Marine-Based Omega-3 Fatty Acids and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Method
2.1. Objective
2.2. Search Strategy
2.3. Study Selection
- (1)
- Randomized controlled trials (RCTs) assessing the impact of omega-3 fatty acids on MetS components in adult populations with diagnosed metabolic syndrome or individuals exhibiting at least one of the components of MetS;
- (2)
- Studies reporting outcomes on at least one of the following: LDL cholesterol, triglycerides, HDL cholesterol, HOMA-IR, fasting plasma glucose, or HbA1c;
- (3)
- Studies published or available in English or Spanish.
2.4. Data Extraction
- The omega-3 treatment dose: low dose (LD): <1000 mg/day, medium dose (MD): 1000–2000 mg/day, and high dose (HD): >2000 mg/day;
- The duration of treatment with omega-3 supplementation: short-term (ST): ≤8 weeks, medium-term (MT): >8 to 12 weeks, and long-term (LT): >12 weeks.
2.5. Quality Assessment
2.6. Data Synthesis and Statistical Analysis
2.6.1. Study Classification
2.6.2. Model Specification
- Parameter change represents the observed change in the outcome variable due to omega-3 supplementation.
- Treatment is a dummy variable, coded 1 for the treatment group (omega-3 users) and 0 for the control group.
- MT and LT are dummy variables indicating medium-term and long-term studies, respectively, with short-term groups serving as the baseline category (both MT = 0 and LT = 0).
- ui is the random error term.
- β0 (Intercept) represents the mean baseline change in the studied parameter among the control groups, reflecting the expected variation in the absence of omega-3 supplementation.
- β1 (Treatment) measures the mean effect of short-term omega-3 supplementation relative to the control groups. Since Treatment = 1 for treated and 0 for controls, β1 indicates the expected change attributable to omega-3 in the short-term (baseline) category, when MT = 0 and LT = 0. A positive and significant β1 shows improvement relative to control; a negative and significant β1 shows a reduction.
- β2 (MT) captures the incremental difference in the parameter change for medium-term interventions compared with short-term ones. A positive and significant β2 suggests a stronger omega-3 effect with medium-term use, while a negative and significant β2 suggests a weaker effect.
- β3 (LT) captures the incremental difference in the parameter change for long-term interventions relative to short-term ones. The sign and magnitude of β3 indicate whether prolonged omega-3 use amplifies or attenuates the effect on the metabolic parameter compared with shorter durations.
2.6.3. Data Simulation and Weighting
2.6.4. Estimation Procedure
2.7. Assessment of Publication Bias
3. Results
3.1. Study Selection
3.2. Study Characteristics
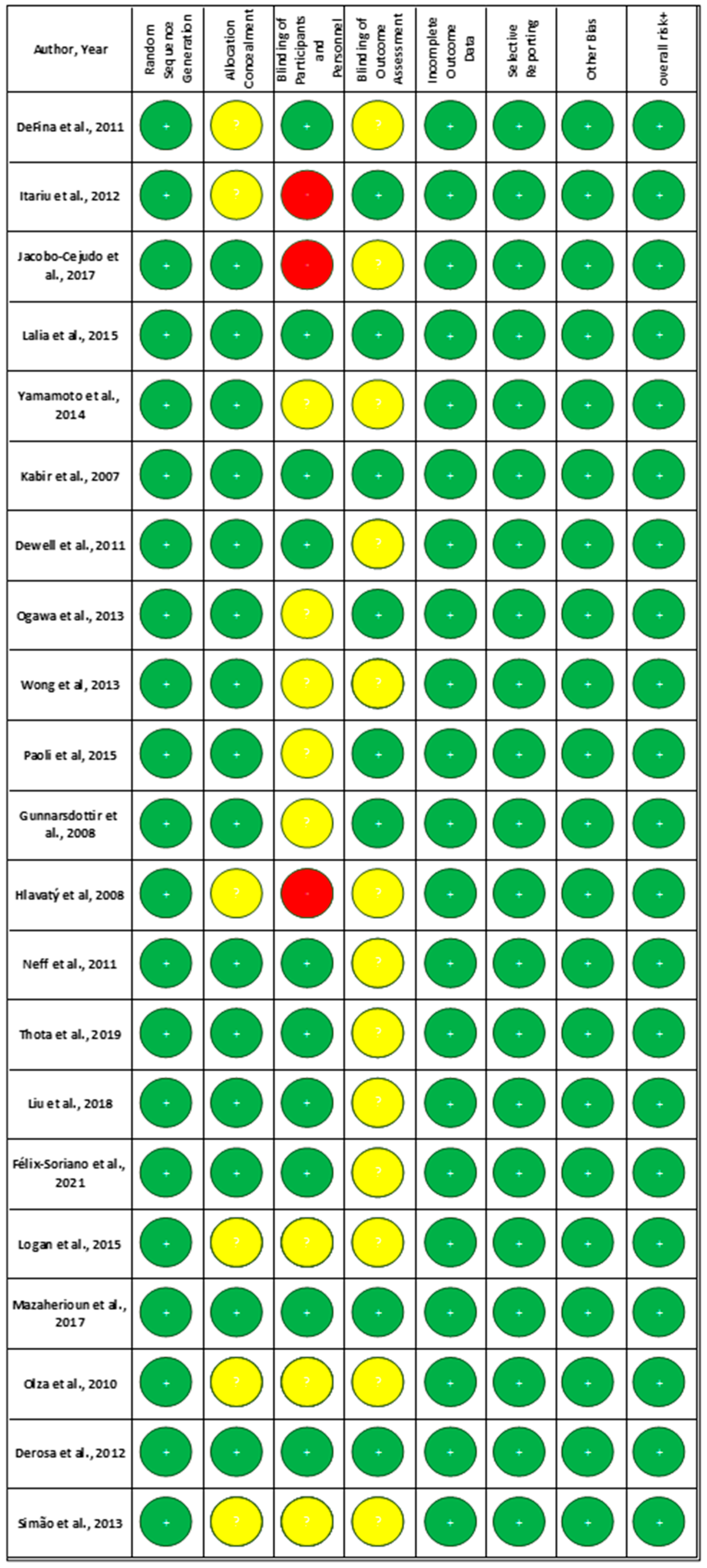
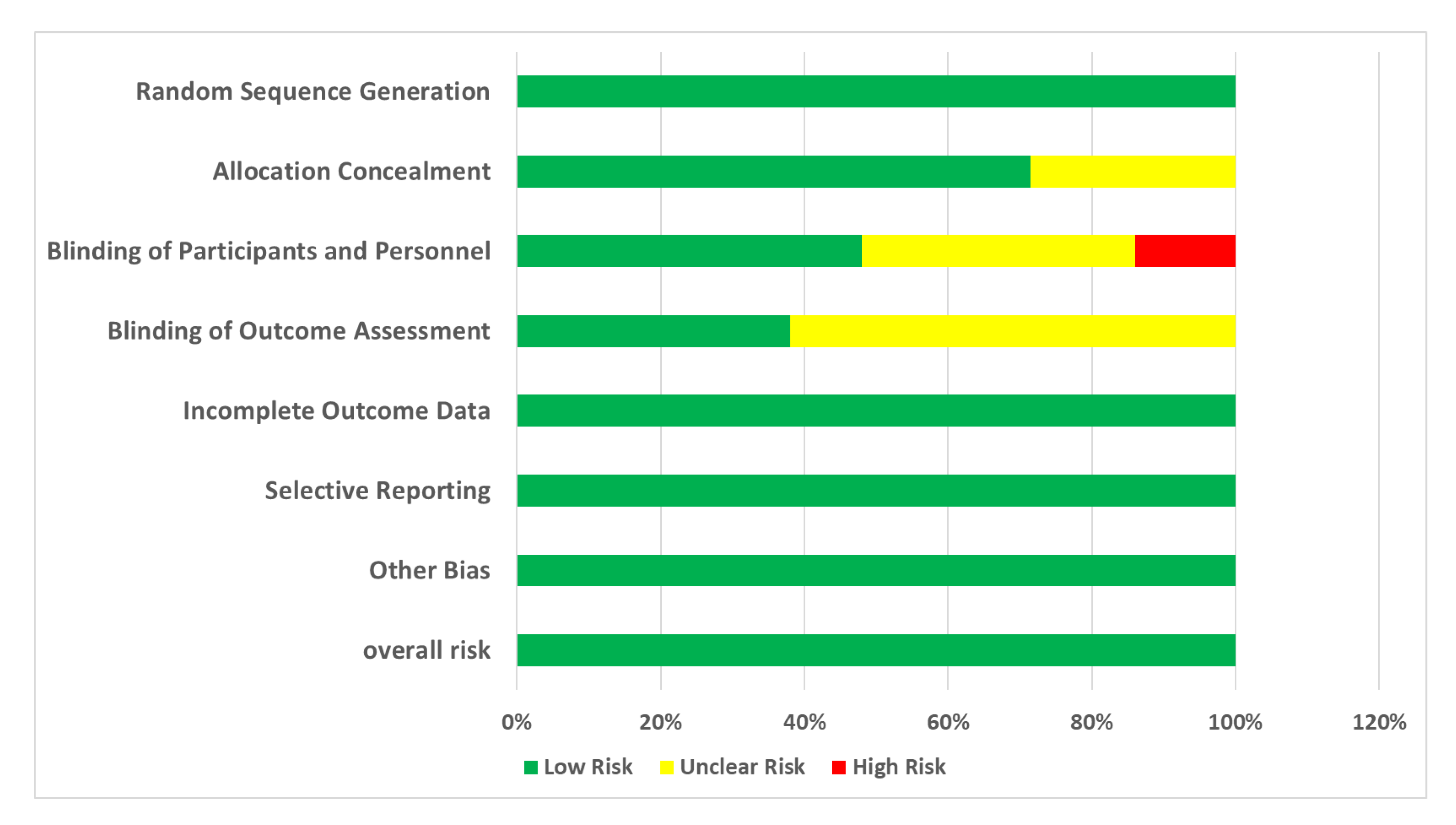
3.3. Risk of Bias in Studies
- Random Sequence Generation: All of the studies (100%) were rated as low risk, indicating that proper randomization methods were commonly used;
- Allocation Concealment: A significant proportion of studies (71%) were rated as low risk, suggesting that many studies adequately report their allocation concealment methods. However, 29% of studies had an unclear risk due to insufficient information.
- Blinding of Participants and Personnel: While a considerable number of studies achieved low risk (48%), a notable portion remained unclear (38%) or high risk (14%), reflecting challenges in maintaining blinding.
- Blinding of Outcome Assessment: Similar trends were observed with blinding of outcome assessors, with 38% rated as low risk and 62% as unclear.
- Incomplete Outcome Data: All included studies (100%) effectively managed attrition bias.
- Selective Reporting: A high percentage of studies (100%) were rated as low risk, indicating comprehensive reporting of outcomes.
- Other Bias: In this domain, 100% of studies are rated as low risk due to the elimination of high-risk studies.
3.4. Publication Bias in Studies
3.5. Results of Individual Studies
- Effects of omega-3 fatty acids on MetS components;
- Triglycerides (TGs).
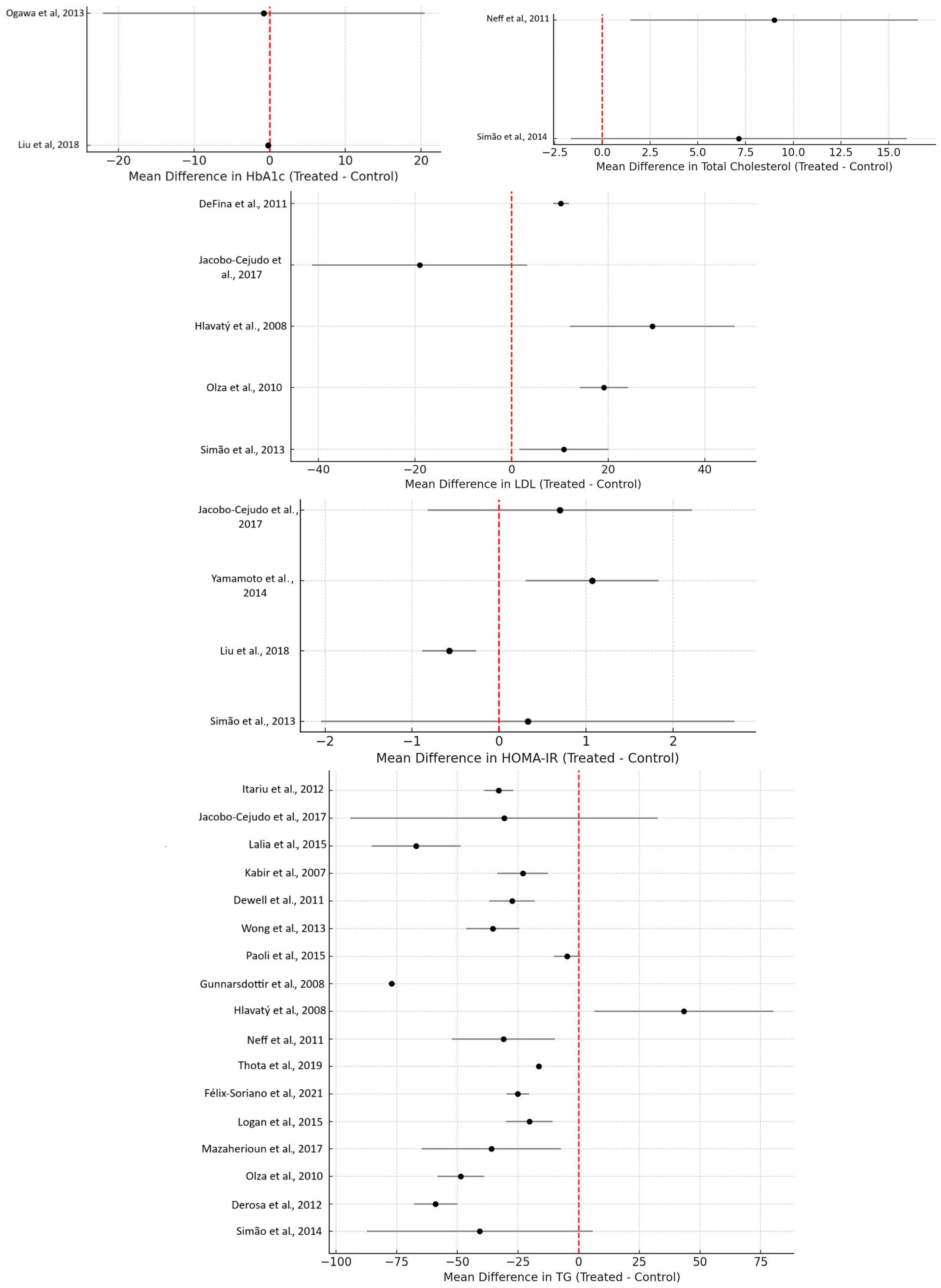
- Itariu et al. (2012) [3]: a significant reduction in TG levels was observed in non-diabetic, severely obese patients (p = 0.03);
- Jacobo-Cejudo et al. (2017) [6]: diabetic patients with a BMI ≤ 30 saw significant reductions in TG levels (p = 0.002);
- Lalia et al. (2015) [7]: insulin-resistant, non-diabetic patients exhibited significant reductions in TG levels;
- Kabir et al. (2007) [9]: a significant decrease in triacylglycerol was observed in diabetic, overweight, post-menopausal women (p = 0.03);
- Dewell et al. (2011) [11]: fish oil supplementation led to a significant decrease in TG levels compared to flaxseed oil in MetS patients (p ≤ 0.01);
- Wong et al. (2013) [13]: obese, dyslipidemic patients in the weight loss + omega-3 group showed a significant reduction in plasma TG compared to the weight loss group alone;
- Paoli et al. (2015) [14]: a significant TG decrease was observed in the KDO3 group compared to the KD group (p < 0.05);
- Gunnarsdottir et al. (2008) [15]: overweight and obese young individuals with central obesity experienced a significant reduction in TG levels in all fish and fish oil groups compared to controls (p = 0.035);
- Neff et al. (2011) [17]: a significant reduction in total TG concentrations was observed in obese patients with prominent abdominal obesity (p = 0.006);
- Thota et al. (2019) [18]: obese individuals with impaired fasting glucose showed a significant reduction in TG levels (p < 0.001);
- Félix-Soriano et al. (2021) [20]: a significant reduction in TG levels was observed in overweight and obese post-menopausal women receiving DHA-rich supplementation (p = 0.035);
- Logan et al. (2015) [21]: healthy women in the fish oil group experienced a significant 29% reduction in TG levels (p = 0.001);
- Mazaherioun et al. (2017) [22]: overweight diabetic patients in the n-3 PUFAs group experienced a significant reduction in TG levels from 172 ± 68 mg/dL to 141 ± 54 mg/dL (p = 0.039);
- Olza et al. (2010) [23]: patients receiving T-Diet Plus® exhibited a significant decrease in TG levels from baseline (185.5 ± 24.2 mg/dL) to 3 months (132.6 ± 16.7 mg/dL) and 6 months (124.8 ± 15.9 mg/dL) (p = 0.002);
- Derosa et al. (2012) [24]: a significant decrease in TG levels was observed in patients with combined dyslipidemia receiving n-3 PUFAs (p < 0.01);
- Simão et al. (2014) [25]: women with MetS experienced a significant decrease in TG levels in the fish oil group over 90 days (p < 0.05).
3.5.1. LDL, HDL, and Total Cholesterol
LDL Cholesterol
HDL Cholesterol
- Hlavatý et al. (2008) [16]: moderately obese non-diabetic women on an omega-3 and low-calorie diet showed a significant increase in HDL levels (p < 0.05);
- Neff et al. (2011) [17]: obese patients with prominent abdominal obesity experienced an increase in the concentration of large HDL particles (p = 0.001);
- Derosa et al. (2012) [24]: patients with combined dyslipidemia receiving n-3 PUFAs showed an increase in HDL levels (p < 0.05).
Total Cholesterol
- Derosa et al. (2012) [24]: total cholesterol levels decreased in patients receiving n-3 PUFAs.
- Systolic and Diastolic Blood Pressure
- In the included studies, the effects of omega-3 on systolic and diastolic blood pressure were mostly insignificant:
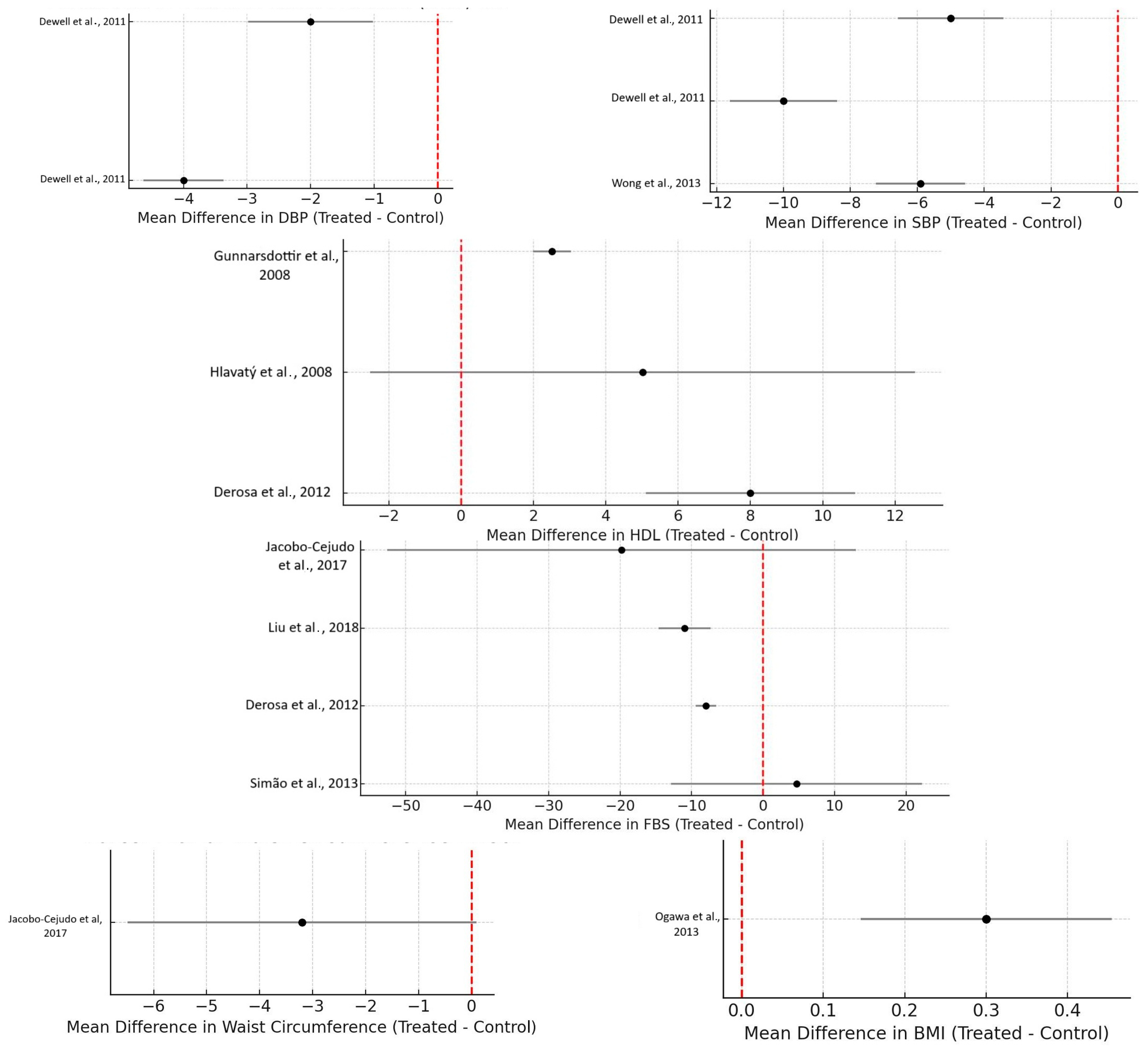
- Dewell et al. (2011) [11]: Significant decreases in systolic blood pressure were noted in high-dose fish oil groups compared to the placebo groups in MetS patients (p ≤ 0.01). Additionally, diastolic blood pressure decreased significantly compared to all other groups (p ≤ 0.02).
- Wong et al. (2013) [13]: a significant reduction in systolic blood pressure was observed in the weight loss + omega-3 group compared to the weight loss group alone (p = 0.018).
- Félix-Soriano et al. (2021) [20]: diastolic blood pressure significantly decreased in the DHA-rich supplementation group (p = 0.038).
- Weight, BMI, and Waist Circumference
- Jacobo-Cejudo et al. (2017) [6]: diabetic patients with a BMI ≤ 30 showed a significant decrease in waist circumference (p = 0.001);
- Kabir et al. (2007) [9]: significant decreases in total fat mass were observed in diabetic, overweight, post-menopausal women treated with fish oil.
- Fasting Blood Sugar (FBS) and Insulin Sensitivity
- Among many studies focusing on the effects of omega-3 on fasting blood glucose (FBS), insulin sensitivity, and insulin resistance, there are different contradictory findings depending on the study design:
- Jacobo-Cejudo et al. (2017) [6]: diabetic patients with a BMI ≤ 30 experienced significant reductions in FBS (p = 0.011) and HbA1c (p = 0.009);
- Ogawa et al. (2013) [10]: a significant decrease in fasting plasma glucose was noted in elderly bedridden patients with type 2 diabetes on enteral nutrition (p < 0.01);
- Jacobo-Cejudo et al. (2017) [6]: a significant reduction in insulin levels (p < 0.001) and HOMA-IR (p < 0.001) was reported in diabetic patients with BMI ≤ 30;
- Lalia et al. (2015) [7]: a modest but significant decrease in hepatic insulin sensitivity was noted in insulin-resistant, non-diabetic patients;
- Yamamoto et al. (2014) [8]: a significant decrease in insulin resistance (HOMA-IR) was reported in a hyperlipidemic population (p < 0.05);
- Paoli et al. (2015) [14]: overweight but healthy participants in the keto diet + omega-3 group showed a significant decrease in insulin levels compared to the keto diet group alone (p < 0.05);
- Liu et al. (2018) [19]: overweight patients newly diagnosed with diabetes exhibited significant reductions in fasting glucose (p = 0.02) and HbA1c (p = 0.03) in the omega-3 group;
- Derosa et al. (2012) [24]: insulin resistance (HOMA-IR) significantly decreased in patients with combined dyslipidemia receiving n-3 PUFAs (p < 0.05).
3.6. Meta-Analysis Results
3.6.1. Triglycerides (TGs)
3.6.2. HDL Cholesterol (HDL-C)
3.6.3. Fasting Blood Glucose (FBG)
3.6.4. Blood Pressure
Systolic Blood Pressure (SBP)
Diastolic Blood Pressure (DBP)
3.6.5. Waist Circumference and BMI
3.6.6. Other MetS-Related Biomarkers
Insulin Resistance (HOMA-IR) and HbA1c
LDL Cholesterol (LDL-C)
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Research
4.3. Strengths and Limitations
4.4. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, M.; Sorkin, J.D.; Mastella, L.; Sutherland, A.; Rhyne, J.; Donnelly, P.; Simpson, K.; Goldberg, A.P. Poly is more effective than monounsaturated fat for dietary management in the metabolic syndrome: The muffin study. J. Clin. Lipidol. 2016, 10, 996–1003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DeFina, L.F.; Marcoux, L.G.; Devers, S.M.; Cleaver, J.P.; Willis, B.L. Effects of omega-3 supplementation in combination with diet and exercise on weight loss and body composition. Am. J. Clin. Nutr. 2011, 93, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Itariu, B.K.; Zeyda, M.; Hochbrugger, E.E.; Neuhofer, A.; Prager, G.; Schindler, K.; Bohdjalian, A.; Mascher, D.; Vangala, S.; Schranz, M.; et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 1137–1149, Erratum in Am. J. Clin. Nutr. 2020, 112, 1405. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Zhang, H.M.; Li, Y.Y.; Xia, S.; Wei, Y.; Yang, L.; Wang, D.; Ye, J.J.; Li, H.X.; Yuan, J.; et al. A combination of omega-3 and plant sterols regulate glucose and lipid metabolism in individuals with impaired glucose regulation: A randomized and controlled clinical trial. Lipids Health Dis. 2019, 18, 106, Erratum in Lipids Health Dis. 2020, 19, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Cejudo, M.G.; Valdés-Ramos, R.; Guadarrama-López, A.L.; Pardo-Morales, R.V.; Martínez-Carrillo, B.E.; Harbige, L.S. Effect of n-3 Polyunsaturated Fatty Acid Supplementation on Metabolic and Inflammatory Biomarkers in Type 2 Diabetes Mellitus Patients. Nutrients 2017, 9, 573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lalia, A.Z.; Johnson, M.L.; Jensen, M.D.; Hames, K.C.; Port, J.D.; Lanza, I.R. Effects of Dietary n-3 Fatty Acids on Hepatic and Peripheral Insulin Sensitivity in Insulin-Resistant Humans. Diabetes Care 2015, 38, 1228–1237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, T.; Kajikawa, Y.; Otani, S.; Yamada, Y.; Takemoto, S.; Hirota, M.; Ikeda, M.; Iwagaki, H.; Saito, S.; Fujiwara, T. Protective effect of eicosapentaenoic acid on insulin resistance in hyperlipidemic patients and on the postoperative course of cardiac surgery patients: The possible involvement of adiponectin. Acta Med. Okayama 2014, 68, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Skurnik, G.; Naour, N.; Pechtner, V.; Meugnier, E.; Rome, S.; Quignard-Boulangé, A.; Vidal, H.; Slama, G.; Clément, K.; et al. Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: A randomized controlled study. Am. J. Clin. Nutr. 2007, 86, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Abe, T.; Nako, K.; Okamura, M.; Senda, M.; Sakamoto, T.; Ito, S.; DIMS Study Group. Eicosapentaenoic acid improves glycemic control in elderly bedridden patients with type 2 diabetes. Tohoku J. Exp. Med. 2013, 231, 63–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dewell, A.; Marvasti, F.F.; Harris, W.S.; Tsao, P.; Gardner, C.D. Low- and high-dose plant and marine (n-3) fatty acids do not affect plasma inflammatory markers in adults with metabolic syndrome. J. Nutr. 2011, 141, 2166–2171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Wang, Y.; Shehzad, Q.; Su, Y.; Xu, L.; Yu, L.; Zeng, W.; Fang, Z.; Wu, G.; Wei, W.; et al. Does omega-3 PUFAs supplementation improve metabolic syndrome and related cardiovascular diseases? A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 64, 9455–9482. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.T.; Chan, D.C.; Barrett, P.H.; Adams, L.A.; Watts, G.F. Supplementation with n3 fatty acid ethyl esters increases large and small artery elasticity in obese adults on a weight loss diet. J. Nutr. 2013, 143, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Moro, T.; Bosco, G.; Bianco, A.; Grimaldi, K.A.; Camporesi, E.; Mangar, D. Effects of n-3 polyunsaturated fatty acids (ω-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar. Drugs 2015, 13, 996–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gunnarsdottir, I.; Tomasson, H.; Kiely, M.; Martinéz, J.A.; Bandarra, N.M.; Morais, M.G.; Thorsdottir, I. Inclusion of fish or fish oil in weight-loss diets for young adults: Effects on blood lipids. Int. J. Obes. 2008, 32, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Hlavatý, P.; Kunešová, M.; Gojová, M.; Tvrzická, E.; Vecka, M.; Roubal, P.; Hill, M.; Hlavatá, K.; Kalousková, P.; Hainer, V.; et al. Change in fatty acid composition of serum lipids in obese females after short-term weight-reducing regimen with the addition of n-3 long chain polyunsaturated fatty acids in comparison to controls. Physiol. Res. 2008, 57 (Suppl. 1), S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Neff, L.M.; Culiner, J.; Cunningham-Rundles, S.; Seidman, C.; Meehan, D.; Maturi, J.; Wittkowski, K.M.; Levine, B.; Breslow, J.L. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. J. Nutr. 2011, 141, 207–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, K.; Wang, B.; Zhou, R.; Lang, H.D.; Ran, L.; Wang, J.; Li, L.; Kang, C.; Zhu, X.H.; Zhang, Q.Y.; et al. Effect of combined use of a low-carbohydrate, high-protein diet with omega-3 polyunsaturated fatty acid supplementation on glycemic control in newly diagnosed type 2 diabetes: A randomized, double-blind, parallel-controlled trial. Am. J. Clin. Nutr. 2018, 108, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Félix-Soriano, E.; Martínez-Gayo, A.; Cobo, M.J.; Pérez-Chávez, A.; Ibáñez-Santos, J.; Palacios Samper, N.; Goikoetxea Galarza, I.; Cuervo, M.; García-Unciti, M.; González-Muniesa, P.; et al. Effects of DHA-Rich n-3 Fatty Acid Supplementation and/or Resistance Training on Body Composition and Cardiometabolic Biomarkers in Overweight and Obese Post-Menopausal Women. Nutrients 2021, 13, 2465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Logan, S.L.; Spriet, L.L. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS ONE 2015, 10, e0144828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mazaherioun, M.; Djalali, M.; Koohdani, F.; Javanbakht, M.H.; Zarei, M.; Beigy, M.; Ansari, S.; Rezvan, N.; Saedisomeolia, A. Beneficial Effects of n-3 Fatty Acids on Cardiometabolic and Inflammatory Markers in Type 2 Diabetes Mellitus: A Clinical Trial. Med. Princ. Pract. 2017, 26, 535–541. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olza, J.; Mesa, M.D.; Aguilera, C.M.; Moreno-Torres, R.; Jiménez, A.; Pérez de la Cruz, A.; Gil, A. Influence of an eicosapentaenoic and docosahexaenoic acid-enriched enteral nutrition formula on plasma fatty acid composition and biomarkers of insulin resistance in the elderly. Clin. Nutr. 2010, 29, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Cicero, A.F.; Fogari, E.; D’Angelo, A.; Bonaventura, A.; Romano, D.; Maffioli, P. Effects of n-3 PUFAs on postprandial variation of metalloproteinases, and inflammatory and insulin resistance parameters in dyslipidemic patients: Evaluation with euglycemic clamp and oral fat load. J. Clin. Lipidol. 2012, 6, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.N.; Lozovoy, M.A.; Dichi, I. Effect of soy product kinako and fish oil on serum lipids and glucose metabolism in women with metabolic syndrome. Nutrition 2014, 30, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Yu, J.H.; Sun, J.H.; Ma, W.Q.; Wang, J.J.; Sun, G.J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.J.; Gao, X.; Guo, X.F.; Li, K.L.; Li, S.; Sinclair, A.J.; Li, D. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin. Nutr. 2021, 40, 4538–4550. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Akinkuolie, A.O.; Ngwa, J.S.; Meigs, J.B.; Djoussé, L. Omega-3 polyunsaturated fatty acid and insulin sensitivity: A meta-analysis of randomized controlled trials. Clin. Nutr. 2011, 30, 702–707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Author, Year | Country | Type of Study | Sample Size | Age | Sex Distribution | Extra Information |
|---|---|---|---|---|---|---|
| DeFina et al., 2011 [2] | USA | Placebo-controlled, randomized clinical trial | 128 (64 participants in the control group; 64 participants in the treated group) | 30–60 | 40 males and 88 females (20 males and 44 females in each group) | The effects of omega-3 and exercise with a calorie-restricted diet on weight loss and body composition. All the participants received dietary and exercise counseling. |
| Itariu et al., 2012 [3] | Austria | Randomized, controlled clinical trial | 46 (23 participants in the control group; 23 participants in the treated group) | 20–65 | 46 females (23 females in each group) | The study mainly focuses on the effects of omega-3 on adipose tissue and systemic inflammation in obese non-diabetic patients. |
| Jacobo-Cejudo et al., 2017 [6] | Mexico | Randomized, single-blind, placebo-controlled pilot study | 65 (31 participants in the placebo group; 34 participants in the fish oil group) | 25–60 | 15 males and 50 females (5 males and 26 females in the placebo group; 10 males and 24 females in the fish oil group) | The study focuses on the effects of omega-3 on metabolic and inflammatory biomarkers in diabetic patients under treatment with oral medication. |
| Lalia et al., 2015 [7] | USA | Randomized, double-blind, placebo-controlled study | 25 (14 participants in the n-3 PUFAs group; 11 participants in the placebo group) | 30–40 | 13 males and 12 females (8 males and 6 females in the n-3 PUFAs group; 5 males and 6 females in the placebo group) | Effects of omega-3 fatty acids on insulin resistance in non-diabetic insulin-resistant participants. |
| Yamamoto et al., 2014 [8] | Japan | Clinical trial Phase II | 60 (31 participants in the EPA group; 29 participants in the placebo group) | 60–80 | 31 males and 29 females (18 males and 13 females in the EPA group; 13 males and 16 females in the placebo group) | EPA is one of the n-3 PUFAs and, in the study, the effects of EPA on insulin resistance in hyperglycemic patients have been assessed. |
| Kabir et al., 2007 [9] | France | Randomized, double-blind, parallel, placebo-controlled trial | 26 (12 participants in the fish oil group; 14 participants in the placebo group) | 40–60 | 26 females | Assessing whether n-3 PUFAs have further impacts on body fat, insulin sensitivity, adipose tissue function (including the production of adipokines and inflammatory and atherogenic factors), and gene expression in type 2 diabetes, as well as other components of metabolic syndrome. |
| Dewell et al., 2011 [11] | USA | Randomized, double-blind, placebo-controlled trial | 100 (20 participants in 5 groups: low-dose flaxseed oil (LFx) group; high-dose flaxseed oil (HFx) group; low-dose fish oil (LFO) group; high-dose fish oil (HFO) group; and placebo group) | 40–60 | 36 females, 64 males (13 males and 7 females in the low-dose flaxseed oil (LFx) group; 12 males and 8 females in the high-dose flaxseed oil (HFx) group; 16 males and 4 females in the low-dose fish oil (LFO) group; 11 males and 9 females in the high-dose fish oil (HFO) group; 12 males and 8 females in the placebo group) | The study compares the effects of plant-based and marine omega-3 on plasma inflammatory markers in adults suffering from metabolic syndrome; in addition, it evaluates the dose-dependent effects of each n-3 PUFA on components of MetS and inflammatory markers. |
| Ogawa et al., 2013 [10] | Japan | Multicenter, prospective, randomized controlled trial | 26 (13 participants in the EPA/DHA group; 13 participants in the control group) | 70–90 | 6 males and 20 females (4 males and 9 females in the EPA/DHA group; 2 males and 11 females in the control group) | In the study, the effects of omega-3 were evaluated on the glycemic control of elderly bedridden patients. |
| Wong et al., 2013 [13] | Australia | Randomized, single-blind intervention trial | 25 (12 participants in the weight loss group; 13 participants in the weight loss + omega-3 group) | 18–75 | 14 males, 11 females (the gender distribution in each group was not reported) | The study investigates how n3 fatty acid ethyl ester supplementation affects arterial elasticity in obese adults undergoing a weight loss diet. |
| Paoli et al., 2015 [14] | Italy | Randomized, controlled, parallel-arm clinical trial | 34 (18 participants in the Keto diet group; 16 participants in the Keto diet + omega-3 group) | 25–65 | 34 males | The population of the study was generally healthy (apart from being overweight), and at the end of the study, the effects of omega-3 on cardiovascular risk factors and metabolic factors were assessed. |
| Gunnarsdottir et al., 2008 [15] | Iceland, Spain, and Ireland | Randomized, controlled intervention trial | 262 (61 participants in the control group; 67 participants in the Cod diet group; 72 participants in the Salmon diet group; 62 participants in the fish oil diet group) | 20–40 | 138 males and 186 females (24 males and 36 females in the control group; 29 males and 38 females in the Cod diet group; 36 males and 36 females in the Salmon diet group; 23 males and 39 females in the fish oil group) | The study evaluates the impact of consuming fish (both lean and oily) and fish oil on blood lipid levels during weight loss. |
| Hlavatý et al., 2008 [16] | Czech Republic | Randomized controlled trial | 40 (20 participants in the low-calorie diet group and 20 participants in the low-calorie + omega-3 diet) | 40–70 | 40 females | Effects of low-calorie diet plus omega-3 in non-diabetic overweight women. |
| Neff et al., 2011 [17] | USA | Randomized, double-blind, placebo-controlled clinical trial | 36 (17 participants in the placebo group; 19 participants in the DHA group) | 18–65 | 15 males, 21 females (6 males and 13 females in the DHA group; 9 males and 8 females in the placebo group) | The main goal of the study was to investigate the effects of DHA on lipoprotein particle size distribution in overweight and obese participants. |
| Thota et al., 2019 [18] | Australia | Randomized, placebo-controlled, double-blind clinical trial | 64 (16 participants in the placebo group (PL); 15 participants in the curcumin group (CC); 17 participants in the fish oil group (FO); 16 participants in the curcumin + fish oil group (CC-FO)) | 30–70 | 26 males and 38 females (7 males and 9 females in the placebo group; 6 males and 9 females in the curcumin group; 7 males and 10 females in the fish oil group; 6 males and 10 females in the curcumin + fish oil group) | The clinical trial focuses on the effects of omega-3 and/or curcumin on insulin resistance and blood lipids in people with a high risk of type 2 diabetes. We used the data reported in the fish oil arm in this systematic review. |
| Liu et al., 2018 [19] | China | Randomized, double-blind, parallel-controlled trial | 122 (30 participants in the control group; 30 participants in the low-carb, high-protein diet group; 31 participants in the omega-3 group; 31 participants in the low-carb, high-protein diet +omega-3 group) | 40–60 | 61 males and 61 females (15 males and 15 females in the control group; 15 males and 15 females in the low-carb, high-protein diet group; 15 males, 16 females in the omega-3 group; 16 males and 15 females in the low-carb, high-protein diet + omega-3 group) | The effects of a low-carb, high-protein diet with or without omega-3 on metabolic markers and glycemic control were studied in patients newly diagnosed with type 2 diabetes. |
| Félix-Soriano et al., 2021 [20] | Spain | Randomized double-blind, placebo-controlled trial | 71 (20 participants in the placebo (PL) group; 15 participants in the DHA-rich n-3 PUFA (n-3) group; 20 participants in the placebo + resistance training (PL + RT) group; 16 participants in the DHA-rich n-3 PUFA + resistance training (n-3 + RT) group) | 55–70 | 71 females | The combination of the resistance training with and without omega-3 was studied. The main goal was to investigate the effects of omega-3 on body composition and cardiometabolic factors in overweight and obese post-menopausal women. |
| Logan et al., 2015 [21] | Canada | Randomized, single-blind, controlled trial (RCT) | 24 (12 participants in the fish oil (FO) group; 12 participants in the placebo (PL) group) | 60–76 | 24 females | Effects of omega-3 on metabolism rate and metabolic markers in older females. |
| Mazaherioun et al., 2017 [22] | Iran | Randomized, double-blind, placebo-controlled clinical trial | 85 (41 participants in the placebo group; 44 participants in the n-3 PUFAs group) | 30–65 | 53 males, 32 females (29 males and 15 females in the n-3 PUFA group; 24 males and 17 females in the placebo group) | The effects of omega-3 on cardiometabolic and inflammatory markers in diabetic patients were assessed. |
| Olza et al., 2010 [23] | Spain | Randomized, experimental, prospective, intention-to-treat comparative study | 65 (33 participants in the control group; 32 participants in the treatment group) | 65 < age | 52 females and 13 males (25 females and 7 males in the T-Diet Plus (treatment) group 27 females and 6 males in the Jevity (control) group) | n-3 polyunsaturated fatty acids and cardiovascular outcomes in the elderly were studied. All the participants needed enteral nutrition. |
| Derosa et al., 2012 [24] | Italy | Randomized, double-blind, controlled clinical trial | 167 (83 participants in the control group; 84 participants in the n-3 PUFAs group) | 18–75 | 82 males and 85 females (41 males and 42 females in the control group; 41 males and 43 females in the n-3 PUFA group) | Effects of omega-3 on inflammatory markers and insulin resistance in dyslipidemic patients were studied. |
| Simão et al., 2014 [25] | Brazil | Randomized, parallel-design, controlled clinical trial | 65 (15 participants in the control group; 15 participants in the Kinako group; 19 participants in the fish oil group; 16 participants in the Kinako + fish oil group) | 35–60 | 65 females | The effects of fish oil as a main source of omega-3 with and without Kinako (Soy) on serum lipids and glucose in women with metabolic syndrome were assessed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basirat, A.; Merino-Torres, J.F. Marine-Based Omega-3 Fatty Acids and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2025, 17, 3279. https://doi.org/10.3390/nu17203279
Basirat A, Merino-Torres JF. Marine-Based Omega-3 Fatty Acids and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2025; 17(20):3279. https://doi.org/10.3390/nu17203279
Chicago/Turabian StyleBasirat, Arghavan, and Juan Francisco Merino-Torres. 2025. "Marine-Based Omega-3 Fatty Acids and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 17, no. 20: 3279. https://doi.org/10.3390/nu17203279
APA StyleBasirat, A., & Merino-Torres, J. F. (2025). Marine-Based Omega-3 Fatty Acids and Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 17(20), 3279. https://doi.org/10.3390/nu17203279






