Assessment of Hidden Nutritional Burden: High Prevalence of Disease-Related Malnutrition in Older Adults Without Cognitive Impairment Living in Nursing Homes in Madrid—A Multicentre Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Anthropometric Assessment
2.4. Analysis of Body Composition
2.5. Laboratory Parameters
2.6. Nutritional Assessment
2.7. Statistical Analysis
3. Results
3.1. Global Data
3.2. Nutritional Risk and Disease-Related Malnutrition Assessment
3.3. Comparison Between Groups According to GLIM Criteria
3.4. Binary Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIA | Bioelectrical impedance analysis |
| BCM | Body cell mass |
| BMI | Body mass index |
| BW | Body weight |
| CC | Calf circumference |
| CRP | Serum C-reactive protein |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| DRM | Disease-related malnutrition |
| ECW | Extracellular water |
| EQ-VAS | EuroQol Visual Analogue Scale |
| FFM | Fat-free mass |
| FM | Fat mass |
| FRAIL | Fatigue, Resistance, Ambulation, Illnesses, and weight loss |
| GLIM | Global Leadership Initiative on Malnutrition |
| ICW | Intracellular water |
| LOS | Length of stay |
| MAC | Mid-arm circumference |
| MAMC | Mid-arm muscle circumference |
| MM | Muscle mass |
| MNA | Mini-Nutritional Assessment |
| PhA | Phase Angle |
| TBW | Total body water |
| TSF | Triceps skinfold thickness |
| UBW | Usual body weight |
References
- United Nations Department of Economic and Social Affairs. 2002. Available online: https://population.un.org/wpp/ (accessed on 28 June 2025).
- Borkent, J.W.; Van Hout, H.P.J.; Feskens, E.J.M.; Naumann, E.; de van der Schueren, M.A.E. Diseases, Health-Related Problems, and the Incidence of Malnutrition in Long-Term Care Facilities. Int. J. Environ. Res. Public Health 2023, 20, 3170. [Google Scholar] [CrossRef]
- Leij-Halfwerk, S.; Verwijs, M.H.; van Houdt, S.; Borkent, J.W.; Guaitoli, P.R.; Pelgrim, T.; Heymans, M.W.; Power, L.; Visser, M.; Corish, C.A.; et al. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults >/=65 years: A systematic review and meta-analysis. Maturitas 2019, 126, 80–89. [Google Scholar] [CrossRef]
- Kokura, Y.; Momosaki, R. Prevalence of Malnutrition Assessed by the GLIM Criteria and Association with Activities of Daily Living in Older Residents in an Integrated Facility for Medical and Long-Term Care. Nutrients 2022, 14, 3656. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pedrolli, C.; Klersy, C.; Bonardi, C.; Quarleri, L.; Cappello, S.; Turri, A.; Rondanelli, M.; Caccialanza, R. Nutritional status in older persons according to healthcare setting: A systematic review and meta-analysis of prevalence data using MNA((R)). Clin. Nutr. 2016, 35, 1282–1290. [Google Scholar] [CrossRef]
- Oliva, S.G.-S.A.; Ramón, M.; Iturburu, M.; Montero, M.; Galdona, N.O.A.; Ulla, S. Short Report: Long Term Care Landscape in Spain; InCARE Project; InCARE IMSERSO and Matia Foundation, 2023; Available online: https://incare.euro.centre.org/publications-tools/ (accessed on 28 June 2025).
- Cuerda, C.; Alvarez, J.; Ramos, P.; Abanades, J.C.; Garcia-de-Lorenzo, A.; Gil, P.; De-la-Cruz, J.J. Prevalencia de desnutrición en sujetos mayores de 65 años en la Comunidad de Madrid: Estudio DREAM+ 65. Nutr. Hosp. 2016, 33, 101. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Urrea, R.; Garcia-Meseguer, M.J. Malnutrition in an elderly population without cognitive impairment living in nursing homes in Spain: Study of prevalence using the Mini Nutritional Assessment test. Gerontology 2013, 59, 490–498. [Google Scholar] [CrossRef]
- Sanz-Paris, A.; Gonzalez Fernandez, M.; Perez-Nogueras, J.; Serrano-Oliver, A.; Torres-Anoro, E.; Sanz-Arque, A.; Arbones-Mainar, J.M. Prevalence of Malnutrition and 1-Year All-Cause Mortality in Institutionalized Elderly Patients Comparing Different Combinations of the GLIM Criteria. J. Parenter. Enteral Nutr. 2021, 45, 1164–1171. [Google Scholar] [CrossRef]
- de Almeida Mello, J.; Schoebrechts, E.; Vandenbulcke, P.A.I.; Declercq, A.; De Lepeleire, J.; Matthys, C.; Declerck, D.; Duyck, J. Insights into the associated risk factors of malnutrition among nursing home residents: A longitudinal study. Clin. Nutr. 2024, 43, 166–173. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, D.; Locquet, M.; Reginster, J.Y.; Cavalier, E.; Bruyere, O.; Beaudart, C. Mortality in malnourished older adults diagnosed by ESPEN and GLIM criteria in the SarcoPhAge study. J. Cachexia Sarcopenia Muscle 2020, 11, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Verbrugghe, M.; Beeckman, D.; Van Hecke, A.; Vanderwee, K.; Van Herck, K.; Clays, E.; Bocquaert, I.; Derycke, H.; Geurden, B.; Verhaeghe, S. Malnutrition and associated factors in nursing home residents: A cross-sectional, multi-centre study. Clin. Nutr. 2013, 32, 438–443. [Google Scholar] [CrossRef]
- Pourhassan, M.; Cederholm, T.; Donini, L.M.; Poggiogalle, E.; Schwab, U.; Nielsen, R.L.; Andersen, A.L.; Malgorzewicz, S.; Volkert, D.; Wirth, R. Severity of Inflammation Is Associated with Food Intake in Hospitalized Geriatric Patients-A Merged Data Analysis. Nutrients 2023, 15, 3079. [Google Scholar] [CrossRef]
- Neyens, J.; Halfens, R.; Spreeuwenberg, M.; Meijers, J.; Luiking, Y.; Verlaan, G.; Schols, J. Malnutrition is associated with an increased risk of falls and impaired activity in elderly patients in Dutch residential long-term care (LTC): A cross-sectional study. Arch. Gerontol. Geriatr. 2013, 56, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Landi, F.; Smoyer, K.E.; Tarasenko, L.; Groarke, J. Association of anorexia/appetite loss with malnutrition and mortality in older populations: A systematic literature review. J. Cachexia Sarcopenia Muscle 2023, 14, 706–729. [Google Scholar] [CrossRef]
- Valmorbida, E.; Trevisan, C.; Imoscopi, A.; Mazzochin, M.; Manzato, E.; Sergi, G. Malnutrition is associated with increased risk of hospital admission and death in the first 18 months of institutionalization. Clin. Nutr. 2020, 39, 3687–3694. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr. Rev. 1996, 54, S59–S65. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1480–1481. [Google Scholar] [CrossRef]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Pellegrino, G.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Vendemiale, G. Comparison of Three Nutritional Screening Tools with the New Glim Criteria for Malnutrition and Association with Sarcopenia in Hospitalized Older Patients. J. Clin. Med. 2020, 9, 1898. [Google Scholar] [CrossRef]
- Enge, M.; Peelen, F.O.; Nielsen, R.L.; Beck, A.M.; Olin, A.O.; Cederholm, T.; Bostrom, A.M.; Paur, I. Malnutrition prevalence according to GLIM and its feasibility in geriatric patients: A prospective cross-sectional study. Eur. J. Nutr. 2024, 63, 927–938. [Google Scholar] [CrossRef]
- Clark, A.B.; Reijnierse, E.M.; Lim, W.K.; Maier, A.B. Prevalence of malnutrition comparing the GLIM criteria, ESPEN definition and MST malnutrition risk in geriatric rehabilitation patients: RESORT. Clin. Nutr. 2020, 39, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sanchez, B.; Sulo, S.; Carnicero, J.A.; Rueda, R.; Rodriguez-Manas, L. Malnutrition Prevalence and Burden on Healthcare Resource Use Among Spanish Community-Living Older Adults: Results of a Longitudinal Analysis. Clin. Outcomes Res. 2020, 12, 355–367. [Google Scholar] [CrossRef]
- Cereda, E.; Zagami, A.; Vanotti, A.; Piffer, S.; Pedrolli, C. Geriatric Nutritional Risk Index and overall-cause mortality prediction in institutionalised elderly: A 3-year survival analysis. Clin. Nutr. 2008, 27, 717–723. [Google Scholar] [CrossRef]
- EuroQol, G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Esquius, M.; Schwartz, S.; Lopez Hellin, J.; Andreu, A.L.; Garcia, E. Anthropometric reference parameters for the aged population. Med. Clin. 1993, 100, 692–698. [Google Scholar]
- de van der Schueren, M.A.E.; Keller, H.; Consortium, G.; Cederholm, T.; Barazzoni, R.; Compher, C.; Correia, M.; Gonzalez, M.C.; Jager-Wittenaar, H.; Pirlich, M.; et al. Global Leadership Initiative on Malnutrition (GLIM): Guidance on validation of the operational criteria for the diagnosis of protein-energy malnutrition in adults. Clin. Nutr. 2020, 39, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Miller, D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J. Nutr. Health Aging 2012, 16, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Rondanelli, M.; Spadaccini, D.; Lenzi, A.; Donini, L.M.; Poggiogalle, E. Are the therapeutic strategies in anorexia of ageing effective on nutritional status? A systematic review with meta-analysis. J. Hum. Nutr. Diet. 2019, 32, 128–138. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B. The Mini Nutritional Assessment (MNA) for grading the nutritional state of elderly patients: Presentation of the MNA, history and validation. Nestle. Nutr. Workshop Ser. Clin. Perform. Programme 1999, 1, 3–12. [Google Scholar]
- Salminen, K.S.; Suominen, M.H.; Soini, H.; Kautiainen, H.; Savikko, N.; Saarela, R.K.T.; Muurinen, S.; Pitkala, K.H. Associations between Nutritional Status and Health-Related Quality of Life among Long-Term Care Residents in Helsinki. J. Nutr. Health Aging 2019, 23, 474–478. [Google Scholar] [CrossRef]
- Winter, J.; Flanagan, D.; McNaughton, S.A.; Nowson, C. Nutrition screening of older people in a community general practice, using the MNA-SF. J. Nutr. Health Aging 2013, 17, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Di Giosia, P.; Stamerra, C.A.; Giorgini, P.; Jamialahamdi, T.; Butler, A.E.; Sahebkar, A. The role of nutrition in inflammaging. Ageing Res. Rev. 2022, 77, 101596. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Sieber, C.; Sobotka, L.; Asselt, D.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Geriatr. Psychol. Neuropsychiatr. Vieil. 2024, 22, 273–315. [Google Scholar] [CrossRef]
- Zhang, X.; Dou, Q.; Zhang, W.; Wang, C.; Xie, X.; Yang, Y.; Zeng, Y. Frailty as a Predictor of All-Cause Mortality Among Older Nursing Home Residents: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2019, 20, 657–663.e4. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ruperto, M.; Barril, G. Clinical Significance of Nutritional Status, Inflammation, and Body Composition in Elderly Hemodialysis Patients-A Case-Control Study. Nutrients 2023, 15, 5036. [Google Scholar] [CrossRef]
- Matias, C.N.; Nunes, C.L.; Francisco, S.; Tomeleri, C.M.; Cyrino, E.S.; Sardinha, L.B.; Silva, A.M. Phase angle predicts physical function in older adults. Arch. Gerontol. Geriatr. 2020, 90, 104151. [Google Scholar] [CrossRef]
- Genton, L.; Norman, K.; Spoerri, A.; Pichard, C.; Karsegard, V.L.; Herrmann, F.R.; Graf, C.E. Bioimpedance-Derived Phase Angle and Mortality Among Older People. Rejuvenation Res. 2017, 20, 118–124. [Google Scholar] [CrossRef]
- Piglowska, M.; Kostka, T.; Guligowska, A. Do Determinants of Quality of Life Differ in Older People Living in the Community and Nursing Homes? Int. J. Environ. Res. Public Health 2023, 20, 916. [Google Scholar] [CrossRef] [PubMed]
- Falke, C.; Karapinar, F.; Bouvy, M.; Emmelot, M.; Belitser, S.; Boland, B.; O’Mahony, D.; Murphy, K.D.; Haller, M.; Salari, P.; et al. The association between medication use and health-related quality of life in multimorbid older patients with polypharmacy. Eur. Geriatr. Med. 2024, 15, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Won, C.W.; Kim, B.S.; Kim, S.; Yoo, J.; Byun, S.; Jang, H.C.; Cho, B.L.; Son, S.J.; Lee, J.H.; et al. EuroQol Visual Analogue Scale (EQ-VAS) as a Predicting Tool for Frailty in Older Korean Adults: The Korean Frailty an Aging Cohort Study (KFACS). J. Nutr. Health Aging 2018, 22, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, G.G.; Shi, Q.L.; Sun, Y.; Zhang, H.; Wang, M.J.; Jia, H.P.; Zhao, Y.L.; Yao, Y. Health-Related Quality of Life and Associated Factors among Oldest-Old in China. J. Nutr. Health Aging 2020, 24, 330–338. [Google Scholar] [CrossRef]
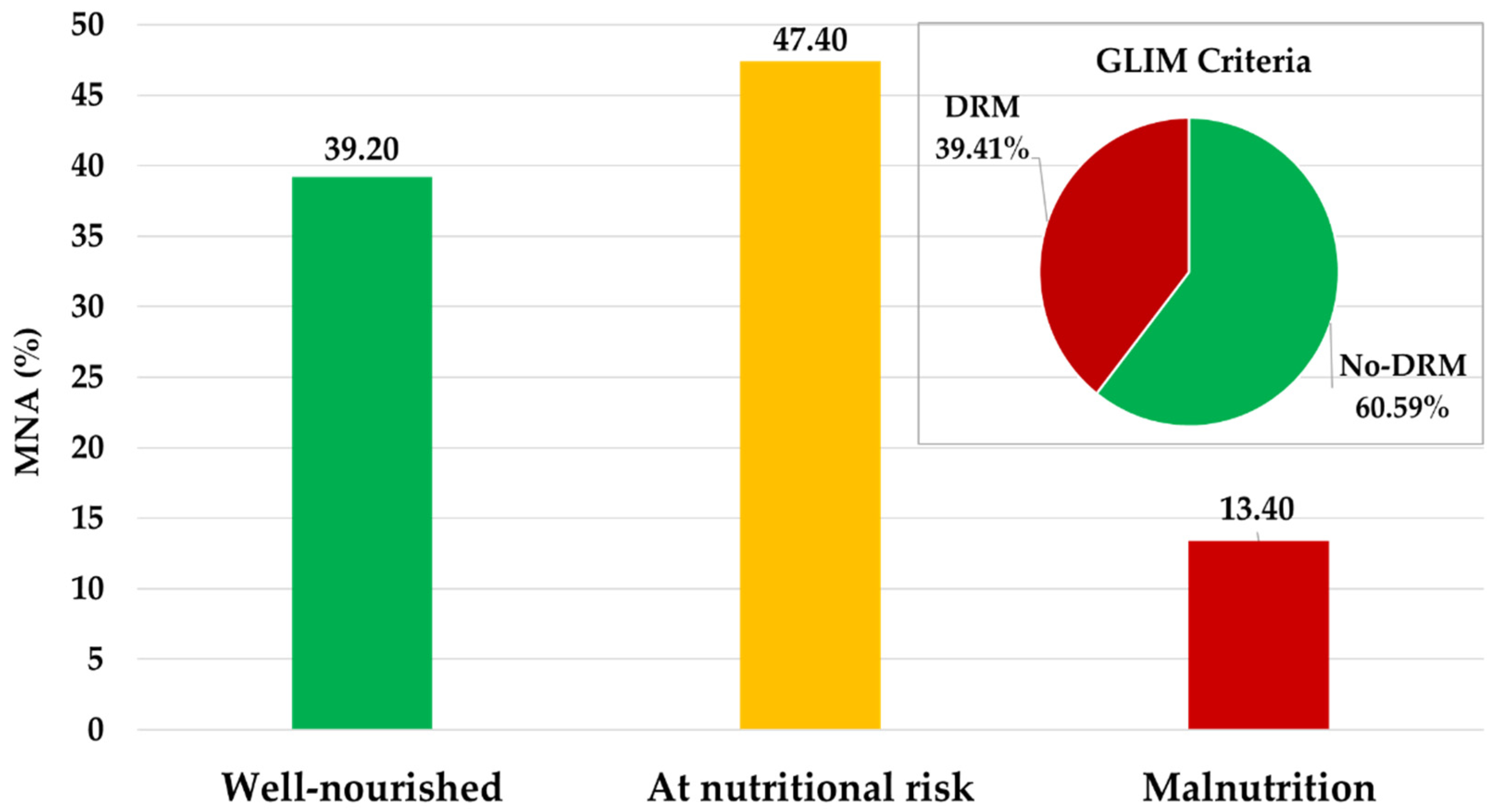
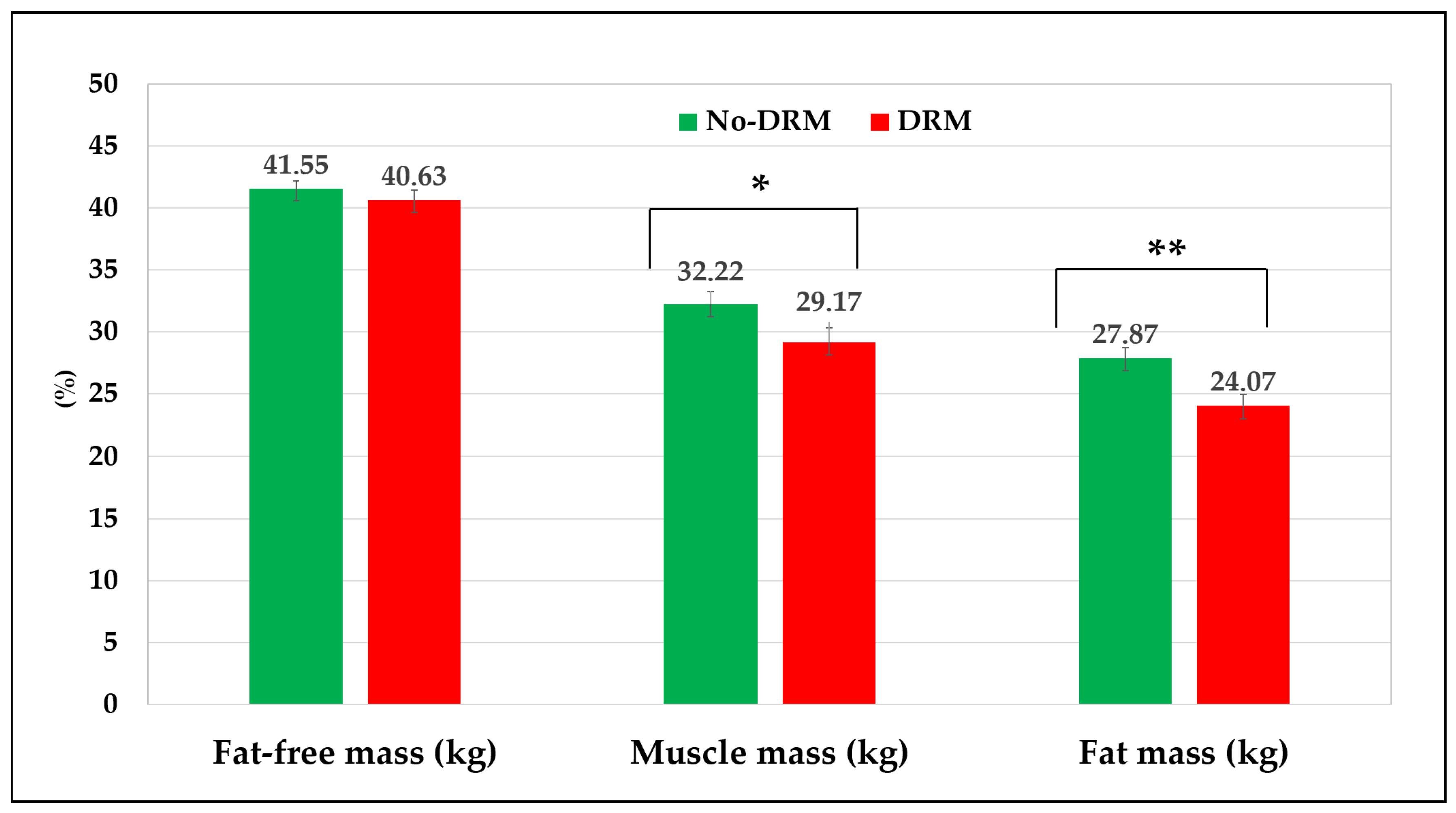
| Variables | Total (n = 340) |
|---|---|
| Gender (Male) n (%) | 96 (28.23) |
| Age (years) | 87.14 ± 6.99 |
| LOS (months) | 36.57 ± 38.70 |
| DM n (%) | 78.0 (25.90) |
| Hypertension (mm Hg) n (%) | 260.0 (76.70) |
| Dyslipidemia n (%) | 183.0 (53.82) |
| Standard Mediterranean diet n (%) | 249.0 (83.30) |
| Oral nutritional supplements n (%) | 40.0 (13.70) |
| EQ-VAS (points) | 72.47 ± 18.40 |
| Variables | No-DRM (n = 206) | DRM (n = 134) | p-Value |
|---|---|---|---|
| Age (years) | 87.23 ± 6.68 | 86.95 ± 7.51 | 0.717 |
| LOS (months) | 31.0 ± 28.35 | 30.52 ± 25.63 | 0.877 |
| MNA (points) | 7.74 ± 4.99 | 10.95 ± 4.33 | <0.001 |
| FRAIL n (%) & | 96.0 (46.60) | 100.0 (74.62) | 0.034 |
| Body weight (kg) | 67.42 ± 12.83 | 62.27 ± 13.47 | 0.001 |
| BMI (kg/m2) | 28.81 ± 4.90 | 26.28 ± 5.41 | <0.001 |
| Weight loss (%) | 1.63 ± 8.58 | 7.37 ± 8.04 | <0.001 |
| TSF (mm) | 21.01 ± 8.58 | 18.71 ± 8.17 | 0.100 |
| MAMC (%) ‡ | 94.86 ± 17.06 | 90.51 ± 14.70 | 0.027 |
| CC (cm) | 33.47 ± 4.28 | 31.80 ± 4.13 | 0.001 |
| Resistance (R) | 559.65 ± 91.22 | 533.64 ± 80.25 | 0.015 |
| Reactance (ꭕc) | 62.57 ± 13.44 | 55.46 ± 15.19 | 0.257 |
| Exchangeable Na/K | 0.89 ± 0.18 | 0.92 ± 0.23 | 0.070 |
| TBW (L) | 33.12 ± 8.10 | 36.02 ± 9.80 | 0.015 |
| ECW (L) | 14.23 ± 3.01 | 15.32 ± 4.75 | 0.045 |
| ICW (L) | 19.30 ± 4.72 | 18.35 ± 4.11 | 0.103 |
| BCM (kg) | 23.58 ± 4.86 | 22.94 ± 5.23 | 0.322 |
| PhA (°) | 6.46 ± 1.13 | 5.23 ± 1.17 | 0.001 |
| Variables | No-DRM (n = 206) | DRM (n = 134) | p-Value |
|---|---|---|---|
| Haemoglobin (g/dL) | 12.56 ± 1.42 | 11.71 ± 1.60 | <0.001 |
| Total lymphocyte count (×103/mm3) | 1977.05 ± 623.93 | 1794.75 ± 677.58 | 0.060 |
| Albumin (g/dL) | 3.90 ± 0.35 | 3.74 ± 0.32 | <0.001 |
| Transferrin (mg/dL) | 219.96 ± 29.04 | 189.37 ± 33.01 | <0.001 |
| CRP (mg/dL) | 1.04 (2.41) | 1.68 (1.98) | 0.004 |
| EQ-VAS (points) | 73.26 ± 18.57 | 67.34 ± 16.57 | 0.036 |
| Variables | OR (95% CI) | p-Value |
|---|---|---|
| Frailty risk (≥3 points) | 3.317 (1.456 to 7.556) | 0.004 |
| Muscle Mass (kg) | 0.732 (0.568 to 0.944) | 0.016 |
| Phase angle (°) | 0.033 (0.002 to 0.500) | 0.014 |
| Albumin (g/dL) | 0.070 (0.012 to 0.394) | 0.003 |
| EQ-VAS (points) | 0.961 (0.926 to 0.996) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruperto, M.; Ongan, D.; Josa, E.; Tsagari, A. Assessment of Hidden Nutritional Burden: High Prevalence of Disease-Related Malnutrition in Older Adults Without Cognitive Impairment Living in Nursing Homes in Madrid—A Multicentre Study. Nutrients 2025, 17, 3325. https://doi.org/10.3390/nu17213325
Ruperto M, Ongan D, Josa E, Tsagari A. Assessment of Hidden Nutritional Burden: High Prevalence of Disease-Related Malnutrition in Older Adults Without Cognitive Impairment Living in Nursing Homes in Madrid—A Multicentre Study. Nutrients. 2025; 17(21):3325. https://doi.org/10.3390/nu17213325
Chicago/Turabian StyleRuperto, Mar, Dilek Ongan, Esmeralda Josa, and Amalia Tsagari. 2025. "Assessment of Hidden Nutritional Burden: High Prevalence of Disease-Related Malnutrition in Older Adults Without Cognitive Impairment Living in Nursing Homes in Madrid—A Multicentre Study" Nutrients 17, no. 21: 3325. https://doi.org/10.3390/nu17213325
APA StyleRuperto, M., Ongan, D., Josa, E., & Tsagari, A. (2025). Assessment of Hidden Nutritional Burden: High Prevalence of Disease-Related Malnutrition in Older Adults Without Cognitive Impairment Living in Nursing Homes in Madrid—A Multicentre Study. Nutrients, 17(21), 3325. https://doi.org/10.3390/nu17213325







