Ultra-Processed Foods and Respiratory and Allergic Diseases in Childhood: Epidemiological Evidence and Mechanistic Insights
Abstract
1. Introduction
2. Ultra-Processed Diets and the Rise of Food Allergies
Ultra-Processed Foods and Eosinophilic Esophagitis
3. UPF Consumption and Respiratory Diseases
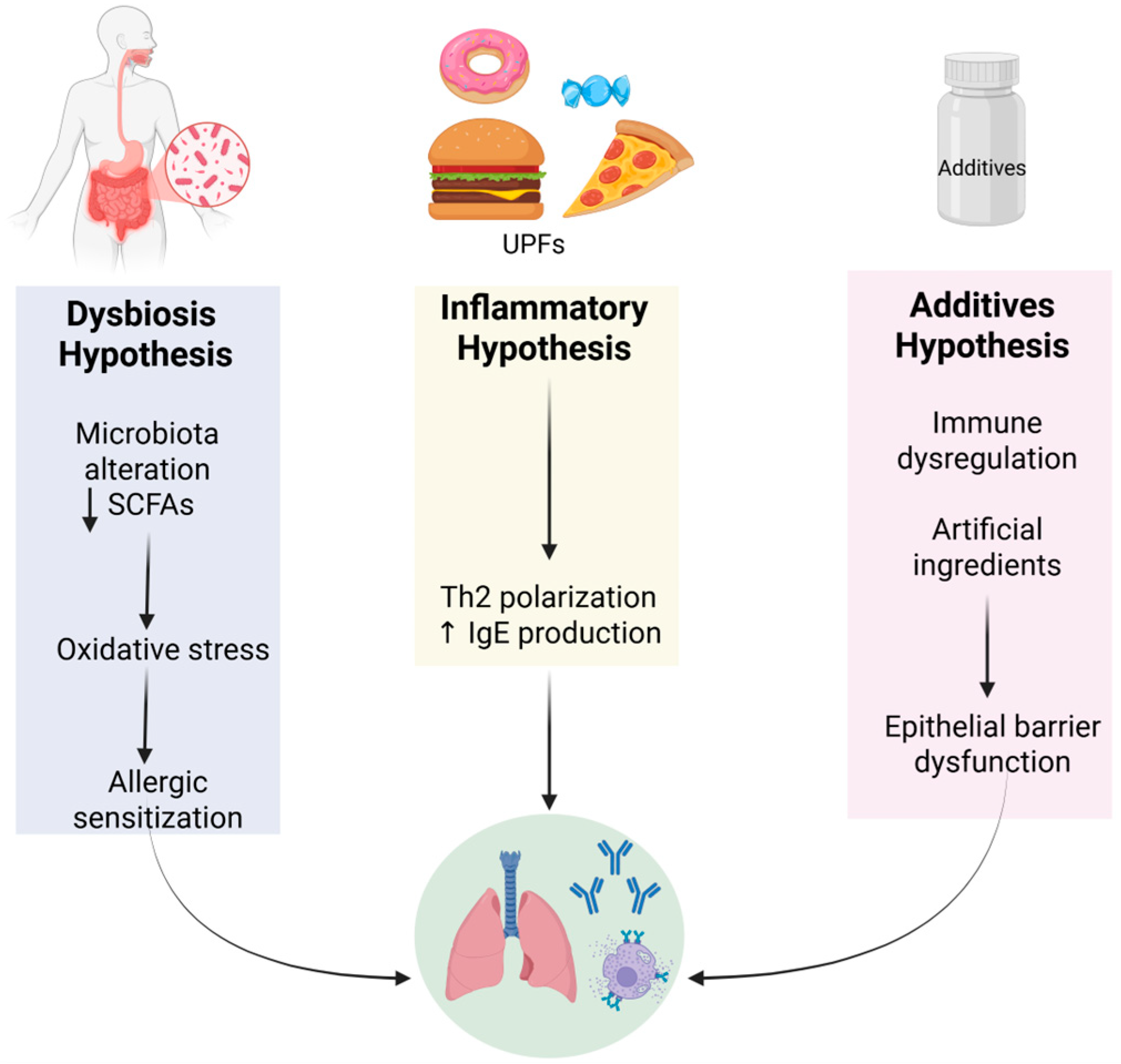
4. Pathophysiological Mechanisms
4.1. Alterations in the Gut Microbiota and Barrier Function
4.2. Role of Advanced Glycation End Products (AGEs)
4.3. Food Additives and Obesity-Driven Epigenetics
4.4. Malnutrition and Mechanistic Interplay
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, S.; Wellens, J.; Yang, N.; Li, D.; Wang, J.; Wang, L.; Yuan, S.; He, Y.; Song, P.; Munger, R.; et al. Ultra-Processed Foods and Human Health: An Umbrella Review and Updated Meta-Analyses of Observational Evidence. Clin. Nutr. 2024, 43, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, M.; Diel, P.; Eisenbrand, G.; Grune, T.; Guth, S.; Henle, T.; Humpf, H.U.; Joost, H.G.; Marko, D.; Raupbach, J.; et al. Dietary Glycation Compounds—Implications for Human Health. Crit. Rev. Toxicol. 2024, 54, 485–617. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Mousavi, S.; Mandhane, P.J.; Simons, E.; Turvey, S.E.; Moraes, T.J.; Subbarao, P.; Miliku, K. Ultraprocessed Food Consumption and Obesity Development in Canadian Children. JAMA Netw. Open 2025, 8, e2457341. [Google Scholar] [CrossRef] [PubMed]
- Dicken, S.J.; Batterham, R.L. Ultra-Processed Food and Obesity: What Is the Evidence? Curr. Nutr. Rep. 2024, 13, 23–38. [Google Scholar] [CrossRef]
- Khoury, N.; Martínez, M.Á.; Garcidueñas-Fimbres, T.E.; Pastor-Villaescusa, B.; Leis, R.; De Las Heras-Delgado, S.; Miguel-Berges, M.L.; Navas-Carretero, S.; Portoles, O.; Pérez-Vega, K.A.; et al. Ultraprocessed Food Consumption and Cardiometabolic Risk Factors in Children. JAMA Netw. Open 2024, 7, E2411852. [Google Scholar] [CrossRef]
- Puig-Vallverdú, J.; Romaguera, D.; Fernández-Barrés, S.; Gignac, F.; Ibarluzea, J.; Santa-Maria, L.; Llop, S.; Gonzalez, S.; Vioque, J.; Riaño-Galán, I.; et al. The Association between Maternal Ultra-Processed Food Consumption during Pregnancy and Child Neuropsychological Development: A Population-Based Birth Cohort Study. Clin. Nutr. 2022, 41, 2275–2283. [Google Scholar] [CrossRef]
- Kotchetkoff, E.C.d.A.; Suano-Souza, F.I.; Neri Gama de Almeida, D.; Barreto, T.L.N.; Mendonça, R.B.; Sarni, R.O.S. Ultra-Processed Food Intake and Food Allergy in Children and Adolescents. Int. J. Food Sci. Nutr. 2024, 75, 317–324. [Google Scholar] [CrossRef]
- Berni Canani, R.; Carucci, L.; Coppola, S.; D’Auria, E.; O’Mahony, L.; Roth-Walter, F.; Vassilopolou, E.; Agostoni, C.; Agache, I.; Akdis, C.; et al. Ultra-Processed Foods, Allergy Outcomes and Underlying Mechanisms in Children: An EAACI Task Force Report. Pediatr. Allergy Immunol. 2024, 35, e14231. [Google Scholar] [CrossRef]
- Paparo, L.; Coppola, S.; Nocerino, R.; Pisapia, L.; Picariello, G.; Cortese, M.; Voto, L.; Maglio, M.; Miele, E.; Carucci, L.; et al. How Dietary Advanced Glycation End Products Could Facilitate the Occurrence of Food Allergy. J. Allergy Clin. Immunol. 2024, 153, 742–758. [Google Scholar] [CrossRef]
- Moreno-Galarraga, L.; Martín-Álvarez, I.; Fernández-Montero, A.; Santos Rocha, B.; Ciriza Barea, E.; Martín-Calvo, N. Consumption of Ultra-Processed Products and Wheezing Respiratory Diseases in Children: The SENDO Project. An. Pediatría (Engl. Ed.) 2021, 95, 18–25. [Google Scholar] [CrossRef]
- Chatzi, L.; Kogevinas, M. Prenatal and Childhood Mediterranean Diet and the Development of Asthma and Allergies in Children. Public Health Nutr. 2009, 12, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Antonogeorgos, G.; Kogias, C.; Douros, K.; Panagiotakos, D. Greater Fruit and Vegetables Consumption, and Adherence to a Mediterranean Type of Diet Reduces the Risk for Asthma in Children; a Systematic Review and Meta-Analysis. Int. J. Food Sci. Nutr. 2024, 75, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G. Do Fast Foods Cause Asthma, Rhinoconjunctivitis and Eczema? Global Findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.; Rezende, L.; Machado, P.; Gouveia, N.; Levy, R. Associations of Ultra-Processed Food and Drink Products with Asthma and Wheezing among Brazilian Adolescents. Pediatr. Allergy Immunol. 2018, 29, 504–511. [Google Scholar] [CrossRef]
- Sampath, V.; Abrams, E.M.; Adlou, B.; Akdis, C.; Akdis, M.; Brough, H.A.; Chan, S.; Chatchatee, P.; Chinthrajah, R.S.; Cocco, R.R.; et al. Food Allergy across the Globe. J. Allergy Clin. Immunol. 2021, 148, 1347–1364. [Google Scholar] [CrossRef]
- Mescoloto, S.B.; Pongiluppi, G.; Domene, S.M.Á. Ultra-Processed Food Consumption and Children and Adolescents’ Health. J. Pediatr. (Rio. J). 2024, 100 (Suppl. S1), S18–S30. [Google Scholar] [CrossRef]
- dos Santos Costa, C.; de Faria, F.R.; Gabe, K.T.; Sattamini, I.F.; Khandpur, N.; Leite, F.H.M.; Steele, E.M.; da Costa Louzada, M.L.; Levy, R.B.; Monteiro, C.A. Nova Score for the Consumption of Ultra-Processed Foods: Description and Performance Evaluation in Brazil. Rev. Saude Publica 2021, 55, 13. [Google Scholar] [CrossRef]
- Wright, L.S.; Rifas-Shiman, S.L.; Oken, E.; Litonjua, A.A.; Gold, D.R. Prenatal and Early Life Fructose, Fructose-Containing Beverages, and Midchildhood Asthma. Ann. Am. Thorac. Soc. 2018, 15, 217–224. [Google Scholar] [CrossRef]
- Maslova, E.; Strøm, M.; Olsen, S.F.; Halldorsson, T.I. Consumption of Artificially-Sweetened Soft Drinks in Pregnancy and Risk of Child Asthma and Allergic Rhinitis. PLoS ONE 2013, 8, e57261. [Google Scholar] [CrossRef]
- Nascimento, J.X.P.T.; Ribeiro, C.C.C.; Batista, R.F.L.; De Britto Alves, M.T.S.S.; Simões, V.M.F.; Padilha, L.L.; Cardoso, V.C.; Vianna, E.O.; Bettiol, H.; Barbieri, M.A.; et al. The First 1000 Days of Life Factors Associated with “Childhood Asthma Symptoms”: Brisa Cohort, Brazil. Sci. Rep. 2017, 7, 16028. [Google Scholar] [CrossRef]
- Bédard, A.; Northstone, K.; Henderson, A.J.; Shaheen, S.O. Maternal Intake of Sugar during Pregnancy and Childhood Respiratory and Atopic Outcomes. Eur. Respir. J. 2017, 50, 1700073. [Google Scholar] [CrossRef]
- Dechristopher, L.R.; Uribarri, J.; Tucker, K.L. Intake of High Fructose Corn Syrup Sweetened Soft Drinks Is Associated with Prevalent Chronic Bronchitis in U.S. Adults, Ages 20–55 Y. Nutr. J. 2015, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Atem, F.; Gelfand, A.; Delclos, G.; Messiah, S.E. Association between Asthma and Sugar-Sweetened Beverage Consumption in the United States Pediatric Population. J. Asthma 2022, 59, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Antonio Buendía, J.; Acuña-Cordero, R.; Patiño, D.G. The Role of High Carbohydrate-Rich Food Intake and Severity of Asthma Exacerbation in Children between 2 to 6 Years Aged. J. Asthma 2023, 60, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.C.; Maskell, J.; Oliver, E.M.; Morris, R.C.G.; Foote, K.D.; Mills, E.N.C.; Margetts, B.M.; Roberts, G. Diet and Food Allergy Development during Infancy: Birth Cohort Study Findings Using Prospective Food Diary Data. J. Allergy Clin. Immunol. 2014, 133, 511–519. [Google Scholar] [CrossRef]
- Machado Azeredo, C.; Cortese, M.; Costa, C.d.S.; Bjornevik, K.; Barros, A.J.D.; Barros, F.C.; Santos, I.S.; Matijasevich, A. Ultra-Processed Food Consumption during Childhood and Asthma in Adolescence: Data from the 2004 Pelotas Birth Cohort Study. Pediatr. Allergy Immunol. 2020, 31, 27–37. [Google Scholar] [CrossRef]
- Scheffers, F.R.; Boer, J.M.A.; Gehring, U.; Koppelman, G.H.; Vonk, J.; Smit, H.A.; Monique Verschuren, W.M.; Wijga, A.H. The Association of Pure Fruit Juice, Sugar-Sweetened Beverages and Fruit Consumption with Asthma Prevalence in Adolescents Growing up from 11 to 20 Years: The PIAMA Birth Cohort Study. Prev. Med. Reports 2022, 28, 101877. [Google Scholar] [CrossRef]
- Venter, C.; Maslin, K.; Holloway, J.W.; Silveira, L.J.; Fleischer, D.M.; Dean, T.; Arshad, S.H. Different Measures of Diet Diversity During Infancy and the Association with Childhood Food Allergy in a UK Birth Cohort Study. J. Allergy Clin. Immunol. Pract. 2020, 8, 2017–2026. [Google Scholar] [CrossRef]
- ISAAC—The International Study of Asthma and Allergies in Childhood. Available online: https://isaac.auckland.ac.nz/ (accessed on 28 June 2025).
- Carucci, L.; Votto, M.; Licari, A.; Marseglia, G.L.; Berni Canani, R. Food Allergy: Cause or Consequence of Pediatric Eosinophilic Esophagitis? Potential Implications of Ultraprocessed Foods in Prevention and Management. Front. Allergy 2023, 4, 1138400. [Google Scholar] [CrossRef]
- Dinardo, G.; Fiocchi, A.; Artesani, M.C.; De Angelis, P.; Rea, F.; Tambucci, R.; Dahdah, L.; Fierro, V.; Valluzzi, R.L.; Arasi, S.; et al. Eosinophilic Esophagitis and Cow’s Milk: Mechanisms, Challenges, and Treatment Perspectives. Nutrients 2025, 17, 265. [Google Scholar] [CrossRef]
- Baker, J.R.; Gangwar, R.S.; Platts-Mills, T.A. The Processed Milk Hypothesis: A Major Factor in the Development of Eosinophilic Esophagitis (EoE)? J. Allergy Clin. Immunol. 2024, 154, 1123–1126. [Google Scholar] [CrossRef]
- da Silva Oliveira, E.K.; Vieira, T.d.S.; de Souza, O.F.; Noll, P.R.e.S.; Bezerra, I.M.P.; Cavalcanti, M.P.E.; de Abreu, L.C.; Riera, A.R.P. Consumption of Ultra-Processed Foods in the Brazilian Amazon during COVID-19. Nutrients 2024, 16, 2117. [Google Scholar] [CrossRef]
- Silveira, V.N.C.; Dos Santos, A.M.; França, A.K.T.C. Determinants of the Consumption of Ultra-Processed Foods in the Brazilian Population. Br. J. Nutr. 2024, 132, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.C.O.A.; Rudakoff, L.C.S.; Muniz, A.K.O.A.; Magalhães, E.I.d.S.; Bragança, M.L.B.M.; da Silva, A.A.M.; Vianna, E.d.S.O.; Bettiol, H.; Barbieri, M.A. Association between the Consumption of Ultra-Processed Foods and Asthma in Adults from Ribeirão Preto, São Paulo, Brazil. Nutrients 2023, 15, 3165. [Google Scholar] [CrossRef] [PubMed]
- Godbharle, S.; Kesa, H.; Jeyakumar, A. Processed Food Consumption and Risk of Non-Communicable Diseases (NCDs) in South Africa: Evidence from Demographic and Health Survey (DHS) VII. J. Nutr. Sci. 2024, 13, e19. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.Y.; Wu, C.H.; Lu, C.H.; Kuo, Y.M.; Li, K.J.; Hsieh, S.C.; Yu, C.L. Advanced Glycation End Products of Bovine Serum Albumin Suppressed Th1/Th2 Cytokine but Enhanced Monocyte IL-6 Gene Expression via MAPK-ERK and MyD88 Transduced NF-ΚB P50 Signaling Pathways. Molecules 2019, 24, 2461. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Racioppo, A.; Santillo, A.; Albenzio, M.; Derossi, A.; Caporizzi, R.; Francavilla, M.; Racca, D.; Flagella, Z.; et al. Ultra-Processed Food and Gut Microbiota: Do Additives Affect Eubiosis? A Narrative Review. Nutrients 2024, 17, 2. [Google Scholar] [CrossRef]
- Kim, C.H. Complex Regulatory Effects of Gut Microbial Short-Chain Fatty Acids on Immune Tolerance and Autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.K.; Shin, H.S.; Kim, G.D. Naturally Derived Bioactive Compounds as Regulators of Oxidative Stress and Inflammation in Asthma. Chin. Med. 2025, 20, 94. [Google Scholar] [CrossRef]
- Baynes, J.W. The Maillard Reaction: Chemistry, Biochemistry and Implications By Harry Nursten (The University of Reading, Reading, U.K.). Royal Society of Chemistry: Cambridge. 2005. Xii + 214 Pp. $199.00. ISBN 0-85404-964-9. J. Am. Chem. Soc. 2005, 127, 14527–14528. [Google Scholar] [CrossRef]
- Yazici, D.; Ogulur, I.; Kucukkase, O.; Li, M.; Rinaldi, A.O.; Pat, Y.; Wallimann, A.; Wawrocki, S.; Celebi Sozener, Z.; Buyuktiryaki, B.; et al. Epithelial Barrier Hypothesis and the Development of Allergic and Autoimmune Diseases. Allergo J. Int. 2022, 31, 91–102. [Google Scholar] [CrossRef]
- Hilmenyuk, T.; Bellinghausen, I.; Heydenreich, B.; Ilchmann, A.; Toda, M.; Grabbe, S.; Saloga, J. Effects of Glycation of the Model Food Allergen Ovalbumin on Antigen Uptake and Presentation by Human Dendritic Cells. Immunology 2010, 129, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Zen, K.; Chen, C.X.-J.; Chen, Y.-T.; Wilton, R.; Liu, Y. Receptor for Advanced Glycation Endproducts Mediates Neutrophil Migration across Intestinal Epithelium. J. Immunol. 2007, 178, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Briceno Noriega, D.; Zenker, H.E.; Croes, C.A.; Ewaz, A.; Ruinemans-Koerts, J.; Savelkoul, H.F.J.; van Neerven, R.J.J.; Teodorowicz, M. Receptor Mediated Effects of Advanced Glycation End Products (AGEs) on Innate and Adaptative Immunity: Relevance for Food Allergy. Nutrients 2022, 14, 371. [Google Scholar] [CrossRef]
- Llauradó-Pont, J.; Stratakis, N.; Fiorito, G.; Handakas, E.; Neumann, A.; Barros, H.; Brantsæter, A.L.; Chang, K.; Chatzi, L.; Felix, J.F.; et al. A Meta-Analysis of Epigenome-Wide Association Studies of Ultra-Processed Food Consumption with DNA Methylation in European Children. Clin. Epigenet. 2025, 17, 3. [Google Scholar] [CrossRef]
- HANCU, A.; MIHALTAN, F.; RADULIAN, G. Asthma and Ultra-Processed Food. Maedica 2019, 14, 402–407. [Google Scholar] [CrossRef]
- Appendix E-3.4: USDA Food Patterns—Adequacy for Young Children. Available online: https://odphp.health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015/advisory-report/appendix-e-3/appendix-e-34 (accessed on 19 September 2025).
- WHO Guideline for Complementary Feeding of Infants and Young Children 6–23 Months of Age. Available online: https://www.who.int/publications/i/item/9789240081864 (accessed on 19 September 2025).
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A. Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Bailey, M.A.; Holscher, H.D. Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv. Nutr. 2018, 9, 93–206. [Google Scholar] [CrossRef]
- De Batlle, J.; Garcia-Aymerich, J.; Barraza-Villarreal, A.; Antó, J.M.; Romieu, I. Mediterranean Diet Is Associated with Reduced Asthma and Rhinitis in Mexican Children. Allergy 2008, 63, 1310–1316. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, A.; Fonseca, J.; Ferraz De Oliveira, J.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Adherence to the Mediterranean Diet and Fresh Fruit Intake Are Associated with Improved Asthma Control. Allergy 2008, 63, 917–923. [Google Scholar] [CrossRef]
- Rice, J.L.; Romero, K.M.; Galvez Davila, R.M.; Meza, C.T.; Bilderback, A.; Williams, D.A.L.; Breysse, P.N.; Bose, S.; Checkley, W.; Hansel, N.N. Association Between Adherence to the Mediterranean Diet and Asthma in Peruvian Children. Lung 2015, 193, 893–899. [Google Scholar] [CrossRef]
- Casas, R.; Ruiz-León, A.M.; Argente, J.; Alasalvar, C.; Bajoub, A.; Bertomeu, I.; Caroli, M.; Castro-Barquero, S.; Crispi, F.; Delarue, J.; et al. A New Mediterranean Lifestyle Pyramid for Children and Youth: A Critical Lifestyle Tool for Preventing Obesity and Associated Cardiometabolic Diseases in a Sustainable Context. Adv. Nutr. 2025, 16, 100381. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Barker, S.; Chang, Y.S.; Chikovani, T.; DunnGalvin, A.; Gerdts, J.D.; Gerth Van Wijk, R.; Gibbs, T.; Villarreal-Gonzalez, R.V.; Guzman-Avilan, R.I.; et al. Harmonizing Allergy Care–Integrated Care Pathways and Multidisciplinary Approaches. World Allergy Organ. J. 2021, 14, 100584. [Google Scholar] [CrossRef]
- Ilieva, G.; Yankova, T.; Ruseva, M.; Dzhabarova, Y.; Klisarova-Belcheva, S.; Dimitrov, A. Consumer Perceptions and Attitudes Towards Ultra-Processed Foods. Appl. Sci. 2025, 15, 3739. [Google Scholar] [CrossRef]
| Health Area | Effect of UPFs |
|---|---|
| Allergies [7,8,13] | More food allergies, asthma, and allergic rhinitis |
| Respiratory system [10,13,14] | Increased risk of wheezing and breathing problems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miraglia del Giudice, M.; Dinardo, G.; Grella, C.; Perrotta, A.; Indolfi, C.; Klain, A. Ultra-Processed Foods and Respiratory and Allergic Diseases in Childhood: Epidemiological Evidence and Mechanistic Insights. Nutrients 2025, 17, 3269. https://doi.org/10.3390/nu17203269
Miraglia del Giudice M, Dinardo G, Grella C, Perrotta A, Indolfi C, Klain A. Ultra-Processed Foods and Respiratory and Allergic Diseases in Childhood: Epidemiological Evidence and Mechanistic Insights. Nutrients. 2025; 17(20):3269. https://doi.org/10.3390/nu17203269
Chicago/Turabian StyleMiraglia del Giudice, Michele, Giulio Dinardo, Carolina Grella, Alessandra Perrotta, Cristiana Indolfi, and Angela Klain. 2025. "Ultra-Processed Foods and Respiratory and Allergic Diseases in Childhood: Epidemiological Evidence and Mechanistic Insights" Nutrients 17, no. 20: 3269. https://doi.org/10.3390/nu17203269
APA StyleMiraglia del Giudice, M., Dinardo, G., Grella, C., Perrotta, A., Indolfi, C., & Klain, A. (2025). Ultra-Processed Foods and Respiratory and Allergic Diseases in Childhood: Epidemiological Evidence and Mechanistic Insights. Nutrients, 17(20), 3269. https://doi.org/10.3390/nu17203269








