The Role of Vitamins in Sepsis: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. The Therapeutic Potential of Vitamins in Sepsis Management
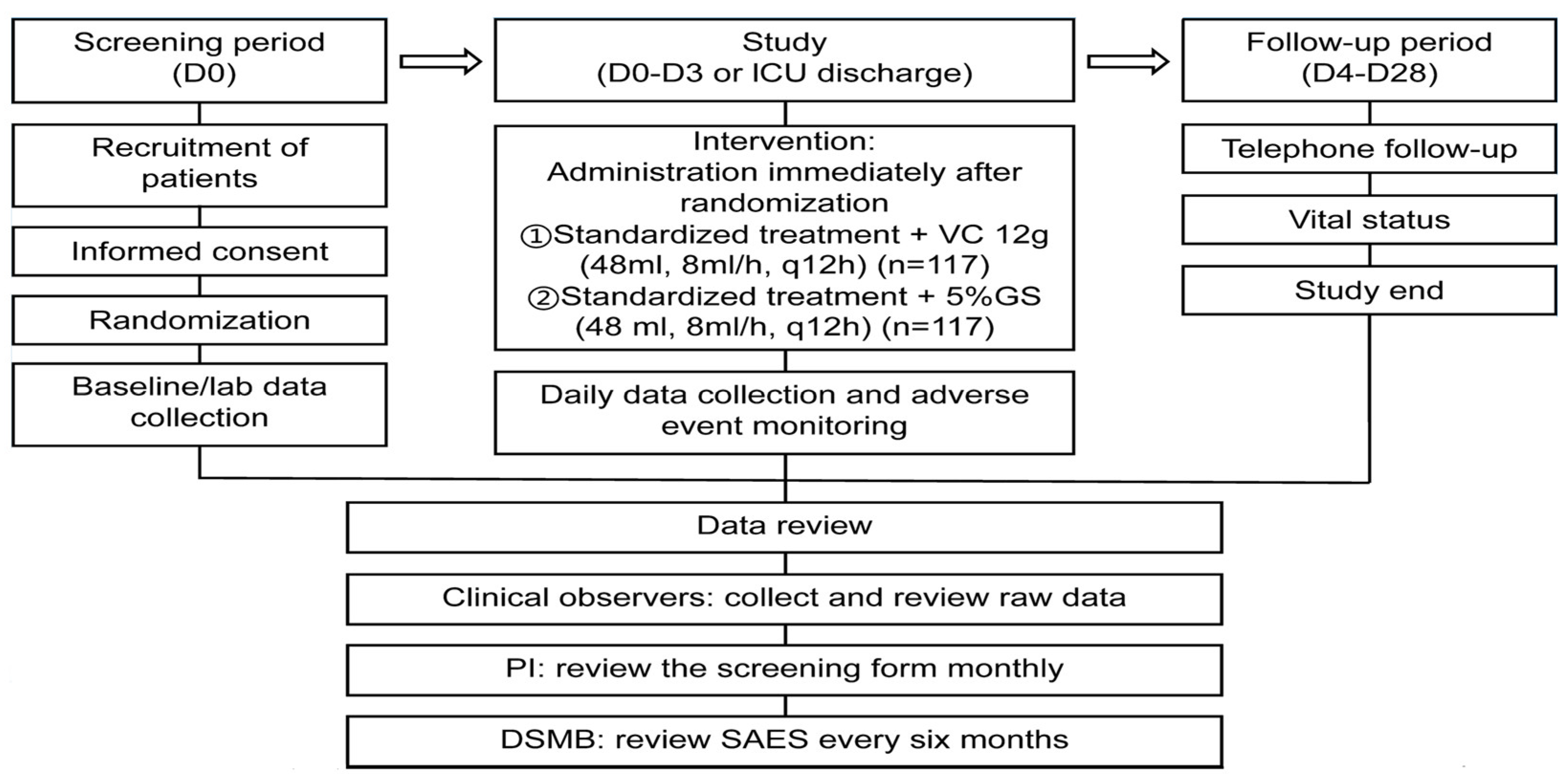
3.1. Vitamin C
3.2. Thiamine
3.3. Vitamin D
3.4. Folic Acid and Other B Vitamins
4. Nutritional Status and Microbiome Quality as Predictors of Mortality in Sepsis
5. Study Limitations
6. Conclusions
- Vitamin C: Large-scale trials have not conclusively proven a mortality benefit, despite its well-documented antioxidant properties and microcirculatory effects.
- Vitamin D: Supplementation trials in sepsis and ICU settings have yielded mixed or inconclusive results. Benefits may be confined to specific, severely deficient subgroups, highlighting the need for a targeted approach.
- Vitamin B1: Benefits are primarily suggested in patients with documented deficiency, where intravenous administration may support metabolic balance, though overall RCT evidence remains inconclusive.
- Vitamins B9 and B12: Evidence is least robust; some studies suggest that measured plasma levels may be misleading due to rapid redistribution and altered binding proteins in the acute phase.
- Patient Selection: Focusing on high-risk subgroups, such as malnourished patients, the elderly, or those with pre-existing conditions (e.g., chronic liver disease or malabsorption) likely to entail deficiency.
- Tissue Sufficiency: Developing methods to measure tissue-level sufficiency rather than relying solely on potentially misleading plasma levels in the acute setting.
- Standardization: Implementing standardized dosing protocols and clearly defined clinical endpoints in well-designed multicenter trials to reliably assess the true role of specific vitamins as adjunctive therapy in sepsis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Sepsis. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/sepsis (accessed on 21 October 2025).
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Prescott, H.C.; Angus, D.C. Enhancing Recovery From Sepsis: A Review. JAMA 2018, 319, 62–75. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Harrison, D.A.; Ferrando-Vivas, P.; Rubenfeld, G.D.; Rowan, K. Risk Factors at Index Hospitalization Associated With Longer-term Mortality in Adult Sepsis Survivors. JAMA Netw. Open 2019, 2, e194900. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J.H. Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; DeWilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Fujii, T.; Luethi, N.; Young, P.J.; Frei, D.R.; Eastwood, G.M.; French, C.J.; Deane, A.M.; Shehabi, Y.; Hajjar, L.A.; Oliveira, G.; et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs. Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA 2020, 323, 423–431. [Google Scholar] [CrossRef]
- Lamontagne, F.; Masse, M.H.; Menard, J.; Sprague, S.; Pinto, R.; Heyland, D.K.; Cook, D.J.; Battista, M.C.; Day, A.G.; Guyatt, G.H.; et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N. Engl. J. Med. 2022, 386, 2387–2398. [Google Scholar] [CrossRef]
- Carr, A.C. Vitamin C administration in the critically ill: A summary of recent meta-analyses. Crit. Care 2019, 23, 265. [Google Scholar] [CrossRef] [PubMed]
- Vandervelden, S.; Cortens, B.; Fieuws, S.; Eegdeman, W.; Malinverni, S.; Vanhove, P.; Monsieurs, K.; Breuls, J.; Hobloue, I.; Stifkens, F.; et al. Early administration of vitamin C in patients with sepsis or septic shock in emergency departments: A multicenter, double-blind, randomized controlled trial: The C-EASIE trial. Crit. Care 2025, 29, 160. [Google Scholar] [CrossRef] [PubMed]
- Rynne, J.; Mosavie, M.; Masse, M.H.; Ménard, J.; Battista, M.C.; Maslove, D.M.; Del Sorbo, L.; St-Arnaud, C.F.; Daragon, F.D.A.; Fox-Robichaud, A.; et al. Sepsis subtypes and differential treatment response to vitamin C: Biological sub-study of the LOVIT trial. Intensive Care Med. 2025, 51, 82–93. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, J. Vitamin C improves 28-day survival in patients with sepsis-associated acute kidney injury in the intensive care unit: A retrospective study. Front. Nutr. 2025, 12, 1600224. [Google Scholar] [CrossRef]
- Sevransky, J.E.; Rothman, R.E.; Hager, D.N.; Bernard, G.R.; Brown, S.M.; Buchman, T.G.; Busse, L.W.; Coopersmith, C.M.; DeWilde, C.; Ely, E.W.; et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial. JAMA 2021, 325, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Guan, J.; Chang, P.; Zeng, Z.; Li, J.; Chen, S.; Liu, Z. Clinical Efficacy of Megadose Vitamin C in Sepsis: Protocol for a Multicenter Randomized Single—Blind Placebo—Controlled Clinical Trial. Intensive Care Res. 2024, 4, 129–136. [Google Scholar] [CrossRef]
- Vine, J.; Lee, J.H.; Kravitz, M.S.; Grossestreuer, A.V.; Balaji, L.; Leland, S.B.; Berlin, N.; Moskowitz, A.; Donnino, M.W. Thiamine administration in septic shock: A post hoc analysis of two randomized trials. Crit. Care 2024, 28, 41. [Google Scholar] [CrossRef]
- Sangla, F.; Verissimo, T.; Faivre, A.; Glauser, T.; Cheah, S.K.; Assouline, B.; Sgardello, S.; Legouis, D. Thiamine as a metabolic resuscitator in septic shock: A meta-analysis of randomized controlled trials with trial sequential analysis. Front. Med. 2023, 10, 1223862. [Google Scholar] [CrossRef]
- Petsakul, S.; Morakul, S.; Tangsujaritvijit, V.; Kunawut, P.; Singhatas, P.; Sanguanwit, P. Effects of thiamine on vasopressor requirements in patients with septic shock: A prospective randomized controlled trial. BMC Anesthesiol. 2020, 20, 280. [Google Scholar] [CrossRef]
- Kanchanasurakit, S.; Suthumpoung, P.; Santimaleeworagun, W.; Nakaranurack, C.; Huynh, N.S.; Srisawat, C.; Nunta, M.; Chirakan, V.; Saokaew, S. Effectiveness of thiamine therapy in mortality rate in patients with septic shock: A systematic review and meta-analysis. Int. J. Crit. Illn. Inj. Sci. 2021, 11, 86–94. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jung, J.W.; Choi, J.C.; Shin, J.W.; Kim, J.Y. Subphenotypes in Patients with Septic Shock Receiving Vitamin C, Hydrocortisone, and Thiamine: A Retrospective Cohort Analysis. Nutrients 2019, 11, 2976. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Delanghe, J.R.; Speeckaert, M.M. Vitamin D Deficiency: An Underestimated Factor in Sepsis? Int. J. Mol. Sci. 2023, 24, 2924. [Google Scholar] [CrossRef]
- Ashoor, T.M.; Abd Elazim, A.E.H.; Mustafa, Z.A.E.; Anwar, M.A.; Gad, I.A.; Mamdouh Esmat, I. Outcomes of High-Dose Versus Low-Dose Vitamin D on Prognosis of Sepsis Requiring Mechanical Ventilation: A Randomized Controlled Trial. J. Intensive Care Med. 2024, 39, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, K.; Ren, Q.; Chen, L.; Zhang, Y.; Wang, G.; Xie, K. Vitamin D supplementation during intensive care unit stay is associated with improved outcomes in critically Ill patients with sepsis: A cohort study. Front. Cell Infect. Microbiol. 2025, 14, 1485554. [Google Scholar] [CrossRef]
- Cutuli, S.L.; Ferrando, E.S.; Cammarota, F.; Franchini, E.; Caroli, A.; Lombardi, G.; Tanzarella, E.S.; Grieco, D.L.; Antonelli, M.; De Pascale, G. Update on vitamin D role in severe infections and sepsis. J. Anesth. Analg. Crit. Care 2024, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, R.; Wang, K.; Leo, C.; Li, M.; Chow, A.; Yang, A.K.; Lu, Y. Evaluation of Vitamin D Supplementation in Critically Ill Patients-A Narrative Review of Randomized Controlled Trials Published in the Last 5 Years. Nutrients 2025, 17, 816. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhu, Y.; Zheng, X.; Li, T.; Niu, K.; Wang, Z.; Lu, X.; Zhang, Y.; Shen, C. Vitamin D Supplementation during Intensive Care Unit Stay Is Associated with Improved Outcomes in Critically Ill Patients with Sepsis: A Cohort Study. Nutrients 2023, 15, 2924. [Google Scholar] [CrossRef]
- Shang, S.; Chen, D.; Wei, Y.; Zou, S.; Chang, Q.; Zhou, H.; Yu, A. The Role of Vitamin D and Vitamin D Receptor in Sepsis. Curr. Issues Mol. Biol. 2025, 47, 500. [Google Scholar] [CrossRef]
- Xu, Q.; Li, L.; Yang, Y.; Zeng, F. Research progress on B vitamins in the treatment of sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2024, 36, 1221–1225. [Google Scholar]
- Deng, J.; Zuo, Q.K.; Venugopal, K.; Hung, J.; Zubair, A.; Blais, S.; Porter, V.; Moskalyk, M.; Heybati, K. Efficacy and Safety of Hydrocortisone, Ascorbic Acid, and Thiamine Combination Therapy for the Management of Sepsis and Septic Shock: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. Arch. Allergy Immunol. 2024, 185, 997–1018. [Google Scholar] [CrossRef]
- Gamarra-Morales, Y.; Molina-López, J.; Herrera-Quintana, L.; Vázquez-Lorente, H.; Planells, E. Folic acid and vitamin B12 as biomarkers of morbidity and mortality in patients with septic shock. Nutr. Hosp. 2022, 39, 247–255. [Google Scholar] [CrossRef]
- Fah, M.; Van Althuis, L.E.; Ohnuma, T.; Winthrop, H.M.; Haines, K.L.; Williams, D.G.A.; Krishnamoorthy, V.; Raghunathan, K.; Wischmeyer, P.E. Micronutrient deficiencies in critically ill patients receiving continuous renal replacement therapy. Clin. Nutr. ESPEN 2022, 50, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, C.S.; Caesar, P.M.; Gundogan, K.; Biesalki, H.K.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Mucha, P.; Kus, F.; Cysewski, D.; Smolenski, R.T.; Tomczyk, M. Vitamin B(12) Metabolism: A Network of Multi-Protein Mediated Processes. Int. J. Mol. Sci. 2024, 25, 8021. [Google Scholar] [CrossRef]
- Pregernig, A.; Held, U.; Schläpfer, M.; Beck-Schimmer, B. Vitamin B12 status and the risk of developing sepsis in patients with bacterial infection: A prospective observational cohort study. BMC Med. 2024, 22, 330. [Google Scholar] [CrossRef]
- Toscano, A.; Bellone, F.; Maggio, N.; Cinquegrani, M.; Spadaro, F.; Bueti, F.M.; Lorello, G.; Marini, H.R.; Lo Gullo, A.; Basile, G.; et al. Unlocking the Predictive Power of Nutritional Scores in Septic Patients. Nutrients 2025, 17, 545. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Spagnuolo, F.; Candelli, M.; Voza, A.; Covino, M.; Gasbarrini, A.; Franceschi, F. The Gut Microbiome in Sepsis: From Dysbiosis to Personalized Therapy. J. Clin. Med. 2024, 13, 6082. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radkowski, P.; Gogojewicz, A.; Charasna, J.; Pilaczyńska-Szcześniak, Ł.; Grabarczyk, Ł. The Role of Vitamins in Sepsis: A Narrative Review. Nutrients 2025, 17, 3330. https://doi.org/10.3390/nu17213330
Radkowski P, Gogojewicz A, Charasna J, Pilaczyńska-Szcześniak Ł, Grabarczyk Ł. The Role of Vitamins in Sepsis: A Narrative Review. Nutrients. 2025; 17(21):3330. https://doi.org/10.3390/nu17213330
Chicago/Turabian StyleRadkowski, Paweł, Anna Gogojewicz, Joanna Charasna, Łucja Pilaczyńska-Szcześniak, and Łukasz Grabarczyk. 2025. "The Role of Vitamins in Sepsis: A Narrative Review" Nutrients 17, no. 21: 3330. https://doi.org/10.3390/nu17213330
APA StyleRadkowski, P., Gogojewicz, A., Charasna, J., Pilaczyńska-Szcześniak, Ł., & Grabarczyk, Ł. (2025). The Role of Vitamins in Sepsis: A Narrative Review. Nutrients, 17(21), 3330. https://doi.org/10.3390/nu17213330






