing the correlation between changes in OM composition and the pathogenesis of different types of NPDs are summarized in Table 2.

Table 2.
Altered oral microbiota (OM) composition in neuropsychiatric diseases.
4. OM-Induced Inflammation Drives Metabolic and Epigenetic Alterations Underlying NPD Pathogenesis
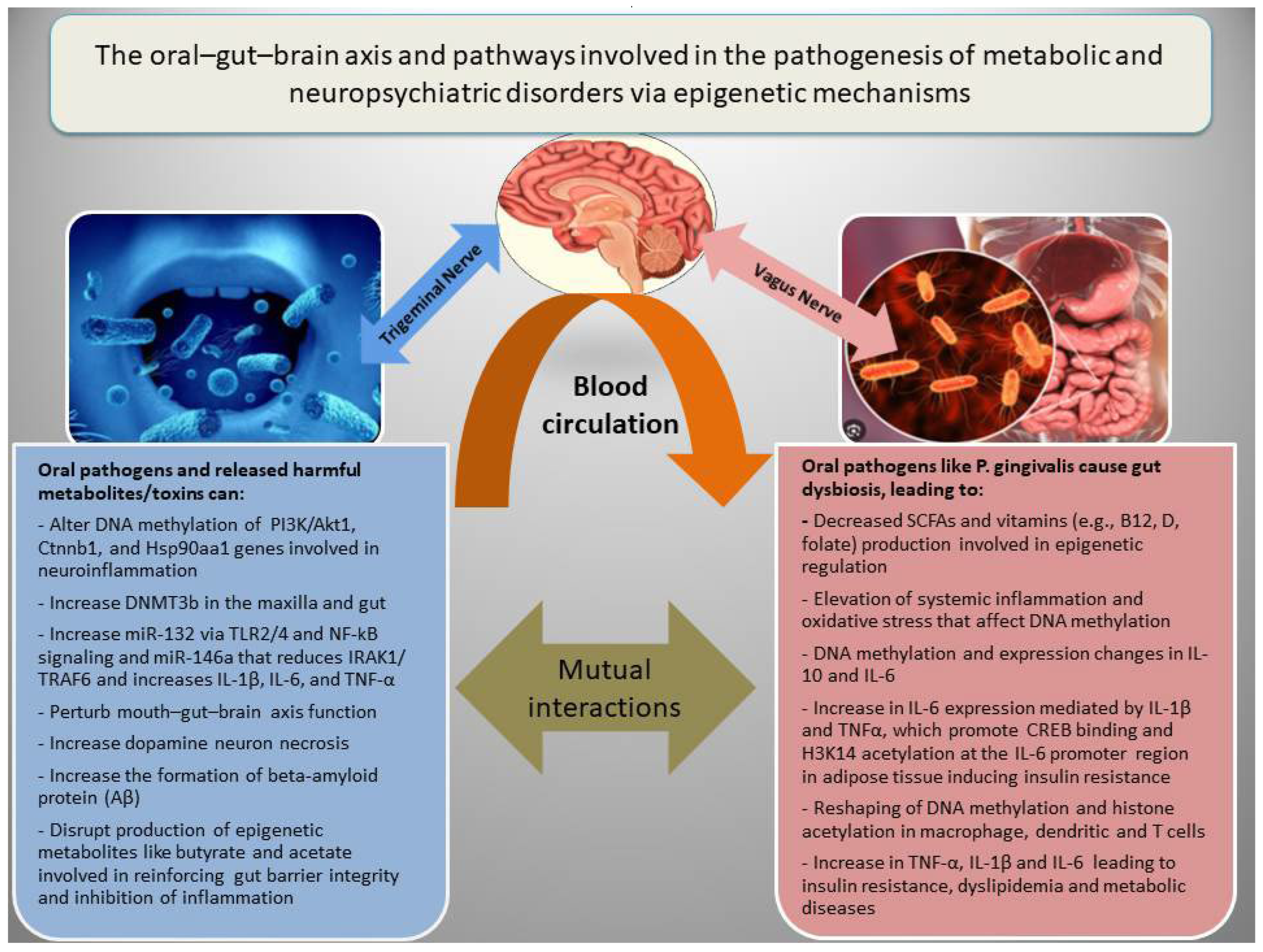
In addition to genetic factors, the pathogenesis of NPDs is associated with epigenetic dysregulation of metabolic and inflammatory genes, which interact with diverse socioeconomic, dietary, ecological, and seasonal conditions [18,20,88]. There are three main epigenetic mechanisms that regulate gene expression, including (a) DNA methylation, which in general inhibits gene expression; (b) histone modifications, which may increase or decrease gene expression depending on the nature of the modification; and (c) RNA interference, which involves miRNAs and lncRNAs, generally associated with the inhibition of gene expression or increased RNA degradation [12]. Epigenetic modifications have been among the major mechanisms for adaptation to environmental changes or fluctuations during ordinary life and throughout evolution. However, adversary environmental factors such as malnutrition, chronic infections, and oral or gut microbial dysbiosis may lead to epigenetic alterations and cause metabolic or mental diseases by inducing oxidative stress and inflammation [89]. Figure 2 shows how the oral–gut–brain axis and its related pathways are involved in the pathogenesis of metabolic diseases and NPDs via epigenetic mechanisms.
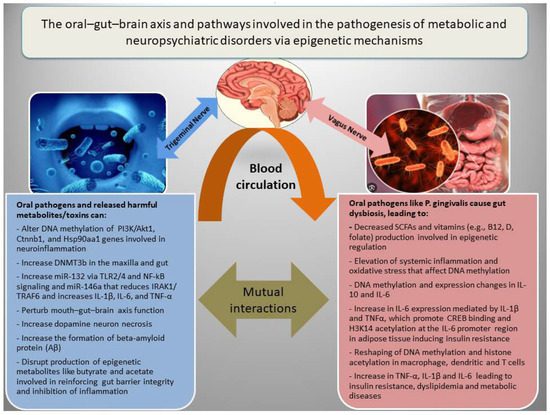
Figure 2.
The oral–gut–brain axis, inflammation, and epigenetic alterations in metabolic and neuropsychiatric disorders. Oral pathogens can promote gut dysbiosis and cooperatively induce inflammation, disrupt gut barrier integrity, and impair the function of the oral–gut–brain axis by affecting the production of short-chain fatty acids (SCFAs) such as butyrate and acetate, as well as vitamins involved in epigenetic modifications in both the brain and metabolic organs.
For instance, experimental studies revealed that orally administered P. gingivalis could induce periodontitis and differentially methylated regions related to PI3K/Akt1, Ctnnb1, and Hsp90aa1 genes involved in inflammation (TNF signaling) and NPDs, in multiple tissues in mice [90]. However, treatment with an anti-TNF-α antibody could mitigate the host response to P. gingivalis and reduce serum TNF-α, IL-6, blood glucose levels, and the size of the P. gingivalis inoculation lesion in a mouse model for type 2 diabetes and obesity [91]. Experimental periodontitis and P. gingivalis gavage could also induce expression of inflammatory markers (BTLA and IL-18R1) and increase DNMT3b, a marker of de novo DNA methylation in the gut and maxilla of C57BL/6 mice [92]. With respect to the causal link between inflammation and epigenetic aberrations, Niwa T and Ushijima reviewed how chronic inflammation (e.g., gastritis and hepatitis) triggers aberrant DNA methylation even in normal tissues. They pinpoint mechanisms like cytokine-stimulated cell proliferation and ROS-driven DNA damage that recruit DNMTs (DNA methyl transferases) to specific gene promoters, creating an “epigenetic field for cancerization” [93]. Another comprehensive review highlighted how inflammatory stimuli (such as LPS and cytokines IFN-γ) or IL-4, an anti-inflammatory cytokine, reshape DNA methylation and histone acetylation in macrophages, dendritic cells, and T cells, which reprogram gene expression during innate memory and T cell polarization [94]. A meta-analysis on epigenome-wide association studies also identified 58 CpG sites whose methylation levels in blood correlate with CRP (a chronic inflammation marker) level, and many of these methylation alterations are linked to cardiometabolic disease [95]. Rodríguez-Ubreva et al. showed that monocytes from septic patients, and even healthy monocytes exposed to LPS (TLR4 stimulus), undergo significant DNA methylation changes associated with IL-10 and IL-6 level alterations [96].
Beyond DNA methylation changes, oral dysbiosis in vivo and exposure of oral epithelial cells to LPS in vitro induce histone modifications, activate the transcriptional coactivators p300/CBP, and promote NF-κB accumulation [97]. Additionally, a clinical study analyzing saliva samples reported downregulation of the histone deacetylase genes HDAC4, HDAC8, and HDAC10 in gingivitis, and HDAC4, HDAC6, HDAC8, and HDAC9 in periodontitis [98]. Remarkably, six months of non-surgical periodontal therapy not only improved clinical periodontal parameters but also upregulated the expression of HDAC2, HDAC4, HDAC6, HDAC8, HDAC9, and HDAC11 in saliva, indicating a causal relationship [99]. Other studies revealed that a shorter duration of such therapy (3 months) could also decrease the levels of MMP-8 (matrix metalloproteinase-8), MAF (macrophage-activating factors), and SIRT1 (an NAD+-dependent histone deacetylase) in saliva, while increasing the serum level of T-SOD (total superoxide dismutase, an antioxidant enzyme) in patients with periodontitis [100].
Inflammatory oral bacteria can affect miRNA expression as well. For example, P. gingivalis infection in THP-1 macrophages upregulates miR-132 via TLR2/4 and NF-κB signaling. Gingival fibroblasts and macrophages exposed to P. gingivalis LPS also show significant upregulation of miR-146a (and 146a-5p), which reduces IRAK1/TRAF6 expression and increases IL-1β, IL-6, and TNF-α secretion [101]. Similarly, in vivo polymicrobial periodontal infection can induce miR-146a in both local periodontal tissues and the spleen of ApoE−/− mice [102]. Experimental evidence also supports that alterations in the OM composition in NPDs, such as ASDs, may be associated with epigenetic alterations [103]. For example, children with ASDs exhibit miRNA and microbiota dysregulations in the saliva, which are connected to their cognitive impairments [104]. Ragusa et al. found an association between changes in specific miRNA expression (e.g., upregulation of miR-29a-3p and miR-141-3p and downregulation of miR-16-5p, let-7b-5p, and miR-451a) and OM composition in children with ASDs (e.g., elevated levels of Actinobacillus, Weeksellaceae, Rothia, Filifactor, Ralstonia, Pasteurellaceae, and Aggregatibacter and reduced levels of Tannerella, Moryella, and the TM7-3 bacterial group) [104]. Their findings showed a negative relationship between salivary miR-141-3p expression and Tannerella abundance in saliva, which was linked to cognitive dysfunction [104].
With respect to metabolic dysfunction, large-scale metagenomic analysis of OM compositional changes in NPDs (such as ASDs) revealed significant alterations in metabolic pathways involved in the degradation of serotonin, GABA, and dopamine; key players in brain function and psychopathogenesis [85]. Reciprocally, impaired dopamine signaling in ASDs is also associated with OM alterations, alongside metabolic and gastrointestinal dysfunction. For example, the ASD-associated variant in the SLC6A3 gene (dopamine transporter, also known as DAT1) leads to a significant reduction in Fusobacterium abundance in the DAT T356M+/+ mouse oral cavity, where increased Fusobacterium abundance is linked to improved glucose handling and reduced body fat [105]. In the saliva of patients with bulimia nervosa and binge-eating disorder, the abundance of specific OM genera (e.g., increased abundance of Bacilli and depletion of Lachnospirales) is linked to exosomal miRNA expression changes (e.g., upregulation of let-7b-5p, mir-15b-5p, mir-429, and mir-221-3p), and DNA hypomethylation of the DAT1 gene [106]. Altogether, these studies provide experimental evidence that OM composition influences inflammatory cytokines and the epigenetic setting related to the pathogenesis of NPDs and their metabolic counterparts. Table 3 presents an overview of the mechanisms by which oral microbiota dysbiosis, through the induction of epigenetic aberrations, may contribute to inflammation and thereby increase the risk of developing metabolic diseases and NPDs.

Table 3.
Experimental evidence of OM-dysbiosis-induced epigenetic modifications and inflammatory responses.
In other mental diseases such as SCZ, patients not only exhibit significant changes in the OM composition, including elevated levels of specific genera such as Neisseria and Porphyromonas, but also display upregulation of key metabolic pathways such as β-alanine metabolism and vitamin digestion and absorption [107]. The findings of this study suggest that elevated levels of certain metabolites, such as L-methionine sulfoxide (L-MetO) and tyramine, resulting from oral dysbiosis, may contribute to the initiation of oxidative stress in SCZ patients [107]. Oxidative stress, in turn, is a well-known driver of inflammation, as well as metabolic and epigenetic alterations [18]. In AD, François et al. reported that disease progression was associated with alterations in oral bacterial composition, including a decreased abundance of Lautropia mirabilis and remarkable changes in vitamin B12 metabolism, and reduced levels of salivary Transcobalamin-1, which binds and protects vitamin B12 (a cofactor in methylation reactions) from degradation by stomach acid [108]. Additionally, while several studies have shown that the disruption of the microbial profiles in the oral cavity is linked to altered functionality of metabolic pathways, it has been shown that the oropharynx microbial profile correlates with the OM profile both in normal conditions and in children with ASDs [109,110]. Since the oropharynx is the exclusive gateway to the GI tract, OM can be considered as the main mediator of the gut microbial composition; it is a well-known contributor to the pathogenesis of NPDs, inflammation, and metabolic diseases [2,110,111]. In line with this, a recent experimental study concluded that “oral-to-gut translocation may be the main route” of environmental microbial translocations to the GI tract [112]. Via this route, the OM is not only directly linked to the pathogenesis of metabolic and mental diseases, but is also indirectly involved in shaping the GM and local or systemic inflammation. For instance, experimental data show that in patients with periodontitis, the salivary microbiota can induce gut dysbiosis because swallowing of the pathogenic OM can perturb the GM composition [113]. Furthermore, Kitamoto et al. experimentally induced periodontitis in a mouse model and found that oral pathobionts can migrate to the colon, significantly worsening inflammation in DSS-induced colitis. The mechanism involved Th17 T cells, initially “primed in oral mucosa–draining lymph nodes”, then trafficked to the gut and reactivated by oral microbes, driving elevated IL-1β and colonic pathology [114]. Research data also support that a dysbiotic OM in periodontitis can trigger excessive secretion of IL-17A by innate and adaptive immune cells; this cytokine fosters both local periodontal destruction and systemic inflammatory diseases [115], and contributes to neuroinflammation, as well as the dysregulation of a wide range of neurotransmitters and neuromodulators, increasing the risk of NPDs [116]. It has been shown that, at least in an AD mouse model, IL-17A induces promoter DNA methylation of the Bmal1 (brain and muscle ARNT-like 1) gene, decreasing its expression and leading to disruption of the circadian rhythm. In this process, the MAPK pathway mediates IL-17A-induced Dnmt1 upregulation, which in turn leads to DNA methylation of the Bmal1 promoter [117].
Based on other animal experiments, oral inoculation with P. gingivalis may also lead to macrophage infiltration into adipose tissue and elevate systemic inflammatory markers, increasing insulin resistance, likely via the oral–gut axis influencing gut barrier function and the microbiome [118]. Periodontal pathogens (e.g., P. gingivalis) can also enter the bloodstream and cause bacteremia/endotoxemia. This stimulates endothelial cells and immune components, triggering systemic cytokine rises (TNF-α, IL-1β, and IL-6), thereby contributing to atherosclerosis, insulin resistance, hypertension, and dyslipidemia [119]. Another study uncovered that while adipose tissue IL-6 is the main contributor to insulin resistance, IL-1β and TNF-α cooperativity increase IL-6 expression by promoting CREB binding and H3K14 acetylation at the IL-6 promoter region [120]. Additionally, a review of several original studies linked oral dysbiosis in periodontitis with autoimmune mechanisms—via pathways like Toll-like receptors, molecular mimicry, and bystander activation—potentially contributing to diseases like rheumatoid arthritis and systemic inflammation beyond the oral cavity [121]. Therefore, from a preventive or therapeutic point of view, while preserving oral health and its microbiota composition is important to prevent inflammation, it is also critical to reshape OM-induced GM alterations to mitigate epigenetic alterations associated with metabolic and mental diseases. The focus of the following section is the use of prebiotics or probiotics (i.e., nutritional agents that modulate oral or GM composition) to prevent or treat OM-induced metabolic and/or associated mental diseases.
5. Therapeutic Remedies Based on Modulation of Oral Microbiome
Given the link between OM and GM with metabolic diseases and NPDs involving epigenetic modifications, in addition to oral hygiene, therapeutic strategies could include microbiota-targeted and nutritional interventions to help mitigate metabolic and epigenetic aberrations in NPDs.
5.1. Oral Hygiene to Prevent or Improve OM-Induced Metabolic Dysfunctions and NPDs
Lack of an adequate hygiene routine and reducing salivary flow due to dehydration or use of different medications, including psychoactive substances or prescribed drugs with anticholinergic side effects (e.g., most traditional antidepressants and antipsychotics), and difficulty in accessing dental health services may increase the growth rate and activity of periodontopathogenic bacteria. As an example, P. gingivalis and T. denticola as well as their toxic proteases (gingipains) have been identified in postmortem brain analyses of AD patients, with the extent of tau and ubiquitin pathology found to correlate with their levels. Additional mechanistic studies revealed that oral infection with P. gingivalis in mice led to brain colonization, increased levels of the amyloid plaque component Aβ1–42, and neurotoxic effects of gingipains on the tau protein. Treatment with a small-molecule gingipain inhibitor reduced brain bacterial load, blocked Aβ1–42 production, decreased neuroinflammation, and prevented hippocampal neuronal loss, indicating a causal role of gingipains in both neuroinflammation and neuronal loss [73]. Improving oral health and oral hygiene practice may be a promising approach to attenuate inflammation during metabolic diseases [122]. For example, frequent tooth brushing decreases the risk of hypertension and T2DM, as brushing at least twice a day may help prevent future occurrences of these conditions [123].
Some oral pathogens are linked to vitamin B deficiency and, hence, the development of various diseases. For instance, Avcu et al. found that subjects with poor Oral Hygiene Index (OHI) scores had the most frequent gastric recurrence of H pylori (58.3%) versus those with fair OHI scores (41.2%) and good OHI scores (4.8%) [124]. Their results also showed that eradication of H pylori in dental plaque could contribute to recovery from anemia with increases in the serum vitamin B12 level [124]. In specific metabolic diseases such as diabetes, which is much more common in patients with major mental diseases such as SCZ and BD [122], oral hygiene has a more critical role in general health status. For example, the relative abundance of P. gingivalis is higher in patients with T2DM [30]. The relative abundance of Firmicutes, a pathogenic bacterium, is also higher in diabetic patients with advanced periodontitis [125]. Another recent study reported higher salivary abundance of Firmicutes in adult diabetic patients with periodontitis as well [126]. Periodontal health problems in diabetic patients with chronic hyperglycemia could induce oral microbial dysbiosis and provoke pathological pathways, such as inflammation, and oxidative stress leading to periodontal tissue damage and interlinked systemic diseases [127]. Altogether, these studies suggest that oral microbial dysbiosis and related dental and gingival diseases not only pose a risk to general health and contribute to gut dysbiosis, but that systemic diseases—particularly metabolic disorders—may also impact oral health, potentially creating a vicious cycle that worsens both conditions. Therefore, the prevention and treatment of periodontal diseases require a systemic approach, including the management of gut dysbiosis.
5.2. Nutritional Interventions and Pre-, Pro-, and Postbiotics to Improve Oral Health and OM-Induced Epigenetic Diseases, Metabolic Diseases, and NPDs
Owing to the critical roles of some dietary factors’ involvement in microbial dysbiosis and dental or gingival erosions, nutritional interventions may contribute to improving oral health and, subsequently, preventing or treating metabolic diseases and related NPDs. For example, the low pH and high titratable acidity of some soft drinks and the metabolizing of their sugar by plaque microorganisms, hence producing organic acids, can cause dental erosion [128]. Vitamin deficiency may accelerate several non-specific oral conditions like glossitis, stomatitis, and mucosal ulceration [129]. As an example, an early sign of vitamin B12 deficiency is glossitis with linear lesions [130]. It appears that correcting disruptions in one-carbon metabolism through the use of various B vitamins may help prevent metabolic diseases and NPDs by supporting a healthy oral or gut microbiota and enhancing the growth and activity of commensal bacteria [131]. Almost all of the B vitamins, such as folate, vitamin B12, vitamin B6, and riboflavin (vitamin B2), are involved in methylation reactions as coenzymes and, hence, their supplementation may contribute to improving NPDs via modulation of the microbiota composition and mitigating epigenetic and metabolic dysregulations [132]. In an interesting study, Wang et al. found that treatment of rats exposed to chronic unpredictable mild stress by the vitamin B complex could prevent homocysteine-induced derangements in DNA methylation and, subsequently, reduce stress-related cognitive decline [133]. Holmes et al. also found that increased levels of homocysteine in subjects with mild cognitive impairment were linked to elevated rates of epigenetic aging, and treatment with B vitamins could hamper accelerated epigenetic aging [134]. Folic acid may also be considered as an adjuvant treatment in patients with periodontal disease [135], as well as in SCZ patients with significant improvement in their core symptoms, as shown in a randomized control trial study [136]. Additionally, adequate intake of folate, vitamin B6, and vitamin B12 is associated with better cognitive performance—as demonstrated in a longitudinal study of patients with MCI—by regulating DNA methylation [137,138]. Considering other vitamins, a recent systematic review and meta-analysis of 16 studies, including nearly 18,000 individuals, concluded that higher vitamin C intake is linked to an almost 50% lower risk of periodontal disease [139]. In another meta-analysis of 11 studies, while circulating vitamin C level correlated inversely (~40% reduced risk) with metabolic syndrome [140], its deficiency was linked to depression and cognitive impairment [141]. One of the main underlying mechanisms of these correlations is epigenetic alterations, since vitamin C is a powerful epigenetic modulator that activates TET enzymes, leading to DNA demethylation at the regulatory regions of genes [142]. Vitamin D deficiency is also linked to periodontitis. For example, a meta-analysis of 16 studies revealed that a lower serum vitamin D level was associated with a higher risk of periodontitis [143]. Another meta-analysis of 23 studies reported that a higher vitamin D level was linked to an almost 20% reduced risk of metabolic syndrome [144]; other meta-analyses have reported that its deficiency is associated with a higher risk of dementia (32%) [145], depression (40%) [146], and SCZ [147]. Interestingly, while Jiang et al. reported that the deficiency of vitamin D metabolites in school-age children with obesity affects DNA methylation of metabolic and vitamin D metabolism genes [148], an animal study revealed that maternal vitamin D deficiency can induce epigenome alterations in multiple generations [149]. Altogether, these data suggest that the deficiency of vitamins linked to dental health is also linked to metabolic and mental diseases, mediated by epigenetic changes bridging the connection between oral health and metabolic and/or mental diseases.
6. Critical Points, Limitations, and Future Perspectives
Critical points regarding OM-induced inflammation triggering epigenetic alterations that drive NPD pathogenesis and interlink metabolic aberrations include the complex interplay between microbial dysbiosis, host immune responses, and epigenetic regulation. Although numerous associations or correlative studies indicate that OM dysbiosis can induce chronic inflammation, potentially altering epigenetic marks in neural and metabolic pathways and contributing to disease development, significant shortcomings remain. Many studies are associative, lacking causal evidence, and most are conducted in preclinical models, which may not fully translate to human physiology [150,151]. Therefore, although several meta-analyses of human studies support the link between OM and metabolic or epigenetic alterations in NPDs, our understanding of the precise molecular mechanisms by which specific oral bacteria influence metabolic or epigenetic changes, as well as the temporal relationship between inflammation and epigenetic remodeling, is still limited. Additional pitfalls include the variability in individual microbiomes, environmental factors, and host genetics, all of which complicate the reproducibility and generalizability of findings. There is also a lack of standardized biomarkers to reliably track metabolic or epigenetic changes in OM-induced inflammation in clinical settings. These gaps hinder the development of targeted therapies and early diagnostics. Future studies need to integrate multi-omics approaches and longitudinal designs to elucidate causal pathways and identify precise therapeutic targets.
Efforts to improve oral hygiene as a way to prevent or mitigate OM-induced metabolic dysfunction and NPDs are grounded in evidence linking OM dysbiosis to systemic inflammation and the pathogenesis of diverse diseases; however, critical shortcomings must be acknowledged. First, interventions like tooth-brushing and mouth rinses, while reducing oral pathogens, may only provide transient improvements in microbial balance, especially if host or environmental factors (e.g., systemic diseases, diet, food preservatives, or contaminants) drive recurring dysbiosis. Second, most current studies are cross-sectional or correlative, which limits definitive conclusions about causality, meaning oral hygiene alone may be insufficient to significantly reduce the risk or severity of metabolic or mental disorders. Finally, individualized host responses and genetic variations influence intervention efficacy, which remains an underexplored area. Therefore, a comprehensive approach is required to deal with these conditions.
Nutritional interventions, including dietary modifications and the use of vitamins, pre-, pro-, and postbiotics, offer a promising approach to improve both oral health and OM-induced epigenetic, metabolic, and neuropsychiatric outcomes. Probiotics (like Lactobacillus and Bifidobacterium) can modulate the oral microbiome, suppress pathogens, reduce inflammation, and improve local and systemic immunity [152]. Nevertheless, challenges exist as the stability and colonization ability of probiotics in the oral environment vary by strain and may be affected by factors such as diet and host immunity. There is also wide variability in dosing, formulation, and treatment duration, and beneficial effects may be temporary if supplementation stops. Additionally, there are potential risks related to overuse or inappropriate combinations of biotic interventions, such as microbial resistance, unintended dysbiosis, or negative immune reactions.
Future research should prioritize large-scale, longitudinal, and interventional studies to clarify the mechanistic links between OM and disease pathogenesis as well as preventive or therapeutic approaches such as oral hygiene, targeted nutritional interventions, and the prevention or mitigation of systemic inflammation, metabolic dysfunction, and NPDs. Once causal links are established between OM dysbiosis, or specific oral bacteria, and metabolic diseases or NPDs, the development of personalized, microbiome-based therapies tailored to individual microbial profiles, seasonal and ecological variations, and host genetics holds great promise for maximizing potential efficacy and minimizing adverse effects. More robust and harmonized methodologies in clinical trials, such as standardizing intervention protocols and outcome measures, are essential to advance the field and allow replication across diverse populations. Innovations such as next-generation probiotics, synbiotics (prebiotic and probiotic combinations), and postbiotics (beneficial microbial metabolites, such as butyrate and acetate) merit further exploration, as do non-invasive biomarkers to monitor response and guide adaptive treatment [153]. Ultimately, integrating oral health into a broader framework of preventive and precision medicine could substantially impact the management of metabolic and neuropsychiatric disorders linked to the oral microbiome.
7. Conclusions
In this article, we reviewed the potential mechanistic links between OM, epigenetic dysregulations, and the pathogenesis of NPDs and metabolic diseases. We propose that oral and gut dysbiosis may contribute to the development of metabolic diseases and NPDs by disrupting metabolic pathways involved in epigenetic regulation. Therefore, simultaneous targeting of metabolic and epigenetic dysregulations using some specific diets, probiotics, and prebiotics may be considered as potential therapeutic approaches for the prevention or treatment of NPDs and metabolic diseases. The concept of the oral–brain axis is an area of interest with ever-increasing growth that may contribute to the prevention or treatment of NPDs, and may contribute to easier diagnosis by providing new microbial signatures and epigenetic marks. Future studies should focus on developing novel therapeutic approaches that simultaneously target metabolic and epigenetic dysregulations, building upon a profound understanding of the potential mechanistic links between NPD pathogeneses and metabolic/epigenetic abnormalities, to achieve more effective treatment of metabolic diseases and NPDs. This field remains largely unexplored, and further investigations with larger sample sizes of unmedicated patients are needed to examine the biological links and interconnections between the OM, GM, and various classes of metabolic diseases and NPDs in order to establish consistent patterns and obtain more definitive findings. Certainly, technological advances in measurement techniques and more precise classifications of the oral and gut microbiota, together with their biological products and effects, will also help identify key disease-specific pathogenic bacterial species and susceptible individuals, thereby informing therapeutic strategies.
Author Contributions
Conceptualization, H.M.A. and S.T.; methodology, H.M.A.; software, S.M.; validation, H.M.A.; investigation, S.M., S.N. and A.P.; writing—original draft preparation, S.M. and S.N.; writing—review and editing, H.M.A. and A.P.; visualization, H.M.A. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge Faria Ashrafi for her assistance with the illustrations in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bransfield, R.C.; Mao, C.; Greenberg, R. Microbes and mental illness: Past, present, and future. In Proceedings of the Healthcare, Kyoto, Japan, 12–14 May 2023; p. 83. [Google Scholar]
- Nohesara, S.; Mostafavi Abdolmaleky, H.; Pirani, A.; Thiagalingam, S. Therapeutic Horizons: Gut Microbiome, Neuroinflammation, and Epigenetics in Neuropsychiatric Disorders. Cells 2025, 14, 1027. [Google Scholar] [CrossRef]
- Shirinian, M.; Chen, C.; Uchida, S.; Jadavji, N.M. The role of epigenetics in neuropsychiatric disorders. Front. Mol. Neurosci. 2022, 15, 985023. [Google Scholar] [CrossRef]
- Barroso, I.; McCarthy, M.I. The genetic basis of metabolic disease. Cell 2019, 177, 146–161. [Google Scholar] [CrossRef]
- Ilieva, M.S. Non-coding RNAs in neurological and neuropsychiatric disorders: Unraveling the hidden players in disease pathogenesis. Cells 2024, 13, 1063. [Google Scholar] [CrossRef]
- Zhu, H.; Guan, A.; Liu, J.; Peng, L.; Zhang, Z.; Wang, S. Noteworthy perspectives on microglia in neuropsychiatric disorders. J. Neuroinflamm. 2023, 20, 223. [Google Scholar] [CrossRef]
- Ngo, J.; Osto, C.; Villalobos, F.; Shirihai, O.S. Mitochondrial heterogeneity in metabolic diseases. Biology 2021, 10, 927. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New insights and potential therapeutic interventions in metabolic diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Morella, I.M.; Brambilla, R.; Morè, L. Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2022, 142, 104892. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Rosell-Valle, C.; Medina-Vera, D.; Navarro, J.A.; Vargas, A.; Rivera, P.; Sanjuan, C.; Rodríguez de Fonseca, F.; Suárez, J. A negative energy balance is associated with metabolic dysfunctions in the hypothalamus of a humanized preclinical model of Alzheimer’s disease, the 5XFAD mouse. Int. J. Mol. Sci. 2021, 22, 5365. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An overview of the epigenetic modifications in the brain under normal and pathological conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Mostafavi Abdolmaleky, H.; Pirani, A.; Thiagalingam, S. Epigenetics and Gut Microbiota in the Pathogenesis and Treatment of Bipolar Disorder (BD). Cells 2025, 14, 1104. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lin, Z.-J.; Li, C.-C.; Lin, X.; Shan, S.-K.; Guo, B.; Zheng, M.-H.; Li, F.; Yuan, L.-Q.; Li, Z.-h. Epigenetic regulation in metabolic diseases: Mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Martin, M.; Zhou, J.-R.; Thiagalingam, S. Epigenetic alterations of brain non-neuronal cells in major mental diseases. Genes 2023, 14, 896. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Tognini, P. Interplay between metabolism, nutrition and epigenetics in shaping brain DNA methylation, neural function and behavior. Genes 2020, 11, 742. [Google Scholar] [CrossRef]
- Huang, K.; Li, S.; Yang, M.; Teng, Z.; Xu, B.; Wang, B.; Chen, J.; Zhao, L.; Wu, H. The epigenetic mechanism of metabolic risk in bipolar disorder. Obes. Rev. 2024, 25, e13816. [Google Scholar] [CrossRef]
- Cui, J.; Zhai, Z.; Wang, S.; Song, X.; Qiu, T.; Yu, L.; Zhai, Q.; Zhang, H. The role and impact of abnormal vitamin levels in autism spectrum disorders. Food Funct. 2024, 15, 1099–1115. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut microbiota dysbiosis, oxidative stress, inflammation, and epigenetic alterations in metabolic diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.J.; Goldin, A.; Fulmer, N.; Tavares, R.; Wands, J.R.; de la Monte, S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J. Alzheimer’s Dis. 2005, 8, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S. Epigenetic aberrations in major psychiatric diseases related to diet and gut microbiome alterations. Genes 2023, 14, 1506. [Google Scholar] [CrossRef]
- Murru, A.; Anmella, G.; Giménez, A.; Vieta, E. Bipolar disorders, obesity, and metabolic disturbances: Mechanisms and implications. In Neurobiology of Bipolar Disorder; Elsevier: Amsterdam, The Netherlands, 2021; pp. 257–274. [Google Scholar]
- Lionaki, E.; Ploumi, C.; Tavernarakis, N. One-carbon metabolism: Pulling the strings behind aging and neurodegeneration. Cells 2022, 11, 214. [Google Scholar] [CrossRef]
- Yu, Y.; Martins, L.M. Mitochondrial One-Carbon Metabolism and Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 6302. [Google Scholar] [CrossRef]
- Frajerman, A.; Urban, M.; Rivollier, F.; Plaze, M.; Chaumette, B.; Krebs, M.-O.; Scoriels, L. Abnormalities in one-carbon metabolism in young patients with psychosis. Front. Psychiatry 2023, 14, 1128890. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Zhou, J.-R.; Thiagalingam, S. Cataloging recent advances in epigenetic alterations in major mental disorders and autism. Epigenomics 2021, 13, 1231–1245. [Google Scholar] [CrossRef]
- Nohesara, S.; Mostafavi Abdolmaleky, H.; Thiagalingam, S. Substance-induced psychiatric disorders, epigenetic and microbiome alterations, and potential for therapeutic interventions. Brain Sci. 2024, 14, 769. [Google Scholar] [CrossRef]
- Tao, K.; Yuan, Y.; Xie, Q.; Dong, Z. Relationship between human oral microbiome dysbiosis and neuropsychiatric diseases: An updated overview. Behav. Brain Res. 2024, 471, 115111. [Google Scholar] [CrossRef]
- Kerstens, R.; Ng, Y.Z.; Pettersson, S.; Jayaraman, A. Balancing the oral–gut–brain axis with diet. Nutrients 2024, 16, 3206. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Malcangi, G.; Semjonova, A.; Inchingolo, A.M.; Patano, A.; Coloccia, G.; Ceci, S.; Marinelli, G.; Di Pede, C.; Ciocia, A.M. Oralbiotica/oralbiotics: The impact of oral microbiota on dental health and demineralization: A systematic review of the literature. Children 2022, 9, 1014. [Google Scholar] [CrossRef]
- Sun, X.; Li, M.; Xia, L.; Fang, Z.; Yu, S.; Gao, J.; Feng, Q.; Yang, P. Alteration of salivary microbiome in periodontitis with or without type-2 diabetes mellitus and metformin treatment. Sci. Rep. 2020, 10, 15363. [Google Scholar] [CrossRef]
- D’Angelo, E.; Fiori, F.; Ferraro, G.A.; Tessitore, A.; Nazzaro, L.; Serpico, R.; Contaldo, M. Autism Spectrum Disorder, Oral Implications, and Oral Microbiota. Children 2025, 12, 368. [Google Scholar] [CrossRef]
- Kisely, S. Periodontal health and psychiatric disorders. Curr. Oral Health Rep. 2023, 10, 111–116. [Google Scholar] [CrossRef]
- D’ambrosio, F.; Caggiano, M.; Schiavo, L.; Savarese, G.; Carpinelli, L.; Amato, A.; Iandolo, A. Chronic stress and depression in periodontitis and peri-implantitis: A narrative review on neurobiological, neurobehavioral and immune–microbiome interplays and clinical management implications. Dent. J. 2022, 10, 49. [Google Scholar] [CrossRef]
- Gupta, U.; Dey, P. The oral microbial odyssey influencing chronic metabolic disease. Arch. Physiol. Biochem. 2024, 130, 831–847. [Google Scholar] [CrossRef]
- Sun, H.-L.; Feng, Y.; Zhang, Q.; Li, J.-X.; Wang, Y.-Y.; Su, Z.; Cheung, T.; Jackson, T.; Sha, S.; Xiang, Y.-T. The microbiome–gut–brain axis and dementia: A bibliometric analysis. Int. J. Environ. Res. Public Health 2022, 19, 16549. [Google Scholar] [CrossRef]
- Alex, A.M.; Levendosky, A.A.; Bogat, G.A.; Muzik, M.; Nuttall, A.K.; Knickmeyer, R.C.; Lonstein, J.S. Stress and mental health symptoms in early pregnancy are associated with the oral microbiome. BMJ Ment Health 2024, 27, e301100. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Piras, E.; Tamburini, S.; Bu, K.; Wallach, D.S.; Remsen, B.; Cantor, A.; Kong, J.; Goetz, D.; Hoffman, K.W. Gut and oral microbiome modulate molecular and clinical markers of schizophrenia-related symptoms: A transdiagnostic, multilevel pilot study. Psychiatry Res. 2023, 326, 115279. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Meng, H.; Gao, X.; Xu, L.; Feng, X. Effect of non-surgical periodontal treatment on short chain fatty acid levels in gingival crevicular fluid of patients with generalized aggressive periodontitis. J. Periodontal Res. 2014, 49, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Leonov, G.E.; Varaeva, Y.R.; Livantsova, E.N.; Starodubova, A.V. The complicated relationship of short-chain fatty acids and oral microbiome: A narrative review. Biomedicines 2023, 11, 2749. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; Oliveira, M.T.; Cruz, S.S.d.; Cerqueira, E.d.M.M.; Trindade, S.C.; Vieira, G.O.; Couto Souza, P.H.; Adan, L.F.F.; Hintz, A.M.; Passos-Soares, J.d.S. Periodontitis is a factor associated with dyslipidemia. Oral Dis. 2022, 28, 813–823. [Google Scholar] [CrossRef]
- Bitencourt, F.V.; Nascimento, G.G.; Costa, S.A.; Orrico, S.R.P.; Ribeiro, C.C.C.; Leite, F.R.M. The role of dyslipidemia in periodontitis. Nutrients 2023, 15, 300. [Google Scholar] [CrossRef]
- Ghitea, T.C. Correlation of periodontal bacteria with chronic inflammation present in patients with metabolic syndrome. Biomedicines 2021, 9, 1709. [Google Scholar] [CrossRef]
- Iwashita, M.; Hayashi, M.; Nishimura, Y.; Yamashita, A. The link between periodontal inflammation and obesity. Curr. Oral Health Rep. 2021, 8, 76–83. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, Z.; Pathak, J.L.; Li, H.; Wu, G.; Xu, S.; Wang, T.; Cheng, H.; Piao, Z.; Jaspers, R.T. Leptin-deficient ob/ob mice exhibit periodontitis phenotype and altered oral microbiome. J. Periodontal Res. 2023, 58, 392–402. [Google Scholar] [CrossRef]
- Sato, K.; Yamazaki, K.; Kato, T.; Nakanishi, Y.; Tsuzuno, T.; Yokoji-Takeuchi, M.; Yamada-Hara, M.; Miura, N.; Okuda, S.; Ohno, H. Obesity-related gut microbiota aggravates alveolar bone destruction in experimental periodontitis through elevation of uric acid. MBio 2021, 12, e0077121. [Google Scholar] [CrossRef]
- Jia, R.; Zhang, Y.; Wang, Z.; Hu, B.; Wang, Z.; Qiao, H. Association between lipid metabolism and periodontitis in obese patients: A cross-sectional study. BMC Endocr. Disord. 2023, 23, 119. [Google Scholar] [CrossRef]
- Wang, M.; Li, L.; Qian, J.; Wang, N.; Bao, J.; Lu, J.; Chen, F.; Li, Y.; Zhang, Y.; Yan, F. Periodontitis salivary microbiota exacerbates nonalcoholic fatty liver disease in high-fat diet-induced obese mice. IScience 2023, 26, 106346. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Minty, M.; Canceill, T.; Loubières, P.; Azalbert, V.; Tercé, F.; Champion, C.; Burcelin, R.; Barthet, P.; Laurencin-Dalicieux, S. Obesity drives an oral microbiota signature of female patients with periodontitis: A pilot study. Diagnostics 2021, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Laurencin-Dalicieux, S.; Minty, M.; Assoulant-Anduze, J.; Vinel, A.; Yanat, N.; Loubieres, P.; Azalbert, V.; Diemer, S.; Burcelin, R. Obesity is associated with the severity of periodontal inflammation due to a specific signature of subgingival microbiota. Int. J. Mol. Sci. 2023, 24, 15123. [Google Scholar] [CrossRef]
- Rahman, B.; Al-Marzooq, F.; Saad, H.; Benzina, D.; Al Kawas, S. Dysbiosis of the subgingival microbiome and relation to periodontal disease in association with obesity and overweight. Nutrients 2023, 15, 826. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Z.; Wang, J.; Su, X.; Yang, J.; Zhang, Q.; Zhang, L. The oral microbiome profile and biomarker in Chinese type 2 diabetes mellitus patients. Endocrine 2020, 68, 564–572. [Google Scholar] [CrossRef]
- Wang, A.; Sun, Y.; Xu, M.; Qin, Q.; Zhu, W.; Xu, Y. The relationship with and effect of oral microbiota on NLRP3 inflammatory pathway in type 2 diabetes mellitus. Arch. Oral Biol. 2023, 155, 105801. [Google Scholar] [CrossRef]
- Guo, X.-j.; Dai, S.-x.; Lou, J.-d.; Ma, X.-x.; Hu, X.-j.; Tu, L.-p.; Cui, J.; Lu, H.; Jiang, T.; Xu, J.-t. Distribution characteristics of oral microbiota and its relationship with intestinal microbiota in patients with type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1119201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qian, F.; Cheng, X.; Wang, D.; Wang, Y.; Pan, Y.; Chen, L.; Wang, W.; Tian, Y. Dysbiosis of oral microbiota and metabolite profiles associated with type 2 diabetes mellitus. Microbiol. Spectr. 2023, 11, e03796-22. [Google Scholar] [CrossRef] [PubMed]
- Prince, Y.; Davison, G.M.; Davids, S.F.; Erasmus, R.T.; Kengne, A.P.; Graham, L.M.; Raghubeer, S.; Matsha, T.E. The relationship between the oral microbiota and metabolic syndrome. Biomedicines 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Dong, T.; Yuan, K.-Y.; Wang, N.-J.; Xia, F.-Z.; Liu, D.; Wang, Z.-M.; Ma, R.; Lu, Y.-L.; Huang, Z.-W. Shifts in the bacterial community of supragingival plaque associated with metabolic-associated fatty liver disease. Front. Cell. Infect. Microbiol. 2020, 10, 581888. [Google Scholar] [CrossRef]
- Takagi, K.; Tamura, Y.; Narita, N.; Komatsu, S.; Yamazaki, S.; Matsumura, A.; Kubota, K.; Matsumiya, T.; Sawada, K.; Nakaji, S. Involvement of Megasphaera in the oral microbiome and dyslipidemia onset: Evidence from a community-based study in Japan. Folia Microbiol. 2025, 1–12, online ahead of print. [Google Scholar] [CrossRef]
- San-Cristobal, R.; Navas-Carretero, S.; Milagro, F.I.; Riezu-Boj, J.I.; Guruceaga, E.; Celis-Morales, C.; Livingstone, K.M.; Brennan, L.; Lovegrove, J.A.; Daniel, H. Gene methylation parallelisms between peripheral blood cells and oral mucosa samples in relation to overweight. J. Physiol. Biochem. 2016, 73, 465–474. [Google Scholar] [CrossRef]
- Kalea, A.; Hoteit, R.; Suvan, J.; Lovering, R.; Palmen, J.; Cooper, J.; Khodiyar, V.; Harrington, Z.; Humphries, S.; D’Aiuto, F. Upregulation of gingival tissue miR-200b in obese periodontitis subjects. J. Dent. Res. 2015, 94, 59S–69S. [Google Scholar] [CrossRef]
- Byun, J.-S.; Lee, H.Y.; Tian, J.; Moon, J.S.; Choi, J.; Lee, S.-H.; Kim, Y.-G.; Yi, H.-S. Effect of salivary exosomal miR-25-3p on periodontitis with insulin resistance. Front. Immunol. 2022, 12, 775046. [Google Scholar]
- Liu, L.; Xiao, Z.; Ding, W.; Wen, C.; Ge, C.; Xu, K.; Cao, S. Relationship between microRNA expression and inflammatory factors in patients with both type 2 diabetes mellitus and periodontal disease. Am. J. Transl. Res. 2022, 14, 6627. [Google Scholar]
- Radović, N.; Nikolić Jakoba, N.; Petrović, N.; Milosavljević, A.; Brković, B.; Roganović, J. Micro RNA-146a and micro RNA-155 as novel crevicular fluid biomarkers for periodontitis in non-diabetic and type 2 diabetic patients. J. Clin. Periodontol. 2018, 45, 663–671. [Google Scholar] [CrossRef]
- Al-Rawi, N.H.; Al-Marzooq, F.; Al-Nuaimi, A.S.; Hachim, M.Y.; Hamoudi, R. Salivary microRNA 155, 146a/b and 203: A pilot study for potentially non-invasive diagnostic biomarkers of periodontitis and diabetes mellitus. PLoS ONE 2020, 15, e0237004. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Wang, P.-Y.; Lin, C.-Y.; Hung, S.-C. Association of the oral microbiome with cognitive function among older adults: Nhanes 2011–2012. J. Nutr. Health Aging 2024, 28, 100264. [Google Scholar] [CrossRef]
- Qiao, Y.; Gong, W.; Li, B.; Xu, R.; Wang, M.; Shen, L.; Shi, H.; Li, Y. Oral microbiota changes contribute to autism spectrum disorder in mice. J. Dent. Res. 2022, 101, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.-y.; Hau, C.C.-f.; Tong, W.-m.; Watt, R.M.; Yiu, C.K.Y.; Shum, K.K.-m. Alterations of oral microbiota in young children with autism: Unraveling potential biomarkers for early detection. J. Dent. 2025, 152, 105486. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xu, M.; Xiao, K.; Yu, K. Association between oral microbiome and depression: A population-based study. J. Affect. Disord. 2025, 379, 441–447. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Shi, C.; Zhai, T.; Zhu, J.; Wei, D.; Shen, J.; Liu, Z.; Jia, K.; Zhao, L. Mechanisms of oral microflora in Parkinson’s disease. Behav. Brain Res. 2024, 474, 115200. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar]
- Panzarella, V.; Mauceri, R.; Baschi, R.; Maniscalco, L.; Campisi, G.; Monastero, R. Oral health status in subjects with amnestic mild cognitive impairment and Alzheimer’s disease: Data from the Zabut Aging Project. J. Alzheimer’s Dis. 2022, 87, 173–183. [Google Scholar]
- Wan, J.; Fan, H. Oral microbiome and alzheimer’s disease. Microorganisms 2023, 11, 2550. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Haditsch, U.; Roth, T.; Rodriguez, L.; Hancock, S.; Cecere, T.; Nguyen, M.; Arastu-Kapur, S.; Broce, S.; Raha, D.; Lynch, C.C. Alzheimer’s disease-like neurodegeneration in Porphyromonas gingivalis infected neurons with persistent expression of active gingipains. J. Alzheimer’s Dis. 2020, 75, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-C.; Tsai, S.-J.; Hsu, J.-W.; Huang, K.-L.; Chen, T.-J.; Chen, M.-H. Risk of periodontitis in adolescents with bipolar disorder: A cohort study of 21,255 subjects. Eur. Child Adolesc. Psychiatry 2024, 33, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Fatima, Z.; Bey, A.; Azmi, S.; Gupta, N.; Khan, A. Mental depression as a risk factor for periodontal disease: A case–control study. Eur. J. Gen. Dent. 2016, 5, 86–89. [Google Scholar] [CrossRef]
- Yolken, R.; Prandovszky, E.; Severance, E.G.; Hatfield, G.; Dickerson, F. The oropharyngeal microbiome is altered in individuals with schizophrenia and mania. Schizophr. Res. 2021, 234, 51–57. [Google Scholar] [CrossRef]
- Ling, Z.; Cheng, Y.; Liu, X.; Yan, X.; Wu, L.; Shao, L.; Gao, J.; Lei, W.; Song, Q.; Zhao, L. Altered oral microbiota and immune dysfunction in Chinese elderly patients with schizophrenia: A cross-sectional study. Transl. Psychiatry 2023, 13, 383. [Google Scholar] [CrossRef]
- Lin, D.; Fu, Z.; Liu, J.; Perrone-Bizzozero, N.; Hutchison, K.E.; Bustillo, J.; Du, Y.; Pearlson, G.; Calhoun, V.D. Association between the oral microbiome and brain resting state connectivity in schizophrenia. Schizophr. Res. 2024, 270, 392–402. [Google Scholar] [CrossRef]
- Huang, H.; Yang, N.; Chen, M.-m.; Chen, X.; Chen, W.; Li, X.; Chen, Y.; Deng, Z.; Zhou, W.; Xu, S.-x. Altered oral health and microbiota in drug-free patients with schizophrenia. BMC Psychiatry 2025, 25, 274. [Google Scholar] [CrossRef]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2023, 29, 1341–1355. [Google Scholar] [CrossRef]
- Na, H.S.; Jung, N.Y.; Song, Y.; Kim, S.Y.; Kim, H.J.; Lee, J.Y.; Chung, J. A distinctive subgingival microbiome in patients with periodontitis and Alzheimer’s disease compared with cognitively unimpaired periodontitis patients. J. Clin. Periodontol. 2024, 51, 43–53. [Google Scholar] [CrossRef]
- Sritana, N.; Phungpinij, A. Analysis of Oral Microbiota in Elderly Thai Patients with Alzheimer’s Disease and Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2024, 21, 1242. [Google Scholar] [CrossRef]
- Mihaila, D.; Donegan, J.; Barns, S.; LaRocca, D.; Du, Q.; Zheng, D.; Vidal, M.; Neville, C.; Uhlig, R.; Middleton, F.A. The oral microbiome of early stage Parkinson’s disease and its relationship with functional measures of motor and non-motor function. PLoS ONE 2019, 14, e0218252. [Google Scholar] [CrossRef] [PubMed]
- Manghi, P.; Filosi, M.; Zolfo, M.; Casten, L.G.; Garcia-Valiente, A.; Mattevi, S.; Heidrich, V.; Golzato, D.; Perini, S.; Thomas, A.M. Large-scale metagenomic analysis of oral microbiomes reveals markers for autism spectrum disorders. Nat. Commun. 2024, 15, 9743. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, B.; Lapsley, C.; McDowell, A.; Miliotis, G.; McLafferty, M.; O’Neill, S.M.; Coleman, S.; McGinnity, T.M.; Bjourson, A.J.; Murray, E.K. Variations in the oral microbiome are associated with depression in young adults. Sci. Rep. 2021, 11, 15009. [Google Scholar] [CrossRef] [PubMed]
- Agranyoni, O.; Rowley, T.; Johnson, S.B.; Volk, H.; Schleif, W.; Hernandez, R.G.; Klein, L.M.; Yolken, R.H.; Sabunciyan, S. Oral microbiome composition is associated with depressive symptoms during pregnancy. Brain Behav. Immun. Health 2025, 45, 100978. [Google Scholar] [CrossRef]
- Nohesara, S.; Abdolmaleky, H.M.; Dickerson, F.; Pinto-Tomas, A.A.; Jeste, D.V.; Thiagalingam, S. Associations of microbiome pathophysiology with social activity and behavior are mediated by epigenetic modulations: Avenues for designing innovative therapeutic strategies. Neurosci. Biobehav. Rev. 2025, 174, 106208. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Nohesara, S.; Zhou, J.-R.; Thiagalingam, S. Epigenetics in evolution and adaptation to environmental challenges: Pathways for disease prevention and treatment. Epigenomics 2025, 17, 317–333. [Google Scholar] [CrossRef]
- Hernandez Martinez, C.d.J.; Glessner, J.; Finoti, L.S.; Silva, P.F.; Messora, M.; Coletta, R.D.; Hakonarson, H.; Palioto, D.B. Methylome-wide analysis in systemic microbial-induced experimental periodontal disease in mice with different susceptibility. Front. Cell. Infect. Microbiol. 2024, 14, 1369226. [Google Scholar] [CrossRef]
- Takano, M.; Nishihara, R.; Sugano, N.; Matsumoto, K.; Yamada, Y.; Takane, M.; Fujisaki, Y.; Ito, K. The effect of systemic anti-tumor necrosis factor-alpha treatment on Porphyromonas gingivalis infection in type 2 diabetic mice. Arch. Oral Biol. 2010, 55, 379–384. [Google Scholar] [CrossRef]
- Palioto, D.B.; Finoti, L.S.; Kinane, D.F.; Benakanakere, M. Epigenetic and inflammatory events in experimental periodontitis following systemic microbial challenge. J. Clin. Periodontol. 2019, 46, 819–829. [Google Scholar] [CrossRef]
- Niwa, T.; Ushijima, T. Induction of epigenetic alterations by chronic inflammation and its significance on carcinogenesis. Adv. Genet. 2010, 71, 41–56. [Google Scholar]
- Claycombe, K.J.; Brissette, C.A.; Ghribi, O. Epigenetics of inflammation, maternal infection, and nutrition. J. Nutr. 2015, 145, 1109S–1115S. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.; Marzi, C.; Aslibekyan, S.; Mendelson, M.M.; Conneely, K.N.; Tanaka, T.; Colicino, E.; Waite, L.L.; Joehanes, R.; Guan, W. DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Sorolla, C.; Garcia-Gomez, A.; Català-Moll, F.; Toledano, V.; Ciudad, L.; Avendaño-Ortiz, J.; Maroun-Eid, C.; Martín-Quirós, A.; Martínez-Gallo, M.; Ruiz-Sanmartín, A. Inflammatory cytokines and organ dysfunction associate with the aberrant DNA methylome of monocytes in sepsis. Genome Med. 2019, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Jiao, Y.; Larsson, L.; Almeida, L.; Garaicoa-Pazmino, C.; Le, J.; Squarize, C.; Inohara, N.; Giannobile, W.V.; Castilho, R. Epigenetic modifications of histones in periodontal disease. J. Dent. Res. 2016, 95, 215–222. [Google Scholar] [CrossRef]
- Liaw, A.; Liu, C.; Bartold, M.; Ivanovski, S.; Han, P. Salivary histone deacetylase in periodontal disease: A cross-sectional pilot study. J. Periodontal Res. 2023, 58, 433–443. [Google Scholar] [CrossRef]
- Liaw, A.; Liu, C.; Bartold, M.; Ivanovski, S.; Han, P. Effect of non-surgical periodontal therapy on salivary histone deacetylases expression: A prospective clinical study. J. Clin. Periodontol. 2024, 51, 926–935. [Google Scholar] [CrossRef]
- Görgülü, N.G.; Doğan, B. Effect of non-surgical periodontal treatment on salivary and serum biomarkers in stage III grade B and C periodontitis. J. Periodontol. 2022, 93, 1191–1205. [Google Scholar] [CrossRef]
- Olsen, I.; Singhrao, S.K.; Osmundsen, H. Periodontitis, pathogenesis and progression: miRNA-mediated cellular responses to Porphyromonas gingivalis. J. Oral Microbiol. 2017, 9, 1333396. [Google Scholar] [CrossRef]
- Nayar, G.; Gauna, A.; Chukkapalli, S.; Velsko, I.; Kesavalu, L.; Cha, S. Polymicrobial infection alter inflammatory microRNA in rat salivary glands during periodontal disease. Anaerobe 2016, 38, 70–75. [Google Scholar] [CrossRef]
- Sharma, R.; Frasch, M.G.; Zelgert, C.; Zimmermann, P.; Fabre, B.; Wilson, R.; Waldenberger, M.; MacDonald, J.W.; Bammler, T.K.; Lobmaier, S.M. Maternal–fetal stress and DNA methylation signatures in neonatal saliva: An epigenome-wide association study. Clin. Epigenetics 2022, 14, 87. [Google Scholar] [CrossRef]
- Ragusa, M.; Santagati, M.; Mirabella, F.; Lauretta, G.; Cirnigliaro, M.; Brex, D.; Barbagallo, C.; Domini, C.N.; Gulisano, M.; Barone, R. Potential associations among alteration of salivary miRNAs, saliva microbiome structure, and cognitive impairments in autistic children. Int. J. Mol. Sci. 2020, 21, 6203. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, G.E.; Mabry, S.J.; Cao, X.; McMillan, C.; Woynaroski, T.G.; Harrison, F.E.; Reddy, I.A.; Matthies, H.J.; Flynn, C.R.; Wallace, M.T. Autism-associated variant in the SLC6A3 gene alters the oral microbiome and metabolism in a murine model. Front. Psychiatry 2021, 12, 655451. [Google Scholar] [CrossRef] [PubMed]
- Mercante, F.; Abbaspour, A.; Pucci, M.; Sabatucci, A.; Rania, M.; Konstantinidou, F.; Gatta, V.; Stuppia, L.; Cifani, C.; Bulik, C.M. Epigenetic alterations and microbiota changes in the saliva of individuals with binge-eating spectrum disorders compared with normal weight healthy controls. Life Sci. 2025, 374, 123695. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, L.; Ren, L.; Zhu, R.; Jiang, Y.; Qiao, Y.; Li, Y. Dysbiosis and Metabolic Dysregulation of Salivary Microbiota in Schizophrenia. J. Multidiscip. Healthc. 2025, 18, 813–825. [Google Scholar] [CrossRef]
- François, M.; Pascovici, D.; Wang, Y.; Vu, T.; Liu, J.-W.; Beale, D.; Hor, M.; Hecker, J.; Faunt, J.; Maddison, J. Saliva Proteome, Metabolome and Microbiome Signatures for Detection of Alzheimer’s Disease. Metabolites 2024, 14, 714. [Google Scholar] [CrossRef]
- Lemon, K.P.; Klepac-Ceraj, V.; Schiffer, H.K.; Brodie, E.L.; Lynch, S.V.; Kolter, R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 2010, 1, e00129-10. [Google Scholar] [CrossRef]
- Hicks, S.D.; Uhlig, R.; Afshari, P.; Williams, J.; Chroneos, M.; Tierney-Aves, C.; Wagner, K.; Middleton, F.A. Oral microbiome activity in children with autism spectrum disorder. Autism Res. 2018, 11, 1286–1299. [Google Scholar] [CrossRef]
- Nohesara, S.; Mostafavi Abdolmaleky, H.; Pirani, A.; Pettinato, G.; Thiagalingam, S. The Obesity–Epigenetics–Microbiome Axis: Strategies for Therapeutic Intervention. Nutrients 2025, 17, 1564. [Google Scholar] [CrossRef]
- Costa, C.F.; Correia-de-Sá, T.; Araujo, R.; Barbosa, F.; Burnet, P.W.; Ferreira-Gomes, J.; Sampaio-Maia, B. The oral-gut microbiota relationship in healthy humans: Identifying shared bacteria between environments and age groups. Front. Microbiol. 2024, 15, 1475159. [Google Scholar] [CrossRef]
- Bao, J.; Li, L.; Zhang, Y.; Wang, M.; Chen, F.; Ge, S.; Chen, B.; Yan, F. Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral Sci. 2022, 14, 32. [Google Scholar] [CrossRef]
- Kitamoto, S.; Nagao-Kitamoto, H.; Jiao, Y.; Gillilland, M.G.; Hayashi, A.; Imai, J.; Sugihara, K.; Miyoshi, M.; Brazil, J.C.; Kuffa, P. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 2020, 182, 447–462.e414. [Google Scholar] [CrossRef] [PubMed]
- Irie, K.; Azuma, T.; Tomofuji, T.; Yamamoto, T. Exploring the role of IL-17A in oral dysbiosis-associated periodontitis and its correlation with systemic inflammatory disease. Dent. J. 2023, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, P.; Xu, F.; Zheng, Y.; Zhao, H. Advances in the study of IL-17 in neurological diseases and mental disorders. Front. Neurol. 2023, 14, 1284304. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Mao, T.; Fan, J.; Shen, Y.; Xue, L.; Du, K.; Li, Y.; Wang, L.; Wang, X. IL-17A Induces Circadian Disruptions Through the Epigenetic Repression of BMAL1 in Mice With Alzheimer’s Disease. J. Cell. Mol. Med. 2025, 29, e70546. [Google Scholar] [CrossRef]
- Schamarek, I.; Anders, L.; Chakaroun, R.M.; Kovacs, P.; Rohde-Zimmermann, K. The role of the oral microbiome in obesity and metabolic disease: Potential systemic implications and effects on taste perception. Nutr. J. 2023, 22, 28. [Google Scholar] [CrossRef]
- Lê, S.; Cecchin-Albertoni, C.; Thomas, C.; Kemoun, P.; Minty, M.; Blasco-Baque, V. The role of dysbiotic oral microbiota in cardiometabolic diseases: A narrative review. Diagnostics 2023, 13, 3184. [Google Scholar] [CrossRef]
- Al-Roub, A.; Al Madhoun, A.; Akhter, N.; Thomas, R.; Miranda, L.; Jacob, T.; Al-Ozairi, E.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. IL-1β and TNFα cooperativity in regulating IL-6 expression in adipocytes depends on CREB binding and H3K14 acetylation. Cells 2021, 10, 3228. [Google Scholar] [CrossRef]
- Suárez, L.J.; Garzón, H.; Arboleda, S.; Rodríguez, A. Oral dysbiosis and autoimmunity: From local periodontal responses to an imbalanced systemic immunity. A review. Front. Immunol. 2020, 11, 591255. [Google Scholar] [CrossRef]
- Luo, H.; Wu, B.; Kamer, A.R.; Adhikari, S.; Sloan, F.; Plassman, B.L.; Tan, C.; Qi, X.; Schwartz, M.D. Oral health, diabetes, and inflammation: Effects of oral hygiene behaviour. Int. Dent. J. 2022, 72, 484–490. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Chen, Y.; Yu, L.; Zhou, J.; Wang, N.; Liu, T.; Fu, C. Associations of oral hygiene with incident hypertension and type 2 diabetes mellitus: A population based cohort study in Southwest China. J. Clin. Hypertens. 2022, 24, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Avcu, N.; Avcu, F.; Beyan, C.; Ural, A.U.; ad Kaptan, K.; Özyurt, M.; Nevruz, O.; Yalçin, A. The relationship between gastric-oral Helicobacter pylori and oral hygiene in patients with vitamin B12–deficiency anemia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Ge, J.; Pan, Q.; Hu, N.; Hua, F. Salivary microbiome variations in type 2 diabetes mellitus patients with different stages of periodontitis. BMC Oral Health 2024, 24, 1424. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Kirakodu, S.S.; Zhang, X.; Dawson, D., III; Miller, C.S. Salivary microbiome and biomarker characteristics of diabetics with periodontitis. Mol. Oral Microbiol. 2025, 40, 37–49. [Google Scholar] [CrossRef]
- Qin, H.; Li, G.; Xu, X.; Zhang, C.; Zhong, W.; Xu, S.; Yin, Y.; Song, J. The role of oral microbiome in periodontitis under diabetes mellitus. J. Oral Microbiol. 2022, 14, 2078031. [Google Scholar] [CrossRef]
- Li, H.; Zou, Y.; Ding, G. Dietary Factors Associated with Dental Erosion: A Meta-Analysis. PLoS ONE 2012, 7, e42626. [Google Scholar] [CrossRef]
- Jagelavičienė, E.; Vaitkevičienė, I.; Šilingaitė, D.; Šinkūnaitė, E.; Daugėlaitė, G. The relationship between vitamin D and periodontal pathology. Medicina 2018, 54, 45. [Google Scholar] [CrossRef]
- Graells, J.; Ojeda, R.M.; Muniesa, C.; Gonzalez, J.; Saavedra, J. Glossitis with linear lesions: An early sign of vitamin B12 deficiency. J. Am. Acad. Dermatol. 2009, 60, 498–500. [Google Scholar] [CrossRef]
- Ramsey, D.; Muskin, P.R. Vitamin deficiencies and mental health: How are they linked. Curr. Psychiatry 2013, 12, 37–43. [Google Scholar]
- Sultana, O.F.; Hia, R.A.; Reddy, P.H. A combinational therapy for preventing and delaying the onset of alzheimer’s disease: A focus on probiotic and vitamin co-supplementation. Antioxidants 2024, 13, 202. [Google Scholar] [CrossRef]
- Wang, S.-D.; Wang, X.; Zhao, Y.; Xue, B.-H.; Wang, X.-T.; Chen, Y.-X.; Zhang, Z.-Q.; Tian, Y.-R.; Xie, F.; Qian, L.-J. Homocysteine-induced disturbances in DNA methylation contribute to development of stress-associated cognitive decline in rats. Neurosci. Bull. 2022, 38, 887–900. [Google Scholar] [CrossRef]
- Holmes, H.E.; Valentin, R.E.; Jernerén, F.; de Jager Loots, C.A.; Refsum, H.; Smith, A.D.; Guarente, L.; Dellinger, R.W.; Sampson, D.; Initiative, A.s.D.N. Elevated homocysteine is associated with increased rates of epigenetic aging in a population with mild cognitive impairment. Aging Cell 2024, 23, e14255. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kuo, H.K.; Lai, Y.L. The association between serum folate levels and periodontal disease in older adults: Data from the National Health and Nutrition Examination Survey 2001/02. J. Am. Geriatr. Soc. 2007, 55, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Roffman, J.L.; Lamberti, J.S.; Achtyes, E.; Macklin, E.A.; Galendez, G.C.; Raeke, L.H.; Silverstein, N.J.; Smoller, J.W.; Hill, M.; Goff, D.C. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry 2013, 70, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Kumar, S.; Vaibhav, K. Role of Folic acid as adjuvant treatment in Schizophrenia: A randomized controlled trial. Eur. Psychiatry 2023, 66, S634–S635. [Google Scholar]
- An, Y.; Feng, L.; Zhang, X.; Wang, Y.; Wang, Y.; Tao, L.; Qin, Z.; Xiao, R. Dietary intakes and biomarker patterns of folate, vitamin B6, and vitamin B12 can be associated with cognitive impairment by hypermethylation of redox-related genes NUDT15 and TXNRD1. Clin. Epigenetics 2019, 11, 139. [Google Scholar] [CrossRef]
- Buzatu, R.; Luca, M.M.; Bumbu, B.A. Does Vitamin C Supplementation Provide a Protective Effect in Periodontal Health? A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 8598. [Google Scholar] [CrossRef]
- Guo, H.; Ding, J.; Liu, Q.; Li, Y.; Liang, J.; Zhang, Y. Vitamin C and metabolic syndrome: A meta-analysis of observational studies. Front. Nutr. 2021, 8, 728880. [Google Scholar] [CrossRef]
- Plevin, D.; Galletly, C. The neuropsychiatric effects of vitamin C deficiency: A systematic review. BMC Psychiatry 2020, 20, 315. [Google Scholar] [CrossRef]
- Blaschke, K.; Ebata, K.T.; Karimi, M.M.; Zepeda-Martínez, J.A.; Goyal, P.; Mahapatra, S.; Tam, A.; Laird, D.J.; Hirst, M.; Rao, A. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature 2013, 500, 222–226. [Google Scholar] [CrossRef]
- Liang, F.; Zhou, Y.; Zhang, Z.; Zhang, Z.; Shen, J. Association of vitamin D in individuals with periodontitis: An updated systematic review and meta-analysis. BMC Oral Health 2023, 23, 387. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, J. Serum vitamin D status and metabolic syndrome: A systematic review and dose-response meta-analysis. Nutr. Res. Pract. 2021, 15, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, K.M.; Checinska, K.; Kulczyk, K.; Janas-Kozik, M. Vitamin D deficiency and depressive symptoms: Meta-analysis of studies. Psychiatr. Pol. 2022, 56, 1327–1344. [Google Scholar]
- Zhu, J.-l.; Luo, W.-w.; Cheng, X.; Li, Y.; Zhang, Q.-z.; Peng, W.-x. Vitamin D deficiency and schizophrenia in adults: A systematic review and meta-analysis of observational studies. Psychiatry Res. 2020, 288, 112959. [Google Scholar] [CrossRef]
- Jiang, X.; Xia, L.; Tang, T.; Fan, X.; Wang, R.; Wang, M.; Yang, W.; Yan, J.; Qi, K.; Li, P. Decreased vitamin D bio-availability with altered DNA methylation of its metabolism genes in association with the metabolic disorders among the school-aged children with degree I, II, and III obesity. J. Nutr. Biochem. 2024, 129, 109627. [Google Scholar] [CrossRef]
- Xue, J.; Schoenrock, S.A.; Valdar, W.; Tarantino, L.M.; Ideraabdullah, F.Y. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin. Epigenetics 2016, 8, 107. [Google Scholar] [CrossRef]
- Fischbach, M.A. Microbiome: Focus on causation and mechanism. Cell 2018, 174, 785–790. [Google Scholar] [CrossRef]
- Walter, J.; Armet, A.M.; Finlay, B.B.; Shanahan, F. Establishing or exaggerating causality for the gut microbiome: Lessons from human microbiota-associated rodents. Cell 2020, 180, 221–232. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Luo, S.-C.; Wei, S.-M.; Luo, X.-T.; Yang, Q.-Q.; Wong, K.-H.; Cheung, P.C.; Zhang, B.-B. How probiotics, prebiotics, synbiotics, and postbiotics prevent dental caries: An oral microbiota perspective. npj Biofilms Microbiomes 2024, 10, 14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).