Single-Cell RNA-Seq Identifies Immune Remodeling in Lungs of β-Carotene Oxygenase 2 Knockout Mice with Improved Antiviral Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Single Cell Isolation and Fixation
2.3. Probe Hybridization and Library Construction
2.4. Processing and Quality Control of scRNA-Seq Data
2.5. Gene Set Variation Analysis (GSVA) Analysis
2.6. Key Visualization and Annotation
2.7. Immunoblotting
2.8. Mouse Infection of SARS-CoV-2
2.9. Statistical Analysis
3. Results
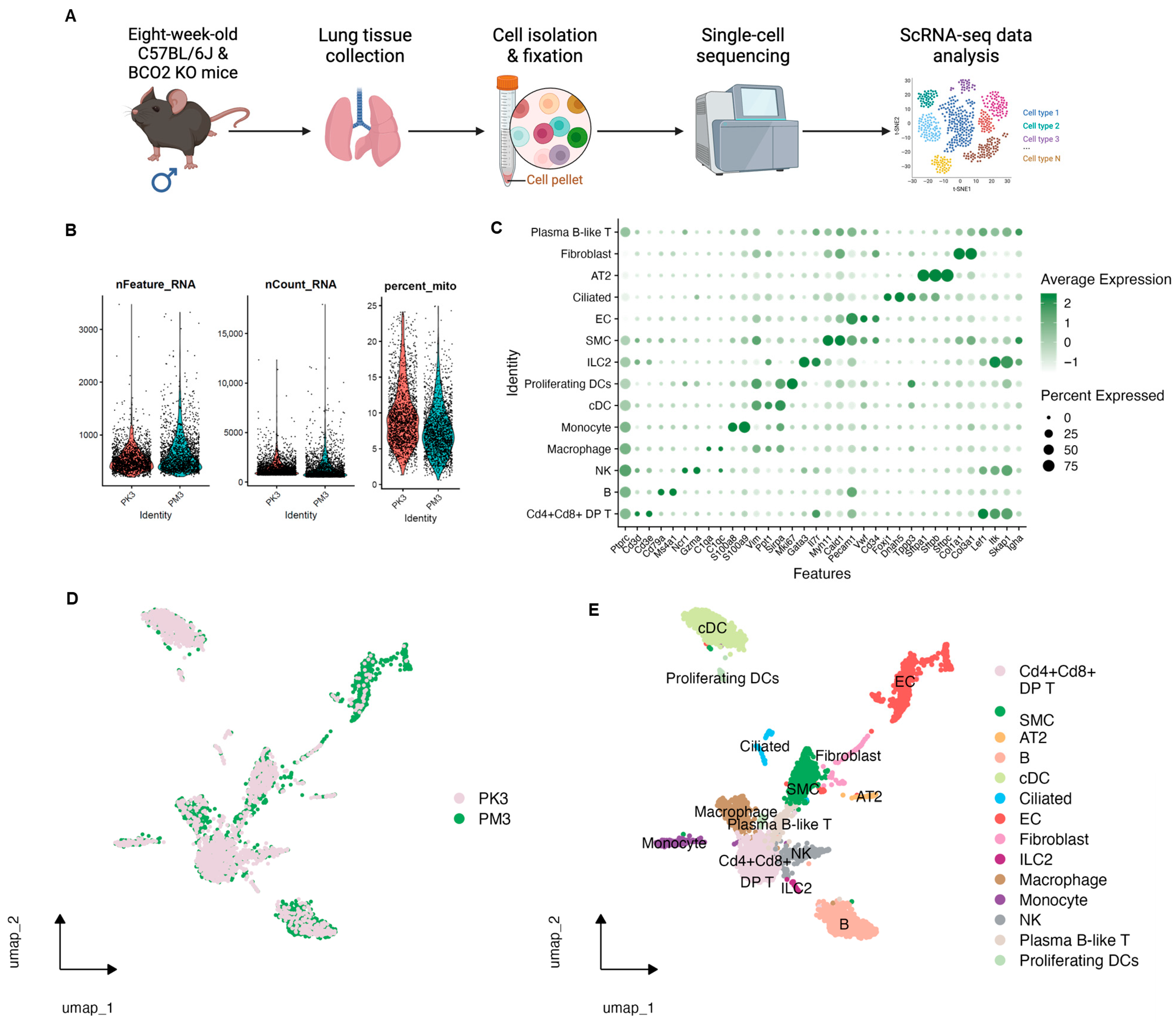
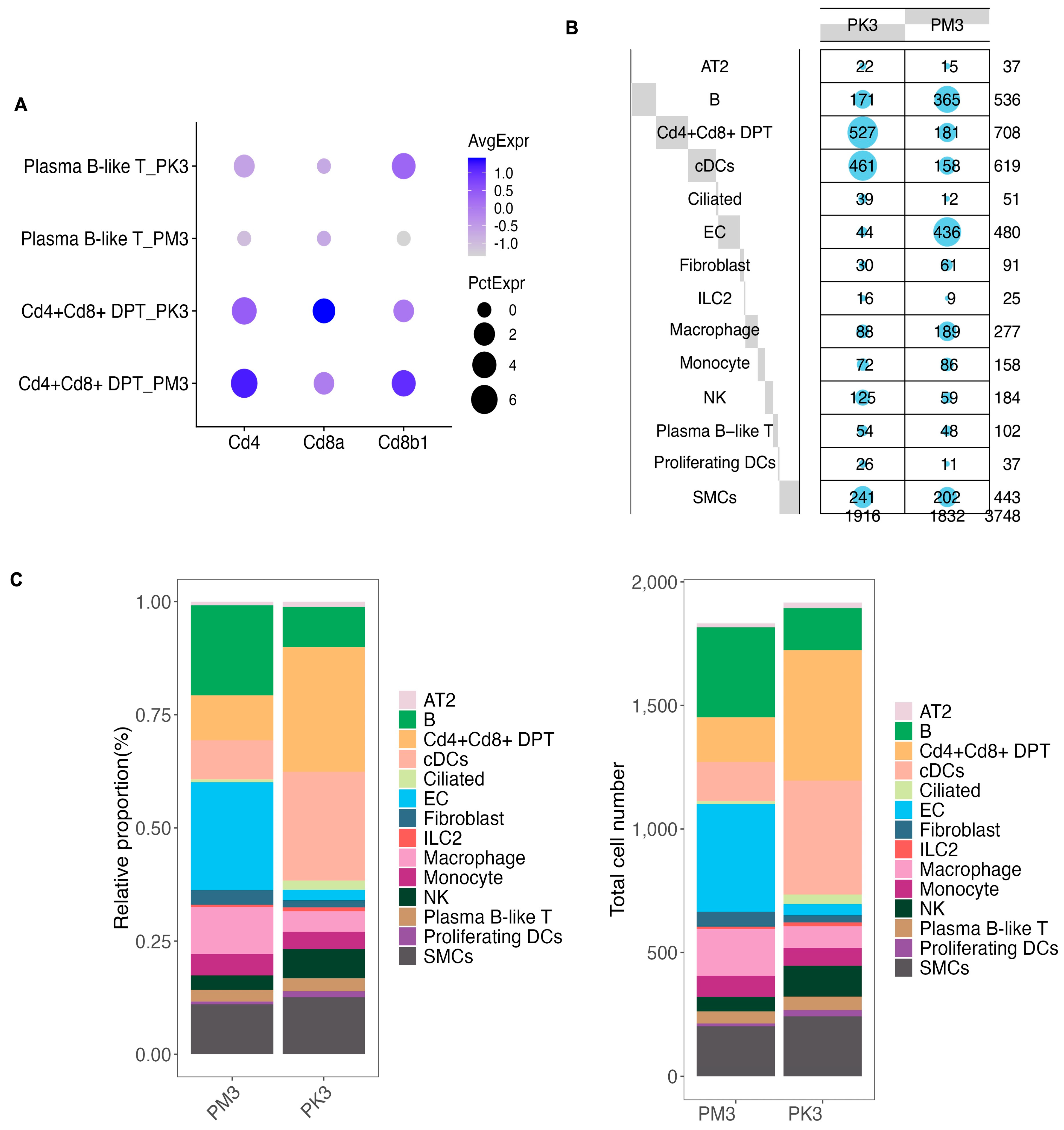
3.1. Single-Cell RNA-Seq Data Quality Control, Clustering, and Annotation
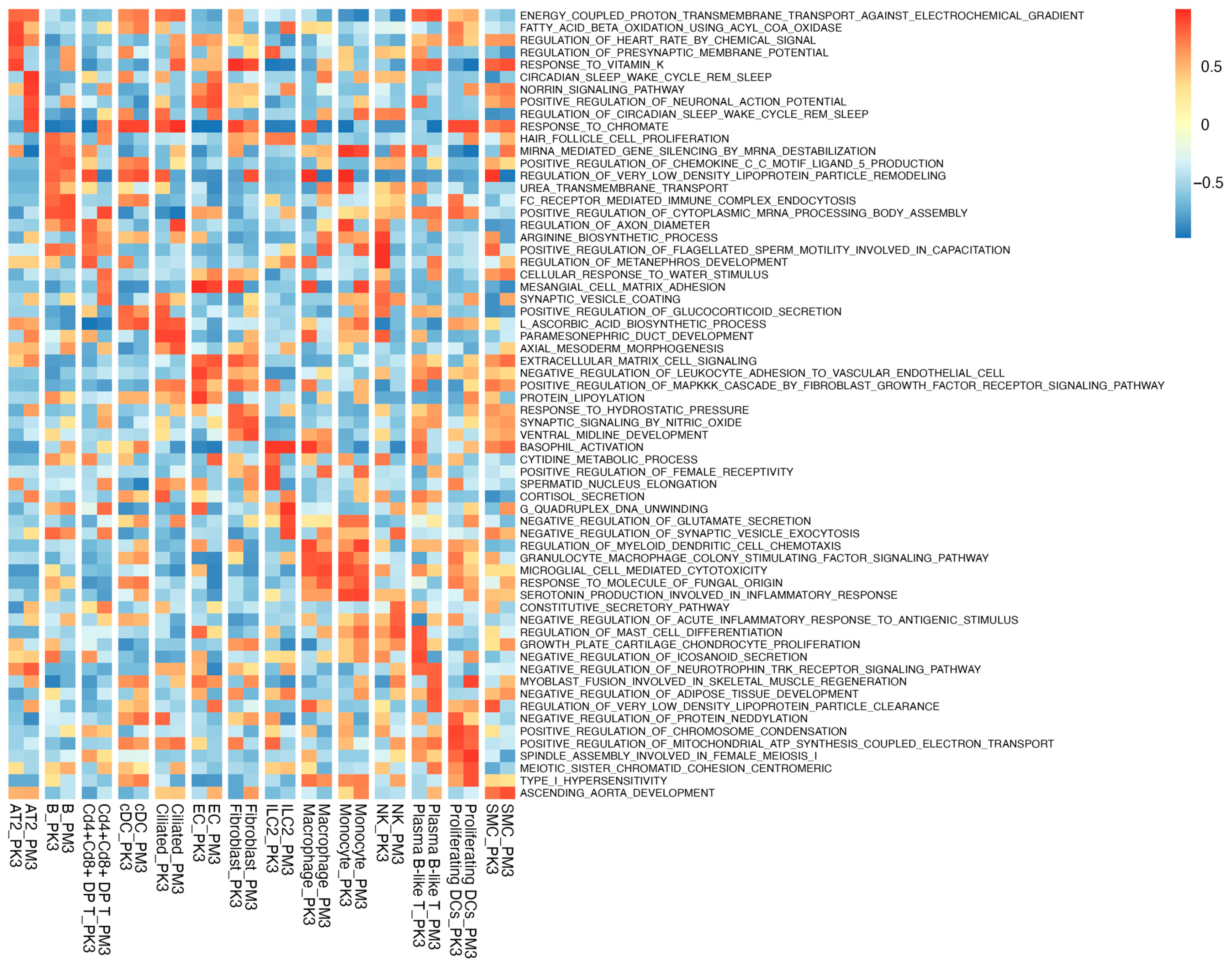
3.2. GSVA Reveals Cell-Type-Specific Pathway Shifts in BCO2 KO
3.3. BCO2 Deficiency Primes IRF3 Signaling and Improves SARS-CoV-2 Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AT2 | Alveolar type II cell |
| BCO2 | β-carotene oxygenase-2 |
| cDC | Conventional dendritic cell |
| cDNA | Complementary DNA |
| cGAS | cyclic GMP–AMP synthase |
| cGAMP | cyclic GMP–AMP |
| DP | Double-positive |
| EC | Endothelial cell |
| ER | Endoplasmic reticulum |
| ETC | Electron transport chain |
| GSVA | Gene set variation analysis |
| ILC2 | Innate lymphoid cell type 2 |
| IRF3 | Interferon regulatory factor 3 |
| ISG | Interferon-stimulated gene |
| KO | Knockout |
| MAVS | Melanoma differentiation-associated protein 5 |
| MDA5 | Mitochondrial antiviral-signaling protein |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer cell |
| OXPHOS | Oxidative phosphorylation |
| RIG-I | Retinoic acid-inducible gene I |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SMC | Smooth muscle cell |
| STINIG | Stimulator of interferon genes |
| TBK1 | TANK-binding kinase 1 |
| UMAP | Uniform Manifold Approximation and Projection |
| UMI | Unique molecular identifier |
| VLDL | Very-low-density lipoprotein |
| WT | Wild-type |
References
- Braciale, T.J.; Sun, J.; Kim, T.S. Regulating the adaptive immune response to respiratory virus infection. Nat. Rev. Immunol. 2012, 12, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.B.; Cowley, C.J.; Fuchs, E. Epithelial cells: Liaisons of immunity. Curr. Opin. Immunol. 2020, 62, 45–53. [Google Scholar] [CrossRef]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011, 25, 948–959. [Google Scholar] [CrossRef]
- Thomas, L.D.; Bandara, S.; Parmar, V.M.; Srinivasagan, R.; Khadka, N.; Golczak, M.; Kiser, P.D.; von Lintig, J. The human mitochondrial enzyme BCO2 exhibits catalytic activity toward carotenoids and apocarotenoids. J. Biol. Chem. 2020, 295, 15553–15565. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Hartson, S.D.; Davis, M.A.; He, H.; Medeiros, D.M.; Wang, W.; Clarke, S.L.; Lucas, E.A.; Smith, B.J.; et al. Lack of β,β-carotene-9′,10′-oxygenase 2 leads to hepatic mitochondrial dysfunction and cellular oxidative stress in mice. Mol. Nutr. Food Res. 2017, 61, 1600576. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lu, P.; Guo, X.; Song, K.; Lyu, Y.; Bothwell, J.; Wu, J.; Hawkins, O.; Clarke, S.L.; Lucas, E.A.; et al. β-Carotene oxygenase 2 deficiency-triggered mitochondrial oxidative stress promotes low-grade inflammation and metabolic dysfunction. Free Radic. Biol. Med. 2021, 164, 271–284. [Google Scholar] [CrossRef]
- He, M.; Cornelis, M.C.; Kraft, P.; van Dam, R.M.; Sun, Q.; Laurie, C.C.; Mirel, D.B.; Chasman, D.I.; Ridker, P.M.; Hunter, D.J.; et al. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lu, P.; Chowanadisai, W.; Smith, B.J.; Salak-Johnson, J.L.; Lucas, E.A.; Clarke, S.L.; Conway, T.; Tang, M.; Lin, D. Mapping the mouse ileum at single-cell resolution: Insights into epithelial differentiation, carotenoid metabolism, and immune crosstalk. J. Nutr. 2025; in press. [Google Scholar] [CrossRef]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef]
- Traxinger, B.R.; Richert-Spuhler, L.E.; Lund, J.M. Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry. Mucosal Immunol. 2022, 15, 398–407. [Google Scholar] [CrossRef]
- Tesfaigzi, Y.; Curtis, J.L.; Petrache, I.; Polverino, F.; Kheradmand, F.; Adcock, I.M.; Rennard, S.I. Does chronic obstructive pulmonary disease originate from different cell types? Am. J. Respir. Cell Mol. Biol. 2023, 69, 500–507. [Google Scholar] [CrossRef]
- Mora Massad, K.; Dai, Z.; Petrache, I.; Ventetuolo, C.E.; Lahm, T. Lung endothelial cell heterogeneity in health and pulmonary vascular disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2025, 328, L877–L884. [Google Scholar] [CrossRef]
- Brazil, J.C.; Quiros, M.; Nusrat, A.; Parkos, C.A. Innate immune cell–epithelial crosstalk during wound repair. J. Clin. Investig. 2019, 129, 2983–2993. [Google Scholar] [CrossRef]
- Weitnauer, M.; Mijošek, V.; Dalpke, A.H. Control of local immunity by airway epithelial cells. Mucosal Immunol. 2016, 9, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Sun, L.; Chen, J.; Chen, Z.J. Detection of microbial infections through innate immune sensing of nucleic acids. Annu. Rev. Microbiol. 2018, 72, 447–478. [Google Scholar] [CrossRef]
- Liu, S.; Cai, X.; Wu, J.; Cong, Q.; Chen, X.; Li, T.; Du, F.; Ren, J.; Wu, Y.-T.; Grishin, N.V.; et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 2015, 347, aaa2630. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, A.; Herzner, A.M.; Urban, C.; Piras, A.; Düster, R.; Mahlberg, J.; Grünewald, A.; Schlee-Guimarães, T.M.; Ciupka, K.; Leka, P.; et al. RNA-binding proteins hnRNPM and ELAVL1 promote type I interferon induction downstream of the nucleic acid sensors cGAS and RIG-I. EMBO J. 2025, 44, 824–853. [Google Scholar] [CrossRef]
- Han, L.; Zhuang, M.W.; Deng, J.; Zheng, Y.; Zhang, J.; Nan, M.L.; Zhang, X.J.; Gao, C.; Wang, P.H. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA5–MAVS, TLR3–TRIF, and cGAS–STING signaling pathways. J. Med. Virol. 2021, 93, 5376–5389. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Zhang, C.S.; Wu, Q.; Yu, X.; Zhou, R.; Meng, F.; Wang, A.; Zhang, F.; Chen, S.; et al. AMPK directly phosphorylates TBK1 to integrate glucose sensing into innate immunity. Mol. Cell 2022, 82, 4519–4536.e7. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Tsang, J.C.; Wang, C.; Clare, S.; Wang, J.; Chen, X.; Brandt, C.; Kane, L.; Campos, L.S.; Lu, L.; et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 2016, 539, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Bell, P.T.; Gelman, A.E. Alveolar macrophage–CD8 T cell interactions after acute lung allograft dysfunction: Insights from single-cell RNA sequencing. J. Heart Lung Transplant. 2024, 43, 1087–1089. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Li, K.; Verma, A.; Li, P.; Ortiz, M.E.; Hawkins, G.M.; Schnicker, N.J.; Szachowicz, P.J.; Pezzulo, A.A.; Wohlford-Lenane, C.L.; Kicmal, T.; et al. Adaptation of SARS-CoV-2 to ACE2H353K mice reveals new spike residues that drive mouse infection. J. Virol. 2024, 98, e0151023. [Google Scholar] [CrossRef]
- Sato, M.; Suemori, H.; Hata, N.; Asagiri, M.; Ogasawara, K.; Nakao, K.; Nakaya, T.; Katsuki, M.; Noguchi, S.; Tanaka, N.; et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 2000, 13, 539–548. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.-M.; Maniatis, T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef]
- Negretti, N.M.; Plosa, E.J.; Benjamin, J.T.; Schuler, B.A.; Habermann, A.C.; Jetter, C.S.; Gulleman, P.; Bunn, C.; Hackett, A.N.; Ransom, M.; et al. A single-cell atlas of mouse lung development. Development 2021, 148, dev199512. [Google Scholar] [CrossRef] [PubMed]
- Alberts, R.; Lu, L.; Williams, R.W.; Schughart, K. Genome-wide analysis of the mouse lung transcriptome reveals novel molecular gene interaction networks and cell-specific expression signatures. Respir. Res. 2011, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Naik, S. Epithelial–immune crosstalk in health and disease. Curr. Opin. Genet. Dev. 2022, 74, 101910. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, M.; Rey-Stolle, F.; Boccard, J.; Reddy, V.P.; García, A.; Cumming, B.M.; Steyn, A.J.C.; Rudaz, S.; Barbas, C. Comprehensive examination of the mouse lung metabolome following Mycobacterium tuberculosis infection using a multiplatform mass spectrometry approach. J. Proteome Res. 2020, 19, 2053–2070. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Tong, Z.; Zou, J.P.; Wang, S.Y.; Luo, W.W.; Wang, Y.Y. Activation of the cGAS–STING–IRF3 axis by type I and II interferons contributes to host defense. Adv. Sci. 2024, 11, e2308890. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern recognition receptor signaling and cytokine networks in microbial defenses and regulation of intestinal barriers: Implications for inflammatory bowel disease. Gastroenterology 2022, 162, 1602–1616.e6. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cui, L.; Nie, X.; Cai, H.; Zhang, W.; Lu, X.; Guo, Y.; Hotaling, J.M.; Cairns, B.R.; Wang, X.; et al. A single-cell transcriptome atlas characterizes the immune landscape of human testes during aging. Aging Cell 2025, 24, e70032. [Google Scholar] [CrossRef]
- Norris, P.A.; Kubes, P. Innate immunity of the lungs in homeostasis and disease. Mucosal Immunol. 2025, 18, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Kombe Kombe, A.J.; Fotoohabadi, L.; Gerasimova, Y.; Nanduri, R.; Lama Tamang, P.; Kandala, M.; Kelesidis, T. The role of inflammation in the pathogenesis of viral respiratory infections. Microorganisms 2024, 12, 2526. [Google Scholar] [CrossRef]
- Lee, I.T.; Nakayama, T.; Wu, C.T.; Goltsev, Y.; Jiang, S.; Gall, P.A.; Liao, C.K.; Shih, L.C.; Schürch, C.M.; McIlwain, D.R.; et al. ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat. Commun. 2020, 11, 5453. [Google Scholar] [CrossRef]
- Kim, B.R.; Rauckhorst, A.J.; Chimenti, M.S.; Rehman, T.; Keen, H.L.; Karp, P.H.; Taylor, E.B.; Welsh, M.J. The oxygen level in air directs airway epithelial cell differentiation by controlling mitochondrial citrate export. Sci. Adv. 2025, 11, eadr2282. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, H.; Jounaidi, Y. Comprehensive snapshots of natural killer cell functions, signaling, molecular mechanisms and clinical utilization. Signal Transduct. Target Ther. 2024, 9, 302. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Fourati, S.; Doni Jayavelu, N.; Patel, R.; Maguire, C.; Dandekar, R.; Qi, J.; Lee, B.H.; van Zalm, P.; Schroeder, A.; et al. Multi-omic longitudinal study reveals immune correlates of clinical course among hospitalized COVID-19 patients. Cell Rep. Med. 2023, 4, 101079. [Google Scholar] [CrossRef] [PubMed]
- Luecke, S.; Sheu, K.M.; Hoffmann, A. Stimulus-specific responses in innate immunity: Multilayered regulatory circuits. Immunity 2021, 54, 1915–1932. [Google Scholar] [CrossRef] [PubMed]
- Tarca, A.L.; Draghici, S.; Bhatti, G.; Romero, R. Down-weighting overlapping genes improves gene set analysis. BMC Bioinform. 2012, 13, 136. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Lin, W.; Chi, X.; Chen, H.; Lin, D.; Chowanadisai, W.; Deng, X.; Lu, P. Single-Cell RNA-Seq Identifies Immune Remodeling in Lungs of β-Carotene Oxygenase 2 Knockout Mice with Improved Antiviral Response. Nutrients 2025, 17, 3329. https://doi.org/10.3390/nu17213329
Tang Y, Lin W, Chi X, Chen H, Lin D, Chowanadisai W, Deng X, Lu P. Single-Cell RNA-Seq Identifies Immune Remodeling in Lungs of β-Carotene Oxygenase 2 Knockout Mice with Improved Antiviral Response. Nutrients. 2025; 17(21):3329. https://doi.org/10.3390/nu17213329
Chicago/Turabian StyleTang, Yashu, William Lin, Xiang Chi, Huimin Chen, Dingbo Lin, Winyoo Chowanadisai, Xufang Deng, and Peiran Lu. 2025. "Single-Cell RNA-Seq Identifies Immune Remodeling in Lungs of β-Carotene Oxygenase 2 Knockout Mice with Improved Antiviral Response" Nutrients 17, no. 21: 3329. https://doi.org/10.3390/nu17213329
APA StyleTang, Y., Lin, W., Chi, X., Chen, H., Lin, D., Chowanadisai, W., Deng, X., & Lu, P. (2025). Single-Cell RNA-Seq Identifies Immune Remodeling in Lungs of β-Carotene Oxygenase 2 Knockout Mice with Improved Antiviral Response. Nutrients, 17(21), 3329. https://doi.org/10.3390/nu17213329






