Protaetia brevitarsis seulensis Larvae Extract Attenuates Inflammatory Osteoclast Differentiation and Bone Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PBE
2.2. Cytokines and Antibodies
2.3. Osteoclast Differentiation Assays
2.4. Western Blot Analysis
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Animal Study
2.7. μ-CT Analysis
2.8. ELISA
2.9. Statistical Analysis
3. Results
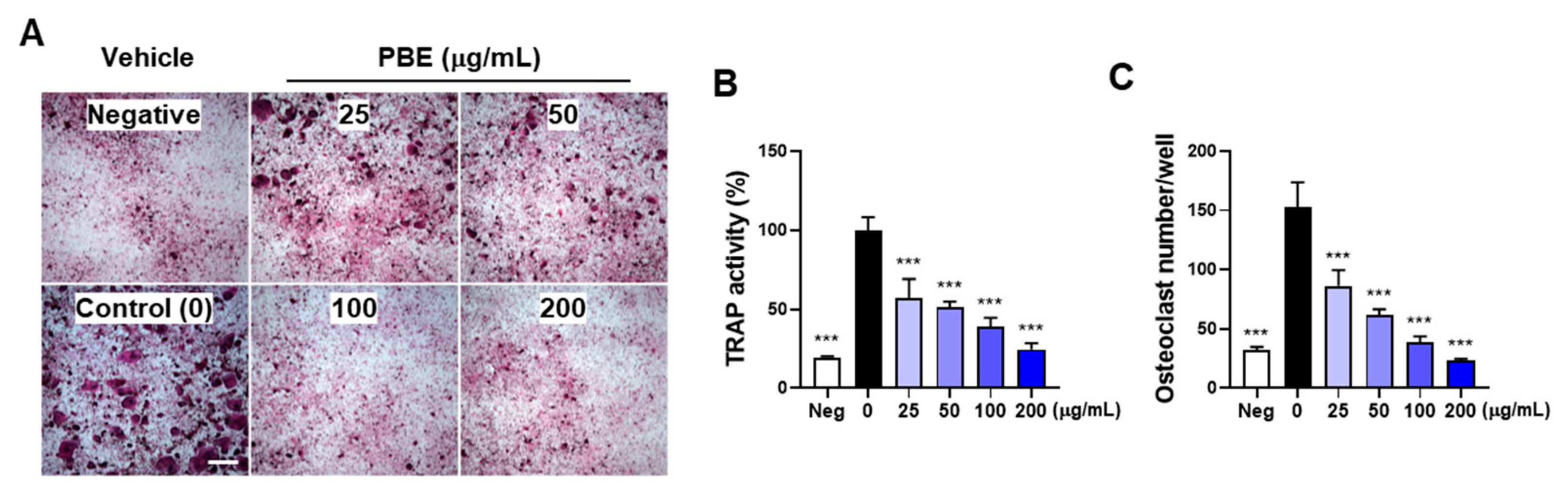
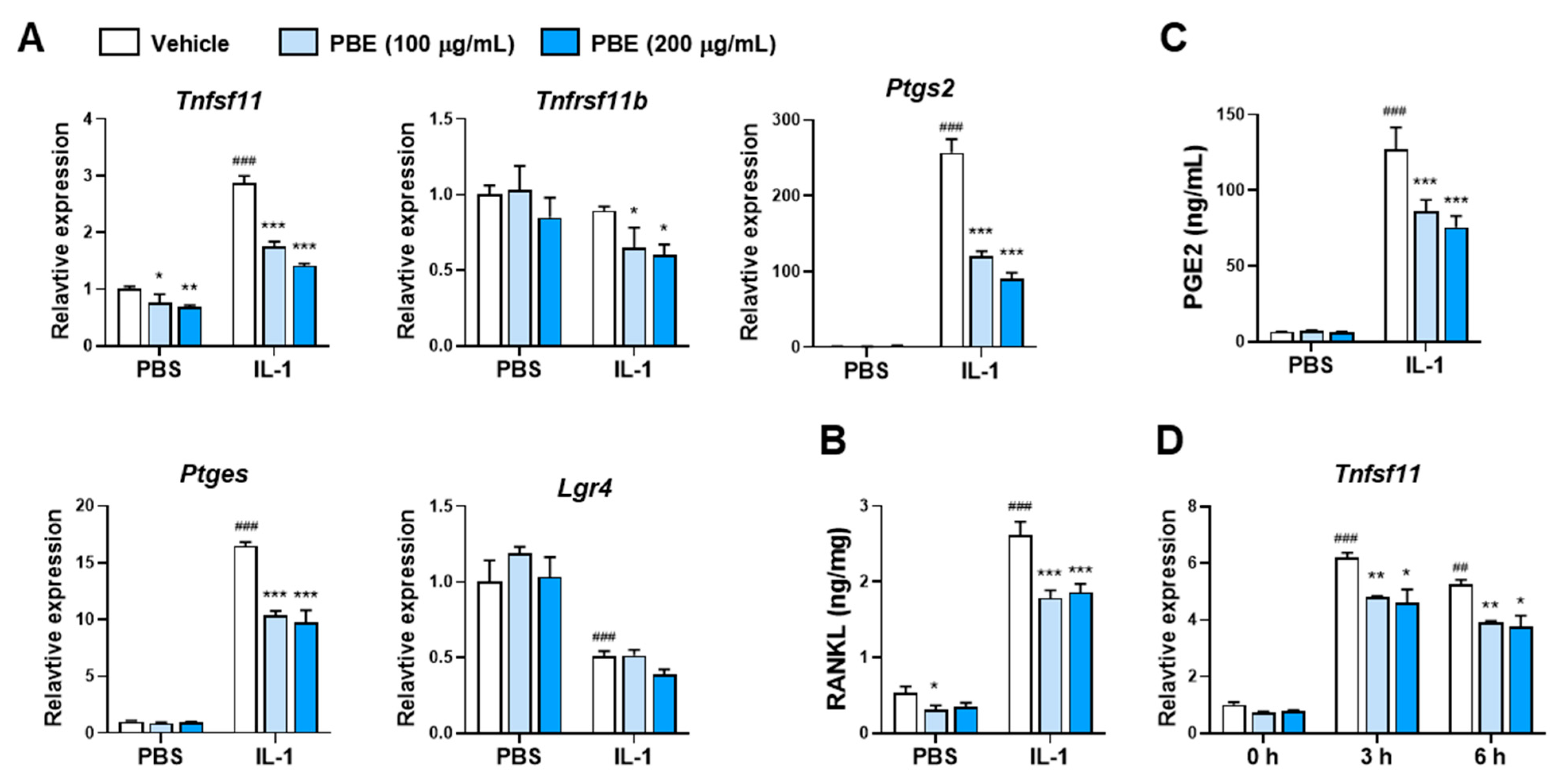
3.1. PBE Inhibits IL-1–Induced Osteoclast Differentiation by Suppressing RANKL Expression
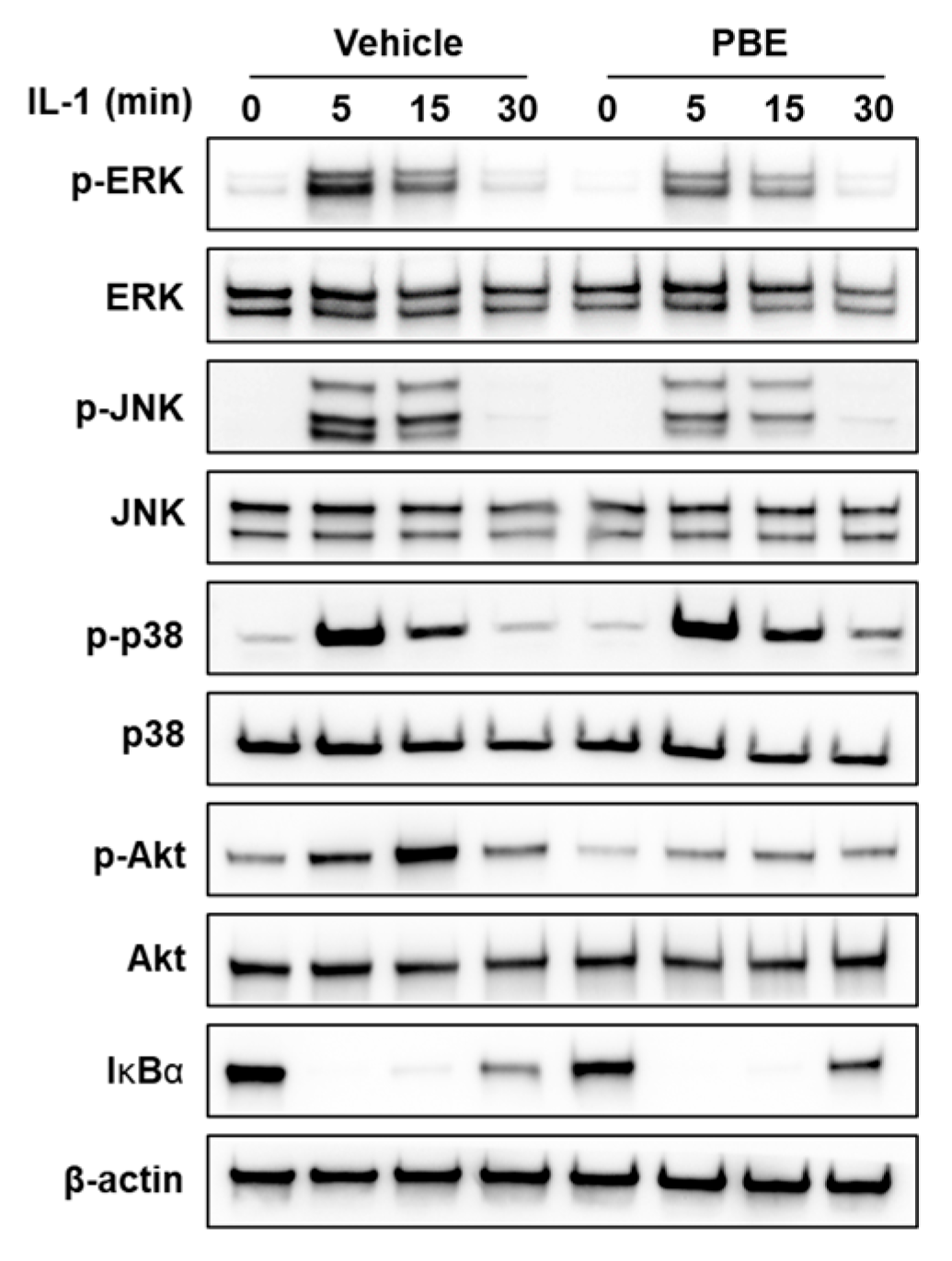
3.2. PBE Attenuates Early IL-1 Signaling in Osteocytic Cells
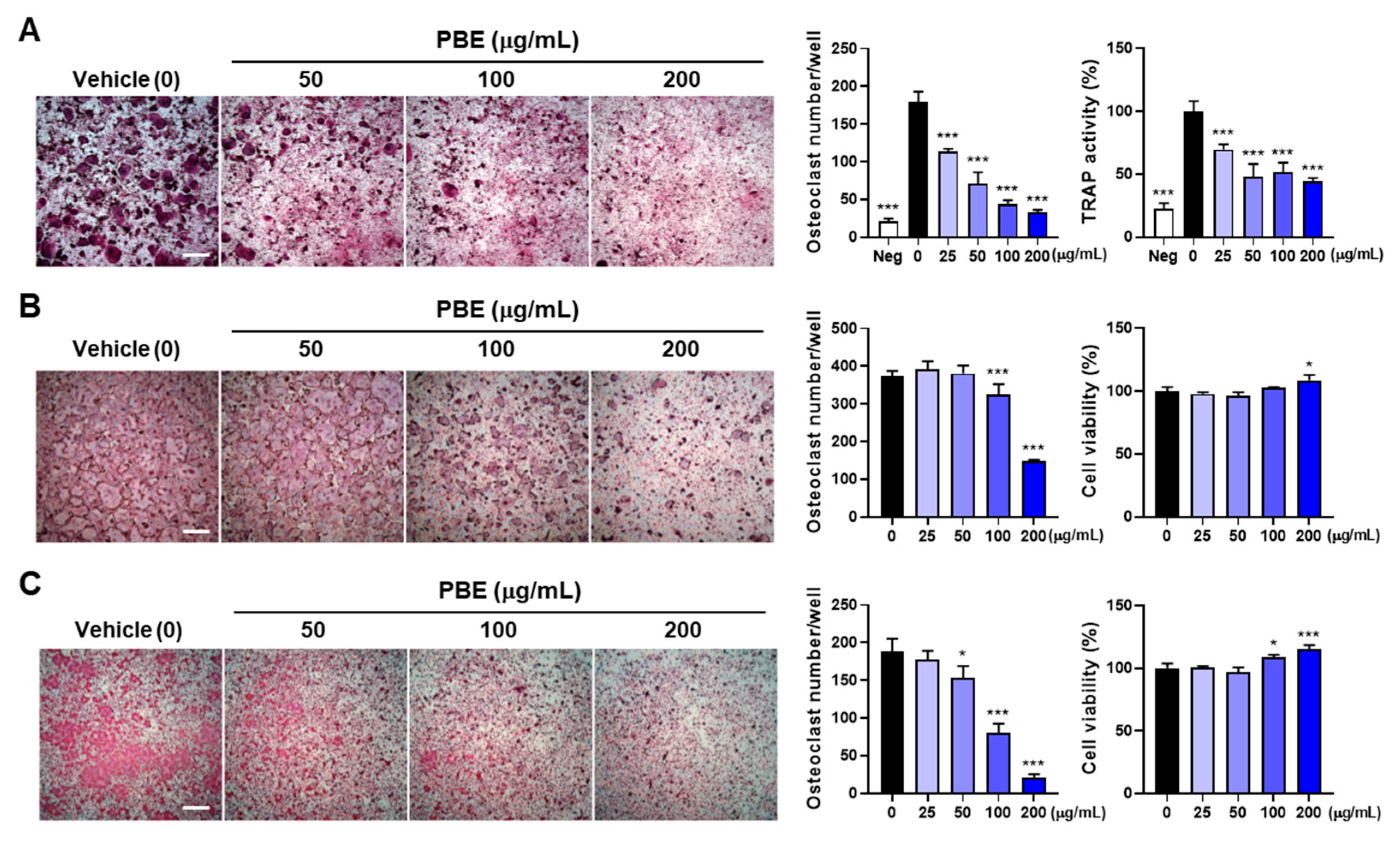
3.3. PBE Directly Suppresses Osteoclast Precursor Differentiation
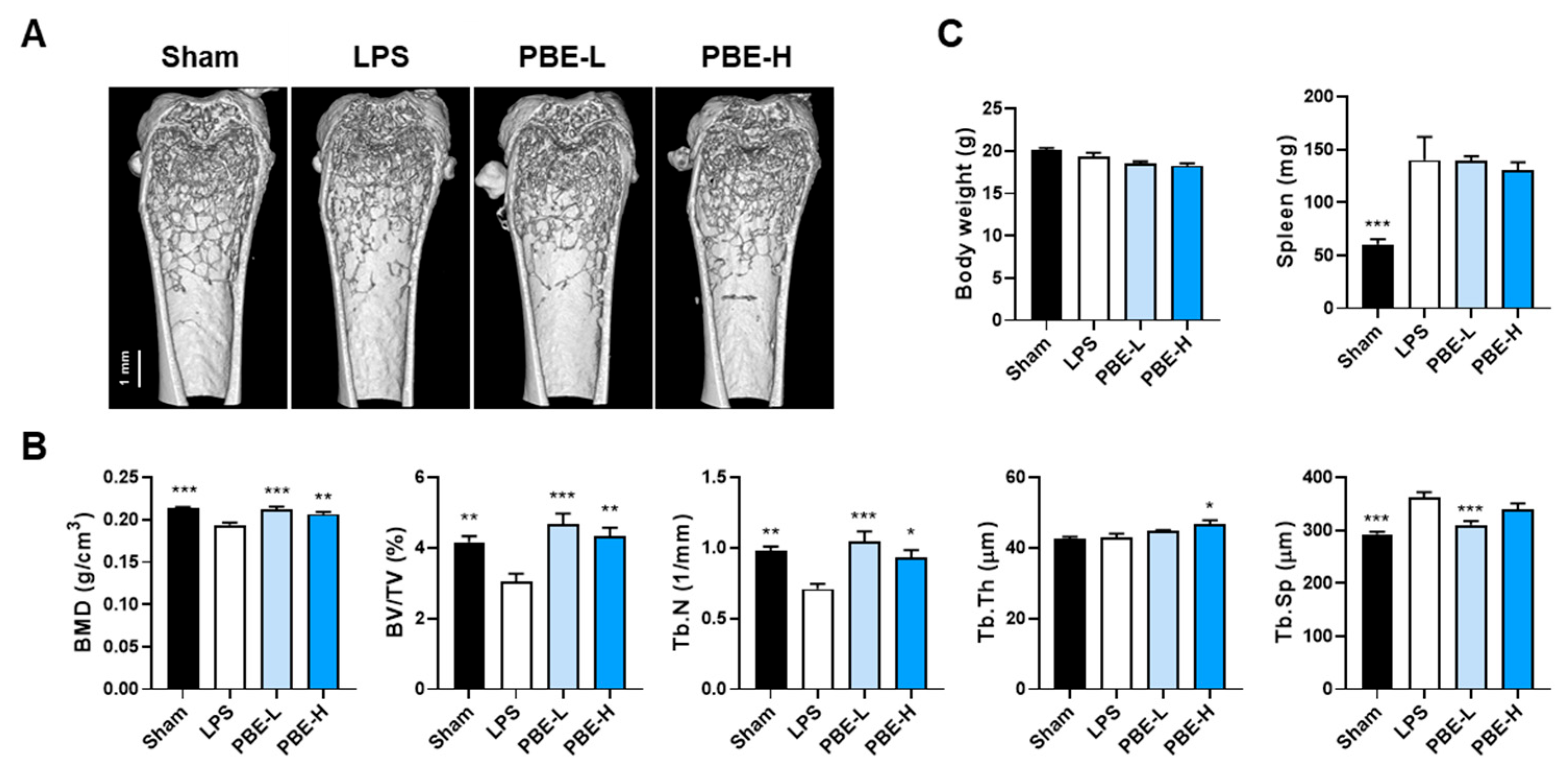
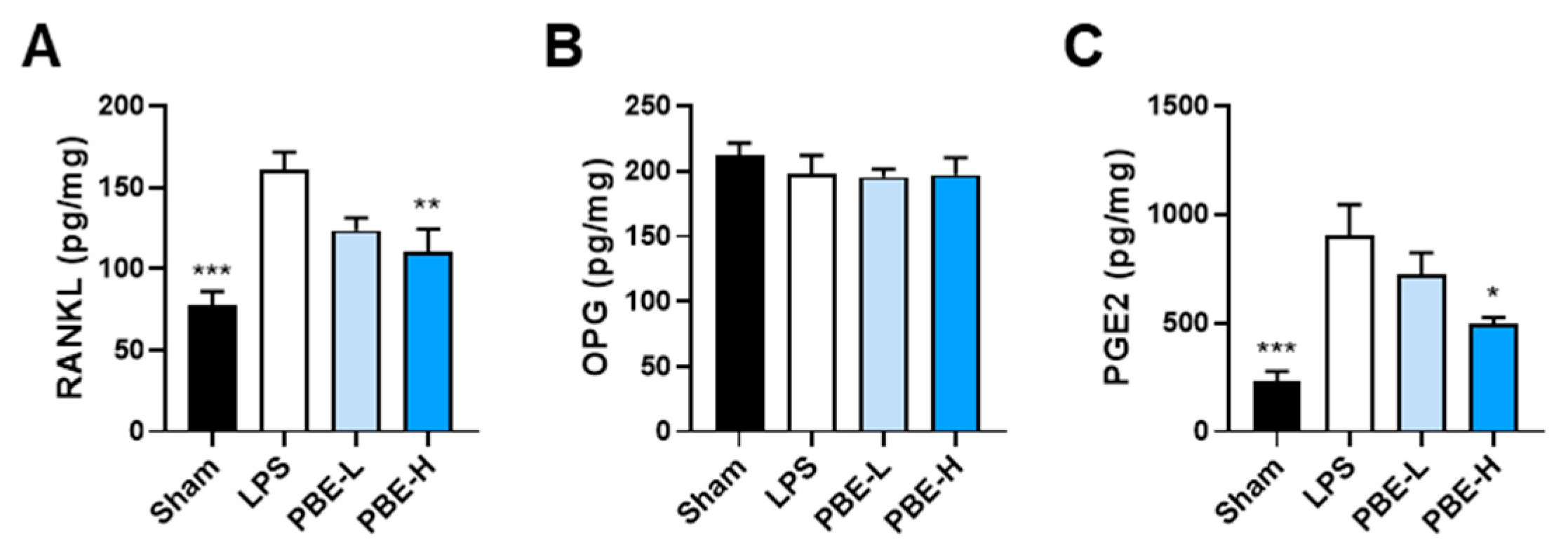
3.4. PBE Mitigates LPS-Induced Bone Loss In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| M-CSF | macrophage colony-stimulating factor |
| RANKL | receptor activator of nuclear factor-κB ligand |
| NF-κB | Nuclear factor kappa B |
| OPG | Osteoprotegerin |
| Lgr4 | Leucine-rich repeat-containing G protein-coupled receptor 4 |
| TNF-α | tumor necrosis factor-α |
| IL-1 | Interleukin-1 |
| LPS | Lipopolysaccharide |
| PGE2 | Prostaglandin E2 |
| PB | Protaetia brevitarsis seulensis |
| PBE | 70% ethanol extract of PB larvae |
| BMM | Bone marrow-derived macrophages |
| TGF-β1 | transforming growth factor-β1 |
| DMSO | Dimethyl sulfoxide |
| TRAP | Tartrate-resistant acid phosphatase |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| DW | Distilled water |
| μ-CT | Micro-computed tomography |
| ELISA | Enzyme-linked immunosorbent assay |
| COX-2 | Cyclooxygenase-2 |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| MAPK | Mitogen-activated protein kinase |
| BMD | Bone mineral density |
| BV/TV | Bone volume per tissue volume |
| Tb.N | Trabecular number |
| Tb.Sp | Trabecular separation |
| Tb.Th | Trabecular thickness |
| cPLA2 | Cytosolic phospholipase A2 |
References
- Tanaka, S.; Nakamura, K.; Takahasi, N.; Suda, T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol. Rev. 2005, 208, 30–49. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004, 15, 457–475. [Google Scholar] [CrossRef]
- Meng, X.; Lin, Z.; Cao, S.; Janowska, I.; Sonomoto, K.; Andreev, D.; Katharina, K.; Wen, J.; Knaup, K.X.; Wiesener, M.S.; et al. Estrogen-mediated downregulation of HIF-1α signaling in B lymphocytes influences postmenopausal bone loss. Bone Res. 2022, 10, 15. [Google Scholar] [CrossRef]
- Yu, W.; Zhong, L.; Yao, L.; Wei, Y.; Gui, T.; Li, Z.; Kim, H.; Holdreith, N.; Jiang, X.; Tong, W.; et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J. Clin. Investig. 2021, 131, e140214. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not Osteoblasts or Lining Cells, are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, J.H.; Kim, H.N.; Kim, H.M.; Kwak, H.B.; Lee, S.; Kim, H.H.; Lee, Z.H. alpha-Lipoic acid inhibits inflammatory bone resorption by suppressing prostaglandin E2 synthesis. J. Immunol. 2006, 176, 111–117. [Google Scholar] [CrossRef]
- Marahleh, A.; Kitaura, H.; Ohori, F.; Kishikawa, A.; Ogawa, S.; Shen, W.R.; Qi, J.; Noguchi, T.; Nara, Y.; Mizoguchi, I. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Front. Immunol. 2019, 10, 2925. [Google Scholar] [CrossRef]
- Ma, T.; Miyanishi, K.; Suen, A.; Epstein, N.J.; Tomita, T.; Smith, R.L.; Goodman, S.B. Human interleukin-1-induced murine osteoclastogenesis is dependent on RANKL, but independent of TNF-α. Cytokine 2004, 26, 138–144. [Google Scholar] [CrossRef]
- Lam, J.; Takeshita, S.; Barker, J.E.; Kanagawa, O.; Ross, F.P.; Teitelbaum, S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 2000, 106, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, R.; Kimble, R.B.; Vannice, J.L.; Kung, V.T.; Pacifici, R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Investig. 1994, 94, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Assuma, R.; Oates, T.; Cochran, D.; Amar, S.; Graves, D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998, 160, 403–409. [Google Scholar] [CrossRef]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef]
- Polzer, K.; Joosten, L.; Gasser, J.; Distler, J.H.; Ruiz, G.; Baum, W.; Redlich, K.; Bobacz, K.; Smolen, J.S.; van den Berg, W.; et al. Interleukin-1 is essential for systemic inflammatory bone loss. Ann. Rheum. Dis. 2010, 69, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Bott, K.N.; Feldman, E.; de Souza, R.J.; Comelli, E.M.; Klentrou, P.; Peters, S.J.; Ward, W.E. Lipopolysaccharide-Induced Bone Loss in Rodent Models: A Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2022, 38, 198–213. [Google Scholar] [CrossRef]
- Ochi, H.; Hara, Y.; Tagawa, M.; Shinomiya, K.; Asou, Y. The roles of TNFR1 in lipopolysaccharide-induced bone loss: Dual effects of TNFR1 on bone metabolism via osteoclastogenesis and osteoblast survival. J. Orthop. Res. 2010, 28, 657–663. [Google Scholar] [CrossRef]
- Inada, M.; Matsumoto, C.; Uematsu, S.; Akira, S.; Miyaura, C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J. Immunol. 2006, 177, 1879–1885. [Google Scholar] [CrossRef]
- Miyaura, C.; Inada, M.; Matsumoto, C.; Ohshiba, T.; Uozumi, N.; Shimizu, T.; Ito, A. An essential role of cytosolic phospholipase A2alpha in prostaglandin E2-mediated bone resorption associated with inflammation. J. Exp. Med. 2003, 197, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Cruz-Monterrosa, R.G.; Liceaga, A.M. Beyond Human Nutrition of Edible Insects: Health Benefits and Safety Aspects. Insects 2022, 13, 1007. [Google Scholar] [CrossRef]
- Yoon, C.H.; Jeon, S.H.; Ha, Y.J.; Kim, S.W.; Bang, W.Y.; Bang, K.H.; Gal, S.W.; Kim, I.S.; Cho, Y.S. Functional Chemical Components in Protaetia brevitarsis Larvae: Impact of Supplementary Feeds. Food Sci. Anim. Resour. 2020, 40, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.K.; Kim, S.W.; Song, D.H.; Kim, H.W.; Kim, I.S. Nutritional Composition of White-Spotted Flower Chafer (Protaetia brevitarsis) Larvae Produced from Commercial Insect Farms in Korea. Food Sci. Anim. Resour. 2021, 41, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Weaver, C.M.; Choi, M.-K. Proximate composition and mineral content of five edible insects consumed in Korea. CyTA—J. Food 2017, 15, 143–146. [Google Scholar] [CrossRef]
- Lee, S.; Seo, Y.H.; Song, J.H.; Kim, W.J.; Lee, J.H.; Moon, B.C.; Ang, M.J.; Kim, S.H.; Moon, C.; Lee, J.; et al. Neuroprotective Effect of Protaetia brevitarsis seulensis’ Water Extract on Trimethyltin-Induced Seizures and Hippocampal Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 679. [Google Scholar] [CrossRef]
- Yang, H.J.; Li, W. Isolation and Quantification of L-Tryptophan from Protaetia brevitarsis seulensis Larvae as a Marker for the Quality Control of an Edible Insect Extract. Insects 2025, 16, 905. [Google Scholar] [CrossRef]
- Park, Y.M.; Noh, E.M.; Lee, H.Y.; Shin, D.Y.; Lee, Y.H.; Kang, Y.G.; Na, E.J.; Kim, J.H.; Yang, H.J.; Kim, M.J.; et al. Anti-diabetic effects of Protaetia brevitarsis in pancreatic islets and a murine diabetic model. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7508–7515. [Google Scholar] [CrossRef]
- Chun, J.M.; Park, J.H.; Moon, B.C.; Baek, S.J. Transcriptomic insights into the anti-inflammatory mechanisms of Protaetia brevitarsis seulensis larvae in IL-1β-driven chondrosarcoma cells. Biomed. Pharmacother. 2025, 183, 117866. [Google Scholar] [CrossRef]
- Nam, H.H.; Kang, S.; Seo, Y.S.; Lee, J.; Moon, B.C.; Lee, H.J.; Lee, J.H.; Kim, B.; Lee, S.; Kim, J.S. Protective effects of an aqueous extract of Protaetia brevitarsis seulensis larvae against radiation-induced testicular injury in mice. Food Sci. Nutr. 2022, 10, 3969–3978. [Google Scholar] [CrossRef]
- Choi, R.Y.; Kim, I.W.; Ji, M.; Paik, M.J.; Ban, E.J.; Lee, J.H.; Hwang, J.S.; Kweon, H.; Seo, M. Protaetia brevitarsis seulensis larvae ethanol extract inhibits RANKL-stimulated osteoclastogenesis and ameliorates bone loss in ovariectomized mice. Biomed. Pharmacother. 2023, 165, 115112. [Google Scholar] [CrossRef]
- Park, W.J.; Oh, E.; Kim, Y. Protaetia brevitarsis larvae extract protects against lipopolysaccharides-induced ferroptosis and inflammation by inhibiting acid sphingomyelinase. Nutr. Res. Pract. 2024, 18, 602–616. [Google Scholar] [CrossRef]
- Gu, D.R.; Yang, H.; Kim, S.C.; Hwang, Y.H.; Ha, H. Water Extract of Angelica dahurica Inhibits Osteoclast Differentiation and Bone Loss. Int. J. Mol. Sci. 2023, 24, 14715. [Google Scholar] [CrossRef]
- Gu, D.R.; Yang, H.; Kim, S.C.; Hwang, Y.H.; Ha, H. Water Extract of Piper longum Linn Ameliorates Ovariectomy-Induced Bone Loss by Inhibiting Osteoclast Differentiation. Nutrients 2022, 14, 3667. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.K.; Harris, S.; Ahuja, S.S.; Bonewald, L.F. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J. Bone Miner. Res. 2002, 17, 2068–2079. [Google Scholar] [CrossRef]
- Sato, N.; Takahashi, N.; Suda, K.; Nakamura, M.; Yamaki, M.; Ninomiya, T.; Kobayashi, Y.; Takada, H.; Shibata, K.; Yamamoto, M.; et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1alpha. J. Exp. Med. 2004, 200, 601–611. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.N.; Yang, D.; Jung, K.; Kim, H.M.; Kim, H.H.; Ha, H.; Lee, Z.H. Trolox prevents osteoclastogenesis by suppressing RANKL expression and signaling. J. Biol. Chem. 2009, 284, 13725–13734. [Google Scholar] [CrossRef]
- Kim, N.; Kadono, Y.; Takami, M.; Lee, J.; Lee, S.H.; Okada, F.; Kim, J.H.; Kobayashi, T.; Odgren, P.R.; Nakano, H.; et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J. Exp. Med. 2005, 202, 589–595. [Google Scholar] [CrossRef]
- Park, H.-J.; Baek, K.; Baek, J.-H.; Kim, H.-R. TNFα Increases RANKL Expression via PGE2-Induced Activation of NFATc1. Int. J. Mol. Sci. 2017, 18, 495. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, Y.; Tanaka, K.; Suda, M.; Komatsu, Y.; Yasoda, A.; Miura, M.; Ozasa, A.; Narumiya, S.; Sugimoto, Y.; Ichikawa, A.; et al. Impaired Bone Resorption by Lipopolysaccharide In Vivo in Mice Deficient in the Prostaglandin E Receptor EP4 Subtype. Infect. Immun. 2000, 68, 6819–6825. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Wasiliew, P.; Kracht, M. Interleukin-1 (IL-1) Pathway. Sci. Signal. 2010, 3, cm1. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Zhou, Y.M.; Xu, J.Z.; Sun, L.H.; Tao, B.; Wang, W.Q.; Wang, J.Q.; Zhao, H.Y.; Liu, J.M. Lgr4 promotes aerobic glycolysis and differentiation in osteoblasts via the canonical Wnt/β-catenin pathway. J. Bone Min. Res. 2021, 36, 1605–1620. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Jin, Y.; Zhang, S.; Ding, Y.; Huo, K.; Yang, J.; Zhao, L.; Nian, B.; Zhong, T.P.; Lu, W.; et al. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 2022, 10, 27. [Google Scholar] [CrossRef]
- Chen, C.; Cao, Z.; Lei, H.; Zhang, C.; Wu, M.; Huang, S.; Li, X.; Xie, D.; Liu, M.; Zhang, L.; et al. Microbial Tryptophan Metabolites Ameliorate Ovariectomy-Induced Bone Loss by Repairing Intestinal AhR-Mediated Gut-Bone Signaling Pathway. Adv. Sci. 2024, 11, e2404545. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Gu, D.R.; Yang, H.J.; Li, W.; Go, Y.; Choi, R.-Y.; Kim, I.-W.; Ha, H. Protaetia brevitarsis seulensis Larvae Extract Attenuates Inflammatory Osteoclast Differentiation and Bone Loss. Nutrients 2025, 17, 3273. https://doi.org/10.3390/nu17203273
Yang H, Gu DR, Yang HJ, Li W, Go Y, Choi R-Y, Kim I-W, Ha H. Protaetia brevitarsis seulensis Larvae Extract Attenuates Inflammatory Osteoclast Differentiation and Bone Loss. Nutrients. 2025; 17(20):3273. https://doi.org/10.3390/nu17203273
Chicago/Turabian StyleYang, Hyun, Dong Ryun Gu, Hye Jin Yang, Wei Li, Younghoon Go, Ra-Yeong Choi, In-Woo Kim, and Hyunil Ha. 2025. "Protaetia brevitarsis seulensis Larvae Extract Attenuates Inflammatory Osteoclast Differentiation and Bone Loss" Nutrients 17, no. 20: 3273. https://doi.org/10.3390/nu17203273
APA StyleYang, H., Gu, D. R., Yang, H. J., Li, W., Go, Y., Choi, R.-Y., Kim, I.-W., & Ha, H. (2025). Protaetia brevitarsis seulensis Larvae Extract Attenuates Inflammatory Osteoclast Differentiation and Bone Loss. Nutrients, 17(20), 3273. https://doi.org/10.3390/nu17203273





