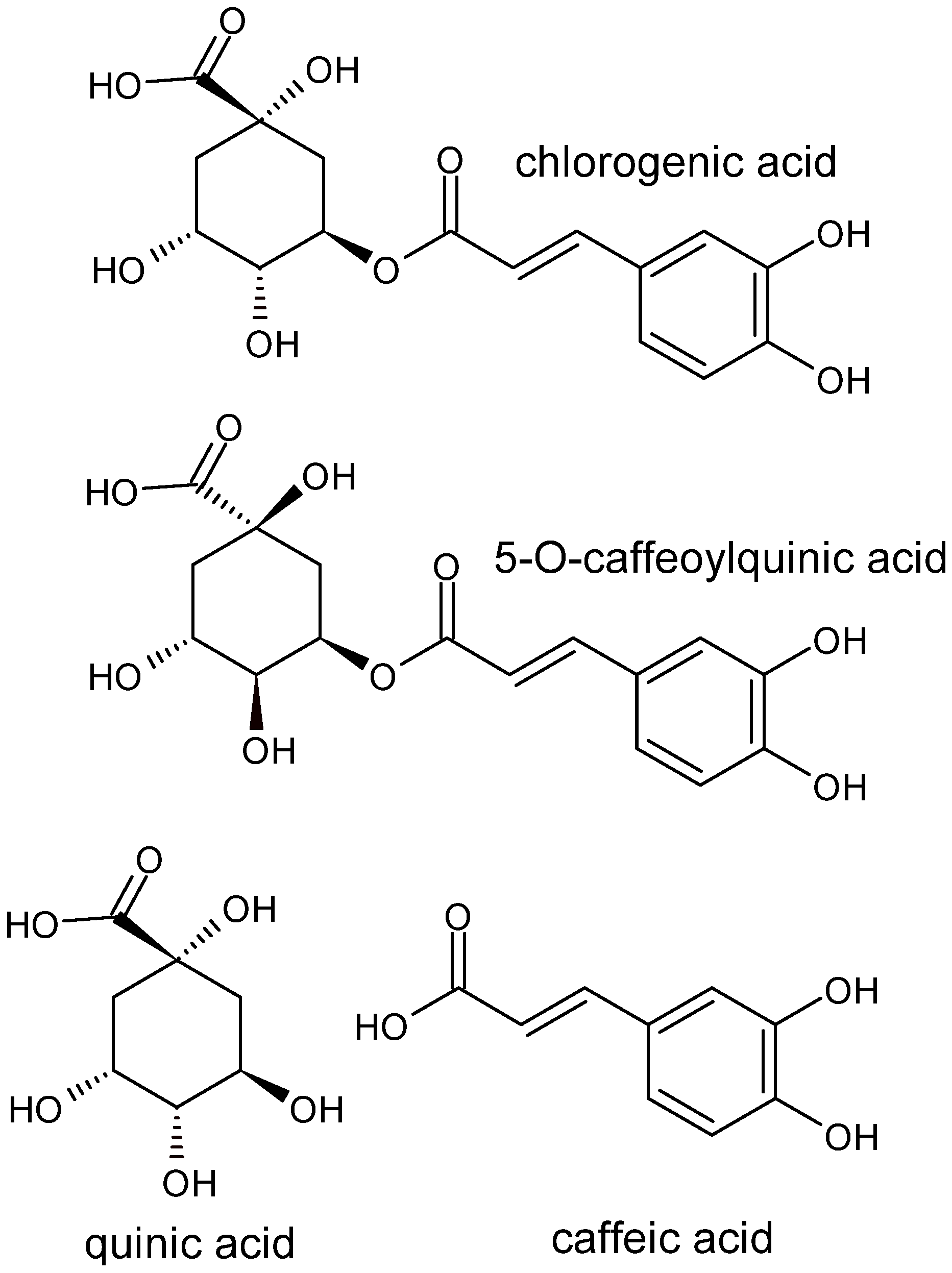
Chlorogenic Acid’s Role in Metabolic Health: Mechanisms and Therapeutic Potential
Abstract
1. Introduction
2. Chlorogenic Acid in the Prevention and Management of Obesity
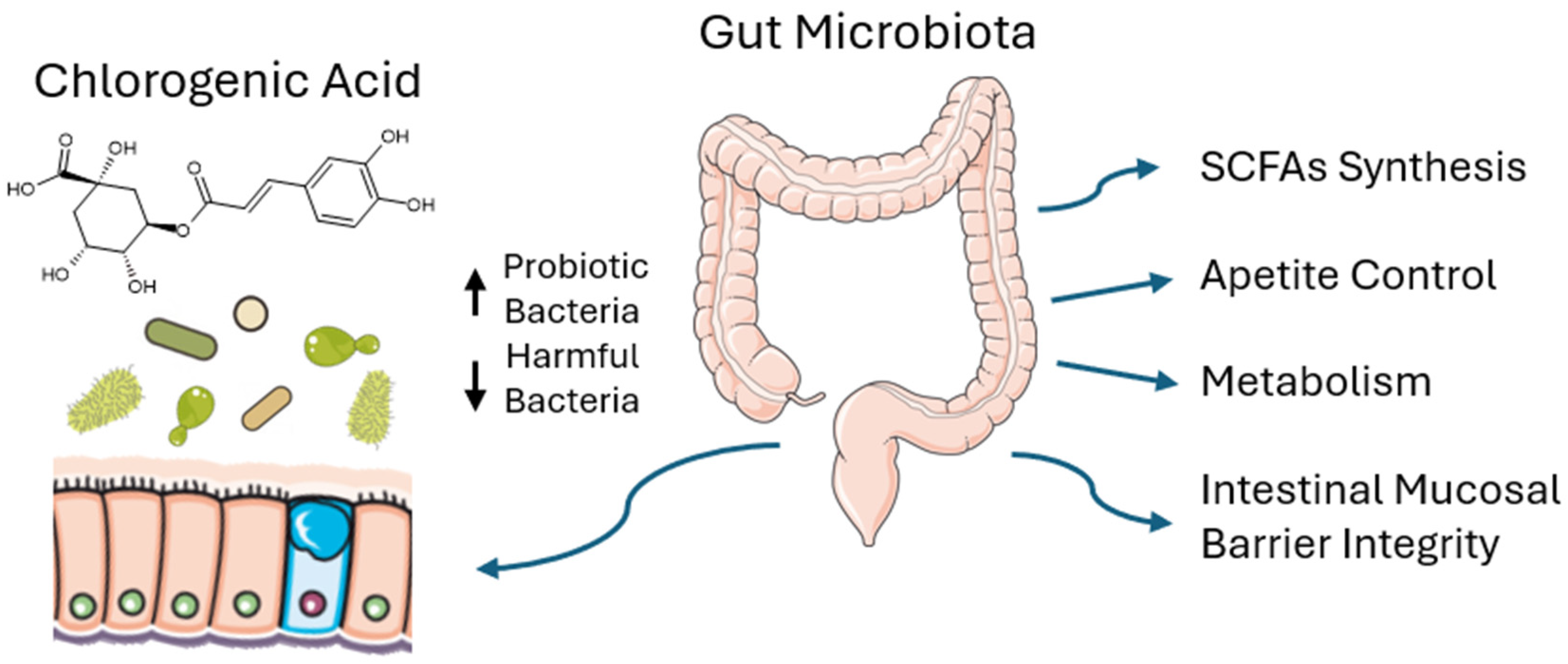
2.1. CGA Effect on Gut Microbiota
2.2. Anti-Inflammatory Effect of CGA
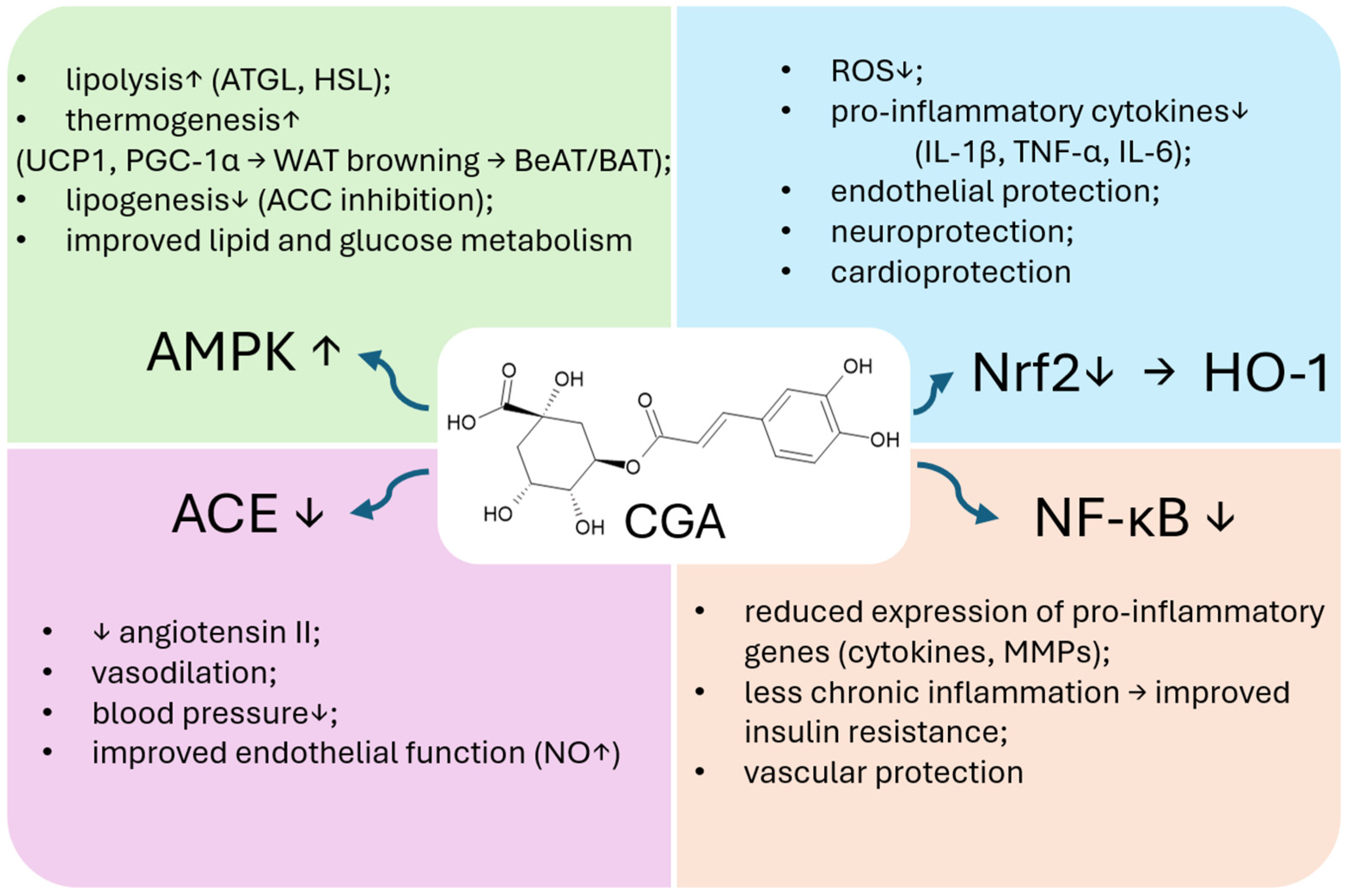
2.3. CGA Effects on Metabolism, Adipogenesis and Thermogenesis
2.4. Synergistic Action of CGA with Other Compounds and Weight Loss Effects
3. Chlorogenic Acid in the Prevention and Management of Hypertension
3.1. Antioxidant and Anti-Inflammatory Effects
3.2. Endothelial Function and Barrier Integrity
3.3. RAAS Inhibition and Blood Pressure Regulation
3.4. Molecular Mechanisms in Vascular Protection
3.5. Antiplatelet and Anti-Thrombotic Activity
3.6. Vascular Remodeling and Cardioprotective Effects
4. Chlorogenic Acid and Its Effects on Lipid Metabolism
4.1. CGA Increases CYP7A1 Enzyme Expression
4.2. CGA Activates AMPK
4.3. CGA Modifies the Expression of Lipid Metabolism Enzymes
4.4. CGA Influences the Lipid Profile via Antioxidant and Anti-Inflammatory Action
5. Chlorogenic Acid as a Modulator of Diabetic Pathophysiology
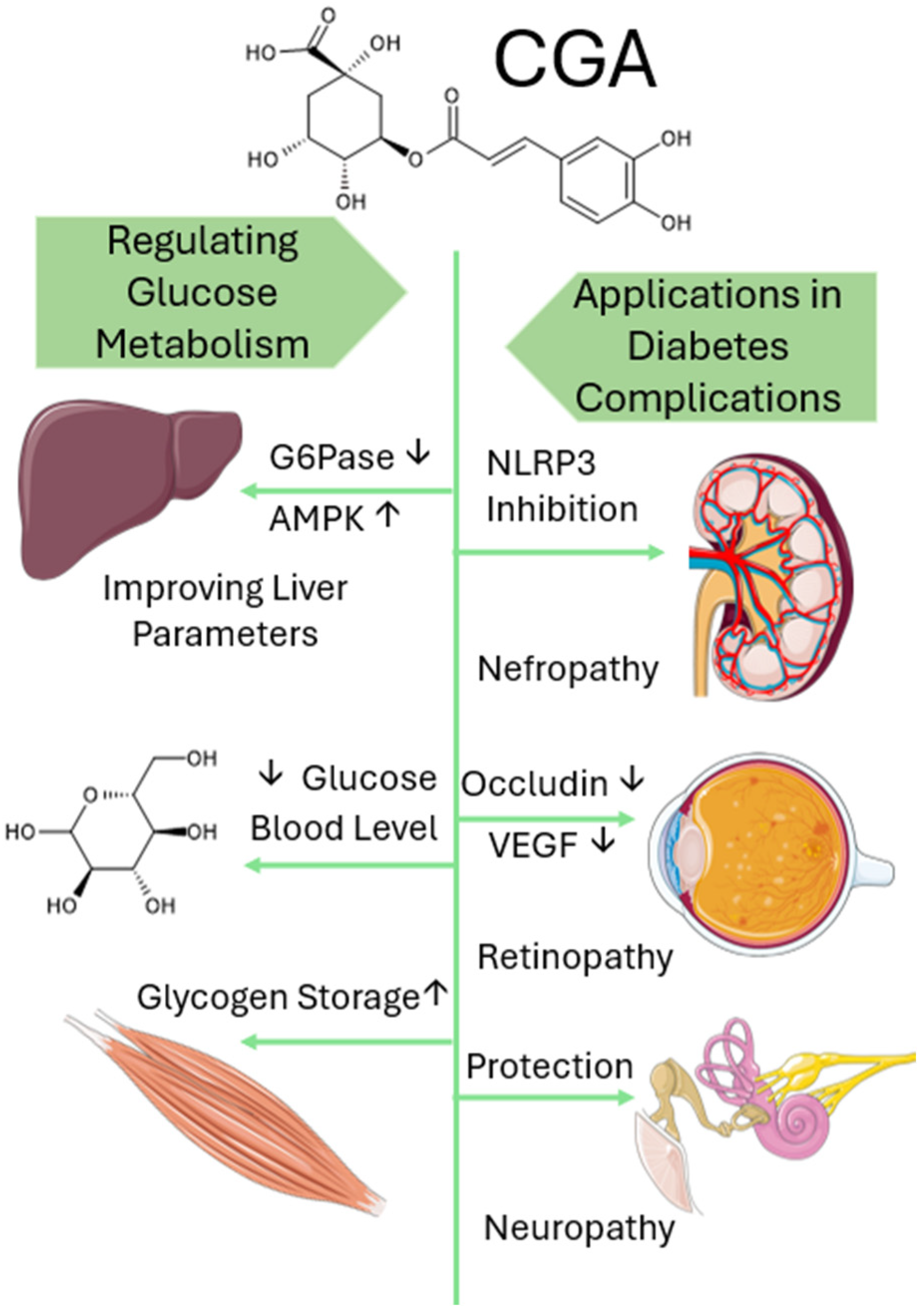
5.1. Chlorogenic Acid’s Role in Regulating Glucose Metabolism
5.1.1. Clinical Studies (Human Trials)
5.1.2. In Vivo Studies (Animal Models)
5.1.3. In Vitro Studies (Cellular Mechanisms)
5.2. Application in Diabetes Complications
5.3. Muscle Atrophy and Tissue Protection
5.4. Wound Healing and Skin Complications
6. Bioavailability and Stability of Chlorogenic Acid
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, Y.; Fang, C.; Shi, J.; Chen, H.; Chen, X.; Yao, X. Antioxidant Potential of Chlorogenic Acid in Age-Related Eye Diseases. Pharmacol. Res. Perspect. 2024, 12, e1162. [Google Scholar] [CrossRef]
- Zeng, L.; Xiang, R.; Fu, C.; Qu, Z.; Liu, C. The Regulatory Effect of Chlorogenic Acid on Gut-Brain Function and Its Mechanism: A Systematic Review. Biomed. Pharmacother. 2022, 149, 112831. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.F.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Yin, X.; He, X.; Wu, L.; Yan, D.; Yan, S. Chlorogenic Acid, the Main Antioxidant in Coffee, Reduces Radiation-Induced Apoptosis and DNA Damage via NF-E2-Related Factor 2 (Nrf2) Activation in Hepatocellular Carcinoma. Oxid. Med. Cell. Longev. 2022, 2022, 4566949. [Google Scholar] [CrossRef]
- Lee, Y.K.; Park, J.E.; Lee, M.; Hardwick, J.P. Hepatic Lipid Homeostasis by Peroxisome Proliferator-Activated Receptor Gamma 2. Liver Res. 2018, 2, 209–215. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic Acid Improves Glucose Tolerance, Lipid Metabolism, Inflammation and Microbiota Composition in Diabetic Db/Db Mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Tang, C.-H.; Ho, T.-L.; Wang, W.-L.; Yao, C.-H. Chlorogenic Acid Prevents Ovariectomized-Induced Bone Loss by Facilitating Osteoblast Functions and Suppressing Osteoclast Formation. Aging 2024, 16, 4832–4840. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Ye, Z.; Cheng, L.; Xuan, Y.; Yu, K.; Li, J.; Gu, H. Chlorogenic Acid Alleviates the Development of Severe Acute Pancreatitis by Inhibiting NLPR3 Inflammasome Activation via Nrf2/HO-1 Signaling. Int. Immunopharmacol. 2025, 151, 114335. [Google Scholar] [CrossRef]
- Xiao, N.; Zhang, T.; Han, M.; Tian, D.; Liu, J.; Li, S.; Yang, L.; Pan, G. Chlorogenic Acid Inhibits Ceramide Accumulation to Restrain Hepatic Glucagon Response. Nutrients 2023, 15, 3173. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic Acid Alleviates Obesity and Modulates Gut Microbiota in High-fat-fed Mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Kanchanasurakit, S.; Saokaew, S.; Phisalprapa, P.; Duangjai, A. Chlorogenic Acid in Green Bean Coffee on Body Weight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Syst. Rev. 2023, 12, 163. [Google Scholar] [CrossRef]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic Acid for Cancer Prevention and Therapy: Current Status on Efficacy and Mechanisms of Action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, L.; Chen, B.; Fang, Y.; Lin, W.; Zhang, T.; Feng, X.; Tao, X.; Wu, Y.; Fu, X.; et al. Chlorogenic Acid Exerts Neuroprotective Effect against Hypoxia-Ischemia Brain Injury in Neonatal Rats by Activating Sirt1 to Regulate the Nrf2-NF-κB Signaling Pathway. Cell Commun. Signal. CCS 2022, 20, 84. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Himshweta; Verma, N.; Trehan, N.; Singh, M. Molecularly Imprinted Polymers in the Analysis of Chlorogenic Acid: A Review. Anal. Biochem. 2024, 694, 115616. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Zock, P.L.; Katan, M.B. Consumption of High Doses of Chlorogenic Acid, Present in Coffee, or of Black Tea Increases Plasma Total Homocysteine Concentrations in Humans. Am. J. Clin. Nutr. 2001, 73, 532–538. [Google Scholar] [CrossRef]
- Cortez, N.; Villegas, C.; Burgos, V.; Ortiz, L.; Cabrera-Pardo, J.R.; Paz, C. Therapeutic Potential of Chlorogenic Acid in Chemoresistance and Chemoprotection in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5189. [Google Scholar] [CrossRef]
- Wei, R.; Su, Z.; Mackenzie, G.G. Chlorogenic Acid Combined with Epigallocatechin-3-Gallate Mitigates D-Galactose-Induced Gut Aging in Mice. Food Funct. 2023, 14, 2684–2697. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Gallo, G.; Desideri, G.; Savoia, C. Update on Obesity and Cardiovascular Risk: From Pathophysiology to Clinical Management. Nutrients 2024, 16, 2781. [Google Scholar] [CrossRef]
- Ruperez, C.; Madeo, F.; de Cabo, R.; Kroemer, G.; Abdellatif, M. Obesity Accelerates Cardiovascular Ageing. Eur. Heart J. 2025, 46, 2161–2185. [Google Scholar] [CrossRef]
- Solsona-Vilarrasa, E.; Vousden, K.H. Obesity, White Adipose Tissue and Cancer. FEBS J. 2025, 292, 2189–2207. [Google Scholar] [CrossRef]
- Yende, A.S.; Sharma, D. Obesity, Dysbiosis and Inflammation: Interactions That Modulate the Efficacy of Immunotherapy. Front. Immunol. 2024, 15, 1444589. [Google Scholar] [CrossRef]
- He, Q.; Zheng, R.; Song, W.; Sun, X.; Lu, C. The Impact of Metabolic Heterogeneity of Obesity and Transitions on Cardiovascular Disease Incidence in Chinese Middle-Aged and Elderly Population: A Nationwide Prospective Cohort Study. Diabetes Obes. Metab. 2025, 27, 501–510. [Google Scholar] [CrossRef]
- Konatham, S.; Goodlett, B.L.; Smith, H.L.; Mitchell, B.M. Hypertension: A Lymphatic Disease? Clin. Sci. Lond. Engl. 1979 2025, 139, 597–603. [Google Scholar] [CrossRef]
- Borozan, S.; Kamrul-Hasan, A.B.M.; Shetty, S.; Pappachan, J.M. Approach to Endocrine Hypertension: A Case-Based Discussion. Curr. Hypertens. Rep. 2025, 27, 8. [Google Scholar] [CrossRef]
- Goorani, S.; Zangene, S.; Imig, J.D. Hypertension: A Continuing Public Healthcare Issue. Int. J. Mol. Sci. 2024, 26, 123. [Google Scholar] [CrossRef]
- Natale, F.; Franzese, R.; Luisi, E.; Mollo, N.; Marotta, L.; Solimene, A.; D’Elia, S.; Golino, P.; Cimmino, G. The Increasing Problem of Resistant Hypertension: We’ll Manage till Help Comes! Med. Sci. 2024, 12, 53. [Google Scholar] [CrossRef]
- Dzau, V.J.; Hodgkinson, C.P. Precision Hypertension. Hypertension 2024, 81, 702–708. [Google Scholar] [CrossRef]
- Islam, K.; Islam, R.; Nguyen, I.; Malik, H.; Pirzadah, H.; Shrestha, B.; Lentz, I.B.; Shekoohi, S.; Kaye, A.D. Diabetes Mellitus and Associated Vascular Disease: Pathogenesis, Complications, and Evolving Treatments. Adv. Ther. 2025, 42, 2659–2678. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Li, Z. tRNA Fragments in Diabetes Mellitus. Clin. Chim. Acta Int. J. Clin. Chem. 2025, 576, 120405. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.; Stennett, D.; Facey, A.; Omoruyi, F.; Mohansingh, S.; Omoruyi, F.O. Diabetes and the Associated Complications: The Role of Antioxidants in Diabetes Therapy and Care. Biomed. Pharmacother. 2024, 181, 117641. [Google Scholar] [CrossRef]
- Arvanitis, M.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2023, 176, ITC81–ITC96. [Google Scholar] [CrossRef]
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2022, 43, 611–653. [Google Scholar] [CrossRef]
- Hoekstra, M.; Van Eck, M. Gene Editing for the Treatment of Hypercholesterolemia. Curr. Atheroscler. Rep. 2024, 26, 139–146. [Google Scholar] [CrossRef]
- Chopra, A.K. Dietary Management of Dyslipidemia. Indian Heart J. 2024, 76 (Suppl. 1), S65–S72. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Cultured Gut Microbiota from Twins Discordant for Obesity Modulate Adiposity and Metabolic Phenotypes in Mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Guan, Z.; Niu, P.; Tan, Q.; Wang, Y.; Deng, S.; Wang, D.; Dong, K.; Xing, J.; You, C. Therapeutic Systems Based on Natural Gut Microbiota Modulators: The Latest Advances in the Treatment of Inflammatory Bowel Disease. Mater. Adv. 2025, 6, 1578–1607. [Google Scholar] [CrossRef]
- Wang, G.; Yu, Y.; Wang, Y.-Z.; Wang, J.-J.; Guan, R.; Sun, Y.; Shi, F.; Gao, J.; Fu, X.-L. Role of SCFAs in Gut Microbiome and Glycolysis for Colorectal Cancer Therapy. J. Cell. Physiol. 2019, 234, 17023–17049. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Anighoro, A.; Quartieri, A.; Amaretti, A.; Tomás-Barberán, F.A.; Rastelli, G.; Rossi, M. Role of Bifidobacteria in the Hydrolysis of Chlorogenic Acid. MicrobiologyOpen 2015, 4, 41–52. [Google Scholar] [CrossRef]
- Pitocco, D.; Di Leo, M.; Tartaglione, L.; De Leva, F.; Petruzziello, C.; Saviano, A.; Pontecorvi, A.; Ojetti, V. The Role of Gut Microbiota in Mediating Obesity and Diabetes Mellitus. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1548–1562. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.-Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia Muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic Acid Improves NAFLD by Regulating Gut Microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate Reduces Appetite and Activates Brown Adipose Tissue via the Gut-Brain Neural Circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Anachad, O.; Taouil, A.; Taha, W.; Bennis, F.; Chegdani, F. The Implication of Short-Chain Fatty Acids in Obesity and Diabetes. Microbiol. Insights 2023, 16, 11786361231162720. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARγ-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut Microbiota and Human NAFLD: Disentangling Microbial Signatures from Metabolic Disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Stremmel, W. Treatment Efficacy of a Probiotic Preparation for Non-Alcoholic Steatohepatitis: A Pilot Trial. J. Dig. Dis. 2017, 18, 698–703. [Google Scholar] [CrossRef]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad, E.G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Seyed Moayed, A. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2016, 35, 500–505. [Google Scholar] [CrossRef]
- Bakinowska, E.; Krompiewski, M.; Boboryko, D.; Kiełbowski, K.; Pawlik, A. The Role of Inflammatory Mediators in the Pathogenesis of Obesity. Nutrients 2024, 16, 2822. [Google Scholar] [CrossRef]
- Du, B.; Xu, B. Natural Bioactive Compounds Exerting Health-Promoting Effects by Ameliorating Oxidative Stress. Antioxidants 2025, 14, 85. [Google Scholar] [CrossRef]
- Gastélum-Estrada, A.; Rabadán-Chávez, G.; Reza-Zaldívar, E.E.; de la Cruz-López, J.L.; Fuentes-Palma, S.A.; Mojica, L.; Díaz de la Garza, R.I.; Jacobo-Velázquez, D.A. Biofortified Beverage with Chlorogenic Acid from Stressed Carrots: Anti-Obesogenic, Antioxidant, and Anti-Inflammatory Properties. Foods 2023, 12, 3959. [Google Scholar] [CrossRef]
- Ohishi, T.; Fukutomi, R.; Shoji, Y.; Goto, S.; Isemura, M. The Beneficial Effects of Principal Polyphenols from Green Tea, Coffee, Wine, and Curry on Obesity. Molecules 2021, 26, 453. [Google Scholar] [CrossRef]
- Goya, L.; Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo, L.; Sarriá, B. Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules 2023, 29, 88. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Relationship of the Phytochemicals from Coffee and Cocoa By-Products with Their Potential to Modulate Biomarkers of Metabolic Syndrome In Vitro. Antioxidants 2019, 8, 279. [Google Scholar] [CrossRef]
- Takahashi, S.; Saito, K.; Li, X.; Jia, H.; Kato, H. iTRAQ-Based Quantitative Proteomics Reveals the Energy Metabolism Alterations Induced by Chlorogenic Acid in HepG2 Cells. Nutrients 2022, 14, 1676. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Zhang, D.; Lv, Y.; Wei, Y.; Wu, W.; Zhou, F.; Tang, M.; Mao, T.; Li, M.; et al. Inhibitory Effect of Blueberry Polyphenolic Compounds on Oleic Acid-Induced Hepatic Steatosis in Vitro. J. Agric. Food Chem. 2011, 59, 12254–12263. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Balcheva-Sivenova, Z.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Caffeic and Chlorogenic Acids Synergistically Activate Browning Program in Human Adipocytes: Implications of AMPK- and PPAR-Mediated Pathways. Int. J. Mol. Sci. 2020, 21, 9740. [Google Scholar] [CrossRef]
- Awais, M.; Akter, R.; Boopathi, V.; Ahn, J.C.; Lee, J.H.; Mathiyalagan, R.; Kwak, G.-Y.; Rauf, M.; Yang, D.C.; Lee, G.S.; et al. Discrimination of Dendropanax Morbifera via HPLC Fingerprinting and SNP Analysis and Its Impact on Obesity by Modulating Adipogenesis- and Thermogenesis-Related Genes. Front. Nutr. 2023, 10, 1168095. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qin, J.; Cong, J.; Yang, Y. Chlorogenic Acids Inhibit Adipogenesis: Implications of Wnt/β-Catenin Signaling Pathway. Int. J. Endocrinol. 2021, 2021, 2215274. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.-Y.; Son, E.; Im, G.; Kim, D.-S. Herbal Combination of Phyllostachys Pubescens and Scutellaria Baicalensis Inhibits Adipogenesis and Promotes Browning via AMPK Activation in 3T3-L1 Adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Choi, S.-I.; Han, X.; Men, X.; Jang, G.-W.; Kwon, H.-Y.; Kang, S.-R.; Han, J.-S.; Lee, O.-H. Radical Scavenging-Linked Anti-Adipogenic Activity of Aster Scaber Ethanolic Extract and Its Bioactive Compound. Antioxidants 2020, 9, 1290. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-S.; Kim, W.-J.; Bae, W.-Y.; Lee, N.-K.; Paik, H.-D. Inula Britannica Inhibits Adipogenesis of 3T3-L1 Preadipocytes via Modulation of Mitotic Clonal Expansion Involving ERK 1/2 and Akt Signaling Pathways. Nutrients 2020, 12, 3037. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The New Role of AMP-Activated Protein Kinase in Regulating Fat Metabolism and Energy Expenditure in Adipose Tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Song, Y.; Xie, H.; Dong, M. An Update on Brown Adipose Tissue and Obesity Intervention: Function, Regulation and Therapeutic Implications. Front. Endocrinol. 2022, 13, 1065263. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Hu, W. Molecular and Cellular Regulation of Thermogenic Fat. Front. Endocrinol. 2023, 14, 1215772. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Pietrzyk, N.; Szustak, M.; Podsędek, A. Viburnum opulus L. Juice Phenolics Inhibit Mouse 3T3-L1 Cells Adipogenesis and Pancreatic Lipase Activity. Nutrients 2020, 12, 2003. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging Role of AMPK in Brown and Beige Adipose Tissue (BAT): Implications for Obesity, Insulin Resistance, and Type 2 Diabetes. Curr. Diab. Rep. 2018, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic Acid Promotes Development of Brown Adipocyte-like Phenotype in 3T3-L1 Adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Huang, Y.; Zhou, C.; Wang, N.; Zhang, L.; Zhang, Z.; Li, B.; He, X.; Wang, K.; et al. Metabolomics Analyses Reveal the Liver-Protective Mechanism of Wang’s Metabolic Formula on Metabolic-Associated Fatty Liver Disease. Heliyon 2024, 10, e33418. [Google Scholar] [CrossRef] [PubMed]
- Ghadieh, H.E.; Smiley, Z.N.; Kopfman, M.W.; Najjar, M.G.; Hake, M.J.; Najjar, S.M. Chlorogenic Acid/Chromium Supplement Rescues Diet-Induced Insulin Resistance and Obesity in Mice. Nutr. Metab. 2015, 12, 19. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Q.; Wang, X.; Wang, H. Mulberry Leaf Polyphenols Alleviated High-Fat Diet-Induced Obesity in Mice. Front. Nutr. 2022, 9, 979058. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic Acid Improves High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Ebrahimian, Z.; Razavi, B.M.; Mousavi Shaegh, S.A.; Hosseinzadeh, H. Exploring the Therapeutic Potential of Chlorogenic Acid in Alleviating Olanzapine-Induced Metabolic Syndrome in Rats: A Key Role of Hypothalamic Satiety Proteins. Nutr. Neurosci. 2025, 28, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Liu, S.; Lu, Y.; Zhang, T.; Wang, X.; Zhang, C.; Hu, C.Y.; Chen, P.; Deng, H.; Meng, Y. Low-Methoxy-Pectin and Chlorogenic Acid Synergistically Promote Lipolysis and β-Oxidation by Regulating AMPK Signaling Pathway in Obese Mice. Int. J. Biol. Macromol. 2024, 280, 135552. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yue, C.; Tian, R.; Yu, L.; Tian, F.; Zhao, J.; Chen, W.; Zhai, Q. Akkermansia muciniphila-Directed Polyphenol Chlorogenic Acid Intervention for Obesity in Mice. Food Sci. Hum. Wellness 2024, 13, 90–100. [Google Scholar] [CrossRef]
- Braojos, C.; Gila-Díaz, A.; Rodríguez-Rodríguez, P.; Monedero-Cobeta, I.; Morales, M.D.; Ruvira, S.; Ramiro-Cortijo, D.; Benítez, V.; Martín-Cabrejas, M.A.; Arribas, S.M. Effect of Supplementation with Coffee and Cocoa By-Products to Ameliorate Metabolic Syndrome Alterations Induced by High-Fat Diet in Female Mice. Foods 2023, 12, 2708. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Lan, N.; Li, S.; Zhang, J.; Wang, S.; Li, C.; Shang, Y.; Huang, T.; Zhang, L. Luteolin Protects against High Fat Diet-Induced Cognitive Deficits in Obesity Mice. Behav. Brain Res. 2014, 267, 178–188. [Google Scholar] [CrossRef]
- Sudeep, H.; Shyam Prasad, K. Supplementation of Green Coffee Bean Extract in Healthy Overweight Subjects Increases Lean Mass/Fat Mass Ratio: A Randomized, Double-Blind Clinical Study. SAGE Open Med. 2021, 9, 20503121211002590. [Google Scholar] [CrossRef]
- Terzo, S.; Amato, A.; Magán-Fernández, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. A Nutraceutical Containing Chlorogenic Acid and Luteolin Improves Cardiometabolic Parameters in Subjects with Pre-Obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de La Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Ochiai, R.; Chikama, A.; Kataoka, K.; Tokimitsu, I.; Maekawa, Y.; Ohishi, M.; Rakugi, H. Effects of hydroxyhydroquinone-reduced coffee on vasoreactivity and blood pressure. Hypertens. Res. 2009, 32, 969–974. [Google Scholar] [CrossRef]
- Kozuma, K.; Tsuchiya, S.; Kohori, J.; Hase, T.; Tokimitsu, I. Antihypertensive Effect of Green Coffee Bean Extract on Mildly Hypertensive Subjects. Hypertens. Res. 2005, 28, 711–718. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The Blood Pressure-Lowering Effect and Safety of Chlorogenic Acid from Green Coffee Bean Extract in Essential Hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyhydroquinone-free coffee: A double-blind, randomized controlled dose–response study of blood pressure. Nutr. Metab. Cardiovasc. Dis. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef]
- Kobylińska, Z.; Biesiadecki, M.; Kuna, E.; Galiniak, S.; Mołoń, M. Coffee as a Source of Antioxidants and an Elixir of Youth. Antioxidants 2025, 14, 285. [Google Scholar] [CrossRef]
- Camargo, L.L.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. Reactive Oxygen Species in Hypertension. Nat. Rev. Cardiol. 2025, 22, 20–37. [Google Scholar] [CrossRef]
- Lukitasari, M.; Saifur Rohman, M.; Nugroho, D.A.; Widodo, N.; Nugrahini, N.I.P. Cardiovascular Protection Effect of Chlorogenic Acid: Focus on the Molecular Mechanism. F1000Research 2020, 9, 1462. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive Effects and Mechanisms of Chlorogenic Acids. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2012, 35, 370–374. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Lehoczki, A.; Laukkanen, J.A. Coffee Consumption, Cancer, and Healthy Aging: Epidemiological Evidence and Underlying Mechanisms. GeroScience 2025, 47, 1517–1555. [Google Scholar] [CrossRef]
- Wuttimongkolchai, N.; Kanlaya, R.; Nanthawuttiphan, S.; Subkod, C.; Thongboonkerd, V. Chlorogenic Acid Enhances Endothelial Barrier Function and Promotes Endothelial Tube Formation: A Proteomics Approach and Functional Validation. Biomed. Pharmacother. 2022, 153, 113471. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-Protective and Antioxidant Properties of Caffeic Acid and Chlorogenic Acid: Mechanistic Role of Angiotensin Converting Enzyme, Cholinesterase and Arginase Activities in Cyclosporine Induced Hypertensive Rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Bello, G.T.; Oyagbemi, A.A. Caffeic and Chlorogenic Acids Modulate Altered Activity of Key Enzymes Linked to Hypertension in Cyclosporine-Induced Hypertensive Rats. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Katada, S.; Watanabe, T.; Mizuno, T.; Kobayashi, S.; Takeshita, M.; Osaki, N.; Kobayashi, S.; Katsuragi, Y. Effects of Chlorogenic Acid-Enriched and Hydroxyhydroquinone-Reduced Coffee on Postprandial Fat Oxidation and Antioxidative Capacity in Healthy Men: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2018, 10, 525. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green Coffee Bean Extract and Its Metabolites Have a Hypotensive Effect in Spontaneously Hypertensive Rats. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic Acid Attenuates Hypertension and Improves Endothelial Function in Spontaneously Hypertensive Rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Spencer, E.A.; Thompson, M.J.; Heneghan, C.J. The Effect of Chlorogenic Acid on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Hum. Hypertens. 2015, 29, 77–81. [Google Scholar] [CrossRef]
- Li, X.-P.; Yu, J.; Luo, J.-Y.; Li, H.-S.; Han, F.-J.; Chen, X.-G.; Hu, Z.-D. Determination and Pharmacokinetic Study of Chlorogenic Acid in Rat Dosed with Yin-Huang Granules by RP-HPLC. Biomed. Chromatogr. BMC 2006, 20, 206–210. [Google Scholar] [CrossRef]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE Pathway by Food Polyphenols: A Nutritional Neuroprotective Strategy for Cognitive and Neurodegenerative Disorders. Mol. Neurobiol. 2011, 44, 192–201. [Google Scholar] [CrossRef]
- Dai, T.; Xiao, Y.; Zhang, H.; Shi, Y.; Wu, F. Chlorogenic Acid Alleviates High Glucose-Induced HK-2 Cell Oxidative Damage through Activation of KEAP1/NRF2/ARE Signaling Pathway. Discov. Med. 2024, 36, 1378–1385. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic Acid: A Review on Its Mechanisms of Anti-Inflammation, Disease Treatment, and Related Delivery Systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Caballero, J.; Alarcón, M.; Rojas, A.; Palomo, I. Chlorogenic Acid Inhibits Human Platelet Activation and Thrombus Formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Kang, H.-J.; Kim, Y.-J.; Lee, D.-H.; Kwon, H.-W.; Kim, Y.-Y.; Park, H.-J. Inhibition of Platelet Aggregation by Chlorogenic Acid via cAMP and cGMP-Dependent Manner. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2012, 23, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Ma, Z.; Ye, H.; Guan, X.; Xiang, Z.; Xia, Y.; Shi, Q. Chlorogenic Acid-Conjugated Nanoparticles Suppression of Platelet Activation and Disruption to Tumor Vascular Barriers for Enhancing Drug Penetration in Tumor. Adv. Healthc. Mater. 2023, 12, e2202205. [Google Scholar] [CrossRef]
- Wang, D.; Hou, J.; Wan, J.; Yang, Y.; Liu, S.; Li, X.; Li, W.; Dai, X.; Zhou, P.; Liu, W.; et al. Dietary Chlorogenic Acid Ameliorates Oxidative Stress and Improves Endothelial Function in Diabetic Mice via Nrf2 Activation. J. Int. Med. Res. 2021, 49, 300060520985363. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, T.; Pei, Q.; Liu, S.; Yuan, H. Pharmacokinetics and Tissue Distribution Study of Chlorogenic Acid from Lonicerae Japonicae Flos Following Oral Administrations in Rats. Evid.-Based Complement. Altern. Med. ECAM 2014, 2014, 979414. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Z.; Chang, C. Chlorogenic Acid Intake Guidance: Sources, Health Benefits, and Safety. Asia Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [CrossRef]
- Li, T.; Matozel, M.; Boehme, S.; Kong, B.; Nilsson, L.-M.; Guo, G.; Ellis, E.; Chiang, J.Y.L. Overexpression of Cholesterol 7α-Hydroxylase Promotes Hepatic Bile Acid Synthesis and Secretion and Maintains Cholesterol Homeostasis. Hepatology 2011, 53, 996–1006. [Google Scholar] [CrossRef]
- Li, T.; Owsley, E.; Matozel, M.; Hsu, P.; Novak, C.M.; Chiang, J.Y.L. Transgenic Expression of Cholesterol 7α-Hydroxylase in the Liver Prevents High-Fat Diet–Induced Obesity and Insulin Resistance in Mice. Hepatology 2010, 52, 678–690. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic Acid Exhibits Anti-Obesity Property and Improves Lipid Metabolism in High-Fat Diet-Induced-Obese Mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Seefeld, K.; Witters, L.A.; Coleman, R.A. AMP-Activated Kinase Reciprocally Regulates Triacylglycerol Synthesis and Fatty Acid Oxidation in Liver and Muscle: Evidence That Sn-Glycerol-3-Phosphate Acyltransferase Is a Novel Target. Biochem. J. 1999, 338, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Carling, D.; Gamblin, S.J. AMP-Activated Protein Kinase: Also Regulated by ADP? Trends Biochem. Sci. 2011, 36, 470–477. [Google Scholar] [CrossRef]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; Green, K.A.; Mustard, K.J.; et al. The Ancient Drug Salicylate Directly Activates AMP-Activated Protein Kinase. Science 2012, 336, 918–922. [Google Scholar] [CrossRef]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and Characterization of a Small Molecule AMPK Activator That Treats Key Components of Type 2 Diabetes and the Metabolic Syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef]
- Tamura, Y.; Morita, I.; Hinata, Y.; Kojima, E.; Sasaki, Y.; Wada, T.; Asano, M.; Fujioka, M.; Hayasaki-Kajiwara, Y.; Iwasaki, T.; et al. Identification of Novel Benzimidazole Derivatives as Highly Potent AMPK Activators with Anti-Diabetic Profiles. Bioorg. Med. Chem. Lett. 2023, 79, 129059. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-Aminoimidazole-4-Carboxamide Ribonucleoside. A Specific Method for Activating AMP-Activated Protein Kinase in Intact Cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of Cells Expressing γ Subunit Variants to Identify Diverse Mechanisms of AMPK Activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef]
- Meng, C.; Zhou, L.; Huang, L.; Gu, Q.; Du, X.; Wang, C.; Liu, F.; Xia, C. Chlorogenic Acid Regulates the Expression of NPC1L1 and HMGCR through PXR and SREBP2 Signaling Pathways and Their Interactions with HSP90 to Maintain Cholesterol Homeostasis. Phytomedicine 2024, 123, 155271. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic Acid Stimulates Glucose Transport in Skeletal Muscle via AMPK Activation: A Contributor to the Beneficial Effects of Coffee on Diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhang, Z.; Liu, J.; Chen, L.; Tian, Y.; Xu, W.; Zeng, T.; Wu, W.; Lu, L. Chlorogenic Acid Alleviates LPS-Induced Inflammation and Oxidative Stress by Modulating CD36/AMPK/PGC-1α in RAW264.7 Macrophages. Int. J. Mol. Sci. 2023, 24, 13516. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single Phosphorylation Sites in Acc1 and Acc2 Regulate Lipid Homeostasis and the Insulin–Sensitizing Effects of Metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Zammit, V.A.; Hardie, D.G. A Common Bicyclic Protein Kinase Cascade Inactivates the Regulatory Enzymes of Fatty Acid and Cholesterol Biosynthesis. FEBS Lett. 1987, 223, 217–222. [Google Scholar] [CrossRef]
- Anthony, N.M.; Gaidhu, M.P.; Ceddia, R.B. Regulation of Visceral and Subcutaneous Adipocyte Lipolysis by Acute AICAR-Induced AMPK Activation. Obesity 2009, 17, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.R.; Hardie, D.G. Regulation of HMG-CoA Reductase: Identification of the Site Phosphorylated by the AMP-Activated Protein Kinase in Vitro and in Intact Rat Liver. EMBO J. 1990, 9, 2439–2446. [Google Scholar] [CrossRef]
- Thampy, K.G. Formation of Malonyl Coenzyme A in Rat Heart. Identification and Purification of an Isozyme of A Carboxylase from Rat Heart. J. Biol. Chem. 1989, 264, 17631–17634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
- Tie, F.; Ding, J.; Gao, Y.; Wang, H. Chlorogenic Acid and Its Isomers Attenuate NAFLD by Mitigating Lipid Accumulation in Oleic Acid-Induced HepG2 Cells and High-Fat Diet- Fed Zebrafish. Chem. Biodivers. 2024, 21, e202400564. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In Vitro and in Vivo Antioxidant Properties of Chlorogenic Acid and Caffeic Acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- La Rosa, G.; Sozio, C.; Pipicelli, L.; Raia, M.; Palmiero, A.; Santillo, M.; Damiano, S. Antioxidant, Anti-Inflammatory and Pro-Differentiative Effects of Chlorogenic Acid on M03-13 Human Oligodendrocyte-like Cells. Int. J. Mol. Sci. 2023, 24, 16731. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Nasution, S.L.; Suhartina, S.; Munshy, U.Z.; Rizal, R.; Widowati, W. Antioxidant and Anti-Inflammatory Activity of Chlorogenic Acid on Lead-Induced Fibroblast Cells. J. Phys. Conf. Ser. 2019, 1374, 012006. [Google Scholar] [CrossRef]
- Rawal, P.; Pandey, B.; Yadav, R.K.; Panta, S. Antioxidant, Alpha-Amylase Inhibitory and Hypoglycemic Activity of Smallanthus Sonchifolius Leaves from Nepal: An Integrated In Vitro, In Vivo, and In Silico Approach. Food Sci. Nutr. 2025, 13, e4672. [Google Scholar] [CrossRef]
- Salamat, S.; Sharif, S.S.; Nazary-Vanani, A.; Kord-Varkaneh, H.; Clark, C.C.T.; Mohammadshahi, M. The Effect of Green Coffee Extract Supplementation on Serum Oxidized LDL Cholesterol and Total Antioxidant Capacity in Patients with Dyslipidemia: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Integr. Med. 2019, 28, 109–113. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Sun, J.; Ren, J.; Hu, X.; Hou, Y.; Yang, Y. Therapeutic Effects of Chinese Herbal Medicines and Their Extracts on Diabetes. Biomed. Pharmacother. 2021, 142, 111977. [Google Scholar] [CrossRef]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic Acid Prevents Diabetic Nephropathy by Inhibiting Oxidative Stress and Inflammation through Modulation of the Nrf2/HO-1 and NF-κB Pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Ouyang, H.; Xie, Y.; Du, A.; Dong, S.; Zhou, S.; Lu, B.; Wang, Z.; Ji, L. Chlorogenic Acid Ameliorates Non-Proliferative Diabetic Retinopathy via Alleviating Retinal Inflammation through Targeting TNFR1 in Retinal Endothelial Cells. Int. Immunopharmacol. 2024, 141, 112929. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Protection against Developing Type 2 Diabetes by Coffee Consumption: Assessment of the Role of Chlorogenic Acid and Metabolites on Glycaemic Responses. Food Funct. 2020, 11, 4826–4833. [Google Scholar] [CrossRef] [PubMed]
- Ihara, Y.; Asahara, S.-I.; Inoue, H.; Seike, M.; Ando, M.; Kabutoya, H.; Kimura-Koyanagi, M.; Kido, Y. Chlorogenic Acid and Caffeine in Coffee Restore Insulin Signaling in Pancreatic Beta Cells. Kobe J. Med. Sci. 2023, 69, E1–E8. [Google Scholar] [PubMed]
- Chen, L.; Teng, H.; Cao, H. Chlorogenic Acid and Caffeic Acid from Sonchus Oleraceus Linn Synergistically Attenuate Insulin Resistance and Modulate Glucose Uptake in HepG2 Cells. Food Chem. Toxicol. 2019, 127, 182–187. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Hsieh, T.-J.; Seres, A.; Zupkó, I. Chlorogenic Acid and Rutin Play a Major Role in the in Vivo Anti-Diabetic Activity of Morus Alba Leaf Extract on Type II Diabetic Rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef]
- Pérez-Nájera, V.C.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Hidalgo-Figueroa, S.; Del-Toro-Sánchez, C.L.; Salazar-Olivo, L.A.; Lugo-Cervantes, E. Smilax aristolochiifolia Root Extract and Its Compounds Chlorogenic Acid and Astilbin Inhibit the Activity of α-Amylase and α-Glucosidase Enzymes. Evid.-Based Complement. Alternat. Med. 2018, 2018, 6247306. [Google Scholar] [CrossRef]
- Saraswat, N.; Sachan, N.; Chandra, P. Anti-Diabetic, Diabetic Neuropathy Protective Action and Mechanism of Action Involving Oxidative Pathway of Chlorogenic Acid Isolated from Selinum Vaginatum Roots in Rats. Heliyon 2020, 6, e05137. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic Acid Decreases Retinal Vascular Hyperpermeability in Diabetic Rat Model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef]
- Singh, A.K.; Rana, H.K.; Singh, V.; Chand Yadav, T.; Varadwaj, P.; Pandey, A.K. Evaluation of Antidiabetic Activity of Dietary Phenolic Compound Chlorogenic Acid in Streptozotocin Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, in Silico Toxicity, in Vitro and in Vivo Studies. Comput. Biol. Med. 2021, 134, 104462. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. Methods Mol. Biol. Clifton N. J. 2020, 2067, 3–7. [Google Scholar] [CrossRef]
- Singh, A.K.; Pandey, A.K. Alleviation of Diabetes Mellitus-Induced Reproductive Dysfunction by Chlorogenic Acid in Male Rats via Combating Redox Imbalance. Indian J. Clin. Biochem. 2025. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Jiang, D.; Wang, Y.-Z.; Duan, M.-Y.; Huang, Y.-W.; Wang, X.-J.; Xiang, Z.-M.; Sheng, J.; Zhu, Q.-Q. Chlorogenic Acid Alleviates Renal Fibrosis by Reducing Lipid Accumulation in Diabetic Kidney Disease through Suppressing the Notch1 and Stat3 Signaling Pathway. Ren. Fail. 2024, 46, 2371988. [Google Scholar] [CrossRef]
- Fujita, H.; Fujishima, H.; Chida, S.; Takahashi, K.; Qi, Z.; Kanetsuna, Y.; Breyer, M.D.; Harris, R.C.; Yamada, Y.; Takahashi, T. Reduction of Renal Superoxide Dismutase in Progressive Diabetic Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 1303–1313. [Google Scholar] [CrossRef]
- Bao, L.; Gong, Y.; Xu, W.; Dao, J.; Rao, J.; Yang, H. Chlorogenic Acid Inhibits NLRP3 Inflammasome Activation through Nrf2 Activation in Diabetic Nephropathy. PLoS ONE 2025, 20, e0316615. [Google Scholar] [CrossRef]
- Cheung, N.; Mitchell, P.; Wong, T.Y. Diabetic Retinopathy. Lancet Lond. Engl. 2010, 376, 124–136. [Google Scholar] [CrossRef]
- Wong, T.Y.; Cheung, C.M.G.; Larsen, M.; Sharma, S.; Simó, R. Diabetic Retinopathy. Nat. Rev. Dis. Primer 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.X.; Fawzi, A.A. Perspectives on Diabetic Retinopathy from Advanced Retinal Vascular Imaging. Eye Lond. Engl. 2022, 36, 319–327. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, F.-L.; Wang, G.-H. Effects of HIF-1α on Diabetic Retinopathy Angiogenesis and VEGF Expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5071–5076. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, M.; Narayanan, S.P.; Somanath, P.R. Regulation of Blood-Retinal Barrier Cell-Junctions in Diabetic Retinopathy. Pharmacol. Res. 2020, 161, 105115. [Google Scholar] [CrossRef]
- Elangovan, S.; Spankovich, C. Diabetes and Auditory-Vestibular Pathology. Semin. Hear. 2019, 40, 292–299. [Google Scholar] [CrossRef]
- Hong, B.N.; Nam, Y.H.; Woo, S.H.; Kang, T.H. Chlorogenic Acid Rescues Sensorineural Auditory Function in a Diabetic Animal Model. Neurosci. Lett. 2017, 640, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Bhushan, S.; Arora, R.; Singh Buttar, H.; Arora, S.; Singh, B. Alternative Treatment Strategies for Neuropathic Pain: Role of Indian Medicinal Plants and Compounds of Plant Origin-A Review. Biomed. Pharmacother. 2017, 92, 634–650. [Google Scholar] [CrossRef]
- Siwi, K.; Tejosukmono, A.; Anggorowati, N.; Arfian, N.; Yunus, J. Chlorogenic Acid Ameliorates Muscle Wasting by Upregulating mRNA Expressions of Calcineurin and PGC-1α in Diabetic Rat Model. Med. J. Malays. 2024, 79, 23–30. [Google Scholar]
- Sandoval-Herrera, I.; Romero-García, J.; Ledezma-Pérez, A.; Alvarado-Canché, C.; Torres-Lubian, R.; De-León, A. Controlled Release of Chlorogenic Acid from Polyvinyl Alcohol/Poly(γ-Glutamic Acid) Blended Electrospun Nanofiber Mats with Potential Applications in Diabetic Foot Treatment. Polymers 2021, 13, 2943. [Google Scholar] [CrossRef]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of Chlorogenic Acids Following Acute Ingestion of Coffee by Humans with an Ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of Dose on the Bioavailability of Coffee Chlorogenic Acids in Humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef]
- Kajikawa, M.; Maruhashi, T.; Hidaka, T.; Nakano, Y.; Kurisu, S.; Matsumoto, T.; Iwamoto, Y.; Kishimoto, S.; Matsui, S.; Aibara, Y.; et al. Coffee with a High Content of Chlorogenic Acids and Low Content of Hydroxyhydroquinone Improves Postprandial Endothelial Dysfunction in Patients with Borderline and Stage 1 Hypertension. Eur. J. Nutr. 2019, 58, 989–996. [Google Scholar] [CrossRef]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of Coffee-Induced Improvements in Human Vascular Function by Chlorogenic Acids and Its Metabolites: Two Randomized, Controlled, Crossover Intervention Trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef]
- Kang, Z.; Li, S.; Kang, X.; Deng, J.; Yang, H.; Chen, F.; Jiang, J.; Zhang, J.; Li, W. Phase I Study of Chlorogenic Acid Injection for Recurrent High-Grade Glioma with Long-Term Follow-Up. Cancer Biol. Med. 2023, 20, 465–476. [Google Scholar] [CrossRef]
- Morton, K.; Knight, K.; Kalman, D.; Hewlings, S. A Prospective Randomized, Double-Blind, Two-Period Crossover Pharmacokinetic Trial Comparing Green Coffee Bean Extract-A Botanically Sourced Caffeine-With a Synthetic USP Control. Clin. Pharmacol. Drug Dev. 2018, 7, 871–879. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, J.; Fang, Z.; Sun, P. Complexing of Chlorogenic Acid with β-Cyclodextrins: Inclusion Effects, Antioxidative Properties and Potential Application in Grape Juice. Food Hydrocoll. 2014, 41, 132–139. [Google Scholar] [CrossRef]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 5063. [Google Scholar] [CrossRef]
- Adeyemo-Salami, O.A.; Afolabi, D.A.; Amuzat, A.A.; Adekanye, J.-P.O.; Oladokun, O.O. Effect of Acute Exposure of Swiss Mice to Chlorogenic Acid. Basic Clin. Pharmacol. Toxicol. 2025, 136, e70017. [Google Scholar] [CrossRef] [PubMed]
- Behne, S.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk Assessment of Chlorogenic and Isochlorogenic Acids in Coffee By-Products. Molecules 2023, 28, 5540. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic Acid Attenuates High-Carbohydrate, High-Fat Diet-Induced Cardiovascular, Liver, and Metabolic Changes in Rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef] [PubMed]
| Patient Data | Intervention Details | Results | Ref. Number | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Illness | n | Dosage (Pure CGA) | CGA Origin | Form | Treatment Duration | BM [%] | BMI [%] | WC [%] | FBG [%] | A1c [%] | TC [%] | HDL [%] | LDL [%] | TG [%] | SBP [%] | DBP [%] | |
| Pre-obesity (BMI 25–30) | 28 | 15–18 mg × 1/day | Nutraceutical (CGA + luteolin) | tablet | 6 months | −2.63 | −3.01 | −3.15 | x | −0.73 | −12.60 | 2.09 | −15.40 | −18.40 | x | x | [88] |
| Pre-obesity (BMI 25–30) | 33 | 250 mg × 2/day | GCE (decaf.) | capsule | 12 weeks | −2.74 | −2.91 | −1.03 | −0.68 | −1.84 | −4.84 | 4.55 | −6.25 | −4.92 | x | x | [87] |
| Impaired glucose tolerance | 14 | 400 mg × 3/day | GCE | capsule | 12 weeks | −3.00 | −3.68 | −1.92 | −3.50 | 0 | −4.44 | 58.82 | −17.39 | −18.75 | −3.90 | −1.74 | [89] |
| Mild hypertension (SBP 140–159, DBP < 99) | 9 | 300 mg × 1/day | HHQ-reduced coffee | adjusted coffee | 8 weeks | −0.73 | −0.83 | x | −3.83 | 1.89 | 3.87 | 3.75 | −2.26 | 26.75 | −6.25 | 0.90 | [90] |
| Mild hypertension * | 29 | 24.84 mg × 1/day | GCE | enriched soup | 28 days | −0.14 | −0.40 | x | x | x | 2.10 | 0 | 2.48 | 1 | −2.19 | −3.15 | [91] |
| Mild hypertension * | 28 | 50.22 mg × 1/day | GCE | enriched soup | 28 days | −0.14 | 0 | x | x | x | −3.44 | 0 | −3.82 | 1.78 | −3.22 | −3.46 | [91] |
| Mild hypertension * | 31 | 99.9 mg × 1/day | GCE | enriched soup | 28 days | 0 | 0 | x | x | x | −3.49 | 0 | −6.50 | −3.93 | −3.84 | −4.22 | [91] |
| Mild hypertension * | 14 | 140 mg × 1/day | GCE | enriched fruit juice | 12 weeks | x | 0 | x | 8.99 | x | 0 | 7.27 | 5.55 | −5 | −6.90 | −7.69 | [92] |
| Mild hypertension ** | 41 | 82 mg × 1/day | HHQ-depleted coffee | adjusted coffee | 4 weeks | x | x | x | x | x | x | x | x | x | −1.86 | −2.96 | [93] |
| Mild hypertension ** | 40 | 172 mg × 1/day | HHQ-depleted coffee | adjusted coffee | 4 weeks | x | x | x | x | x | x | x | x | x | −1.96 | −2.53 | [93] |
| Mild hypertension ** | 40 | 299 mg × 1/day | HHQ-depleted coffee | adjusted coffee | 4 weeks | x | x | x | x | x | X | x | x | x | −2.29 | −3.07 | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalewska, K.; Kulawik, M.; Gierszewska, J.; Gramala, Z.; Kalus, O.; Karpiński, M.; Maćkowiak, J.; Staniewski, A.; Szymańska, Z.; Zalewska, B.; et al. Chlorogenic Acid’s Role in Metabolic Health: Mechanisms and Therapeutic Potential. Nutrients 2025, 17, 3303. https://doi.org/10.3390/nu17203303
Zalewska K, Kulawik M, Gierszewska J, Gramala Z, Kalus O, Karpiński M, Maćkowiak J, Staniewski A, Szymańska Z, Zalewska B, et al. Chlorogenic Acid’s Role in Metabolic Health: Mechanisms and Therapeutic Potential. Nutrients. 2025; 17(20):3303. https://doi.org/10.3390/nu17203303
Chicago/Turabian StyleZalewska, Katarzyna, Maciej Kulawik, Julia Gierszewska, Zofia Gramala, Oliwia Kalus, Michał Karpiński, Joanna Maćkowiak, Antoni Staniewski, Zofia Szymańska, Barbara Zalewska, and et al. 2025. "Chlorogenic Acid’s Role in Metabolic Health: Mechanisms and Therapeutic Potential" Nutrients 17, no. 20: 3303. https://doi.org/10.3390/nu17203303
APA StyleZalewska, K., Kulawik, M., Gierszewska, J., Gramala, Z., Kalus, O., Karpiński, M., Maćkowiak, J., Staniewski, A., Szymańska, Z., Zalewska, B., Lu, W., Cielecka-Piontek, J., & Zalewski, P. (2025). Chlorogenic Acid’s Role in Metabolic Health: Mechanisms and Therapeutic Potential. Nutrients, 17(20), 3303. https://doi.org/10.3390/nu17203303









