Natural Small-Molecule Bergapten Ameliorates Amyloid-β Pathology and Neuroinflammation in Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
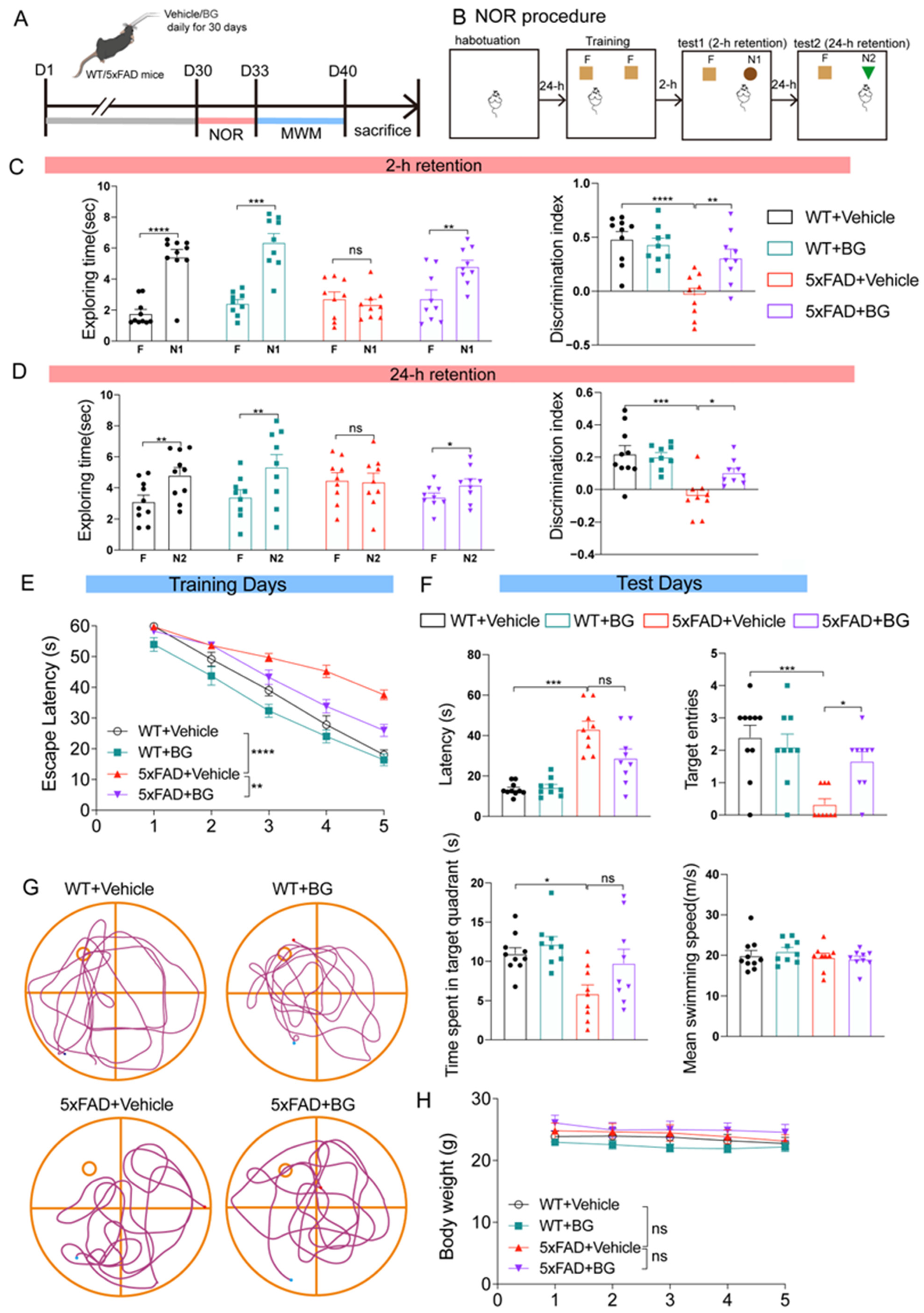
2.3. Experimental Design of BG Administration
2.4. Behavior Tests
2.4.1. Novel Object Recognition (NOR) Test
2.4.2. Morris Water Maze (MWM) Test
2.5. Preparation of Oligomeric Aβ1–42 and BG
2.6. Cell Culture and Treatment
2.6.1. BV2 Cells Culture and Treatment
2.6.2. Cell-Counting Kit-8 (CCK-8) Assay
2.7. Immunofluorescence
2.8. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.9. Western Blot
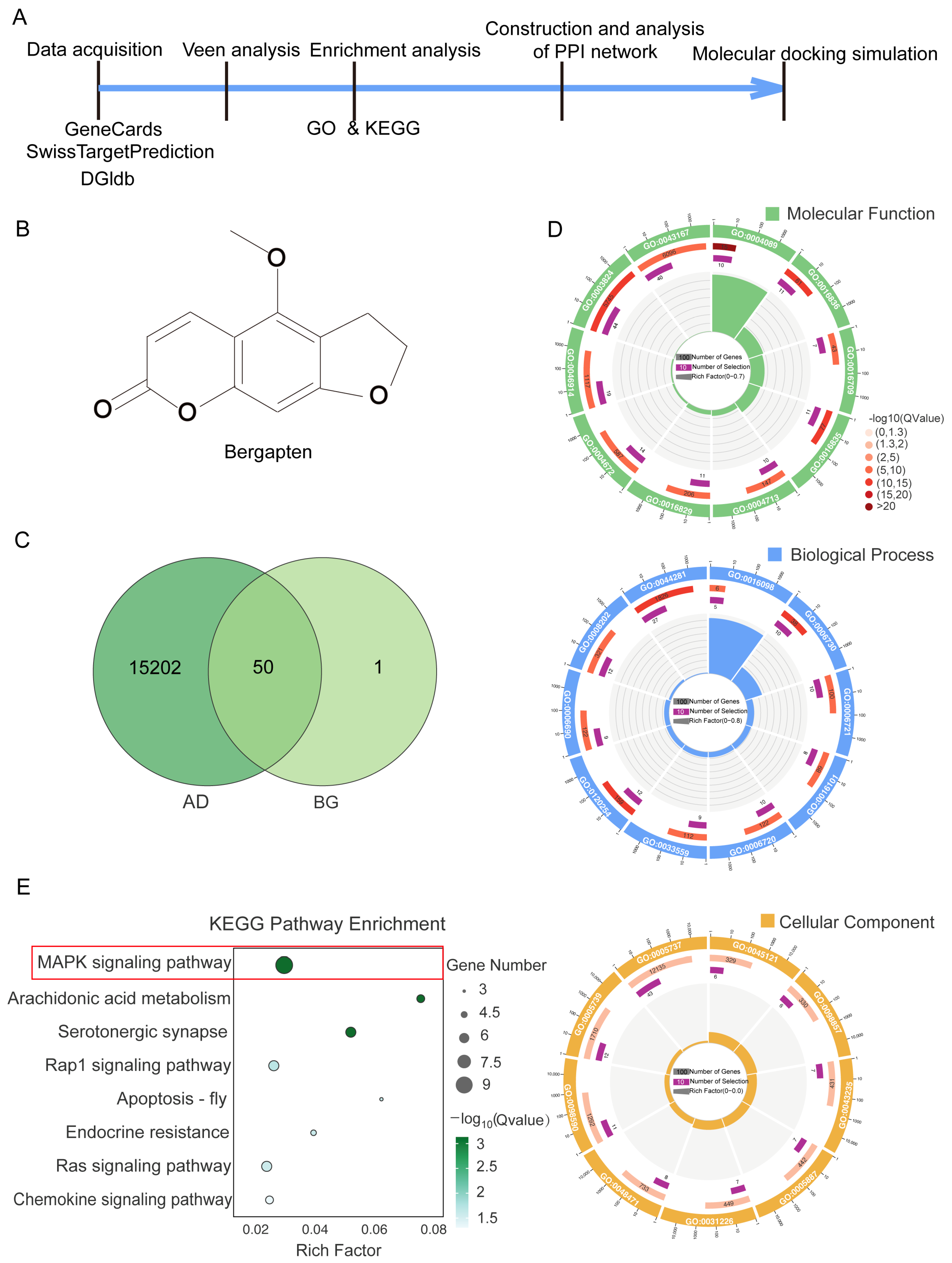
2.10. Bioinformatics Analysis
2.10.1. Target Screening of AD and BG
2.10.2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analysis
2.10.3. Protein–Protein Interaction (PPI) Network Analysis
2.10.4. Molecular Docking Simulation
2.11. Statistical Analysis
3. Results
3.1. Analysis of Publicly Available Databases Revealed That BG Modulates the MAPK Signaling Pathway in AD
3.2. PPI Network Analysis and Molecular Docking Suggested BG’s Modulation of MAPK Signaling Pathway Proteins
3.3. BG Treatment Ameliorates Cognitive Deficits in 5×FAD Mice
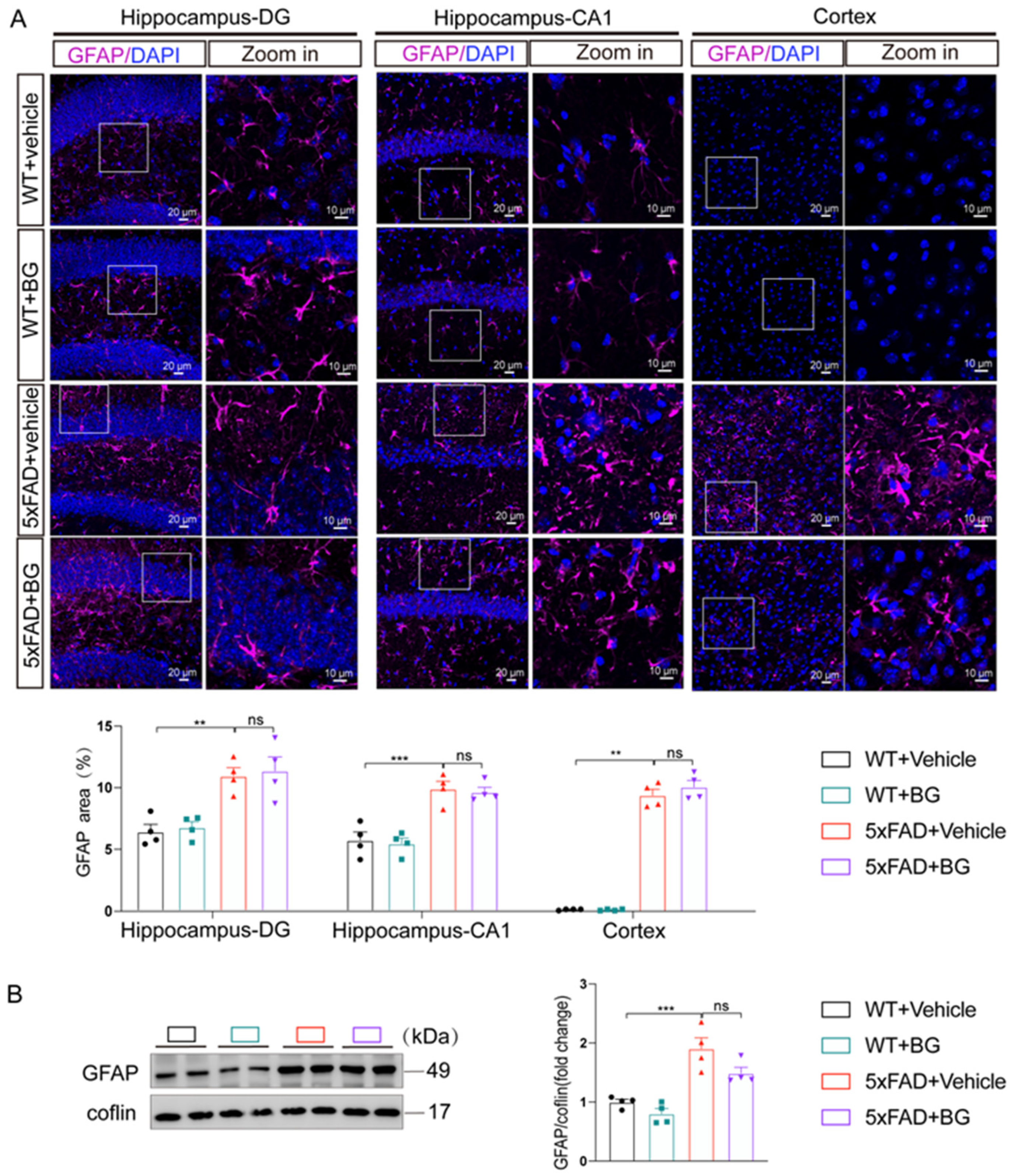
3.4. BG Attenuates Aβ Burden and Microglial Activation in 5×FAD Mice
3.5. BG Reduced Pro-Inflammatory Cytokine Expression in 5×FAD Mice and Aβ-Stimulated BV2 Cells
3.6. BG Attenuates Microglial Activation by Suppressing MAPK Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BG | Bergapten |
| CNS | Central nervous system |
| Il-6 | Interleukin-6 |
| Il-1β | Interleukin-1β |
| Tnfα | Tumor necrosis factor α |
| NF-κB | Nuclear factor-κB |
| MAPK | Mitogen-activated protein kinase |
| LPS | Lipopolysaccharide |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes |
| PPI | Protein–Protein Interaction |
| p-ERK | phosphorylated ERK |
| PTK2 | Serine/threonine-protein kinase 2 |
| MET | Hepatocyte growth factor receptor |
| PDGFRB | Platelet-derived growth factor receptor beta |
| MAP3K8 | Mitogen-activated protein kinase kinase kinase 8 |
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms, and therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, J.; Yong, K.X.X.; Apostolova, L.G.; Bouwman, F.H.; Carrillo, M.; Dickerson, B.C.; Rabinovici, G.D.; Schott, J.M.; Jones, D.T.; Murray, M.E. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021, 20, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer disease. Nat. Rev. Immunol. 2024, 25, 321–352. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Almutary, A.G.; Begum, M.Y.; Kyada, A.K.; Gupta, S.; Jyothi, S.R.; Chaudhary, K.; Sharma, S.; Sinha, A.; Abomughaid, M.M.; Imran, M.; et al. Inflammatory signaling pathways in Alzheimer’s disease: Mechanistic insights and possible therapeutic interventions. Ageing Res. Rev. 2024, 104, 102548. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, Y.; Wang, J.-Z. Updates in Alzheimer’s disease: From basic research to diagnosis and therapies. Transl. Neurodegener. 2024, 13, 45. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Karch, C.M.; Cruchaga, C.; Goate, A.M. Alzheimer’s disease genetics: From the bench to the clinic. Neuron 2014, 83, 11–26. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, G.; Holtzman, D.M. Antibody Therapeutics Targeting Aβ and Tau. Cold Spring Harb. Perspect. Med. 2017, 7, a024331. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef]
- Kaur, A.; Garg, S.; Shiekh, B.A.; Singh, N.; Singh, P.; Bhatti, R. In Silico Studies and In Vivo MAOA Inhibitory Activity of Coumarins Isolated from Angelica archangelica Extract: An Approach toward Antidepressant Activity. ACS Omega 2020, 5, 15069–15076. [Google Scholar] [CrossRef]
- Yan, M.; Bo, X.; Zhang, J.; Liu, S.; Li, X.; Liao, Y.; Liu, Q.; Cheng, Y.; Cheng, J. Bergapten alleviates depression-like behavior by inhibiting cyclooxygenase 2 activity and NF-κB/MAPK signaling pathway in microglia. Exp. Neurol. 2023, 365, 114426. [Google Scholar] [CrossRef]
- Darcourt, G.; Feuillade, P.; Bistagnin, Y.; Robert, P.; Pringuey, D.; Touari, M.; Merdji, Y.; Bensmaïl, B. Antidepressant effect of 5-methoxypsoralen: The melatonin synchronizer hypothesis. Eur. Psychiatry 1995, 10, 142–154. [Google Scholar] [CrossRef]
- Salvati, E.; Belugou, J.L.; Robert, P.; Brunet, G.; Darcourt, G.; Souêtre, E. Antidepressant effect of 5-methoxypsoralen: A preliminary report. Psychopharmacology 1988, 95, 430–431. [Google Scholar] [CrossRef]
- Xie, J.; Sun, S.; Zhou, Z.; Ma, X.; Li, Q.; Li, S.; Luo, Y.; Zhang, G.; Hu, Y.; Wang, G.; et al. Natural small molecule bergapten ameliorates rheumatoid arthritis by targeting STAT3. Pharmacol. Res. 2025, 219, 107878. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Li, Y.; Ding, X.; Zhang, Y.; Jiang, X.; Lai, H.; Shi, J. Novel application of bergapten and quercetin with anti-bacterial, osteogenesis-potentiating, and anti-inflammation tri-effects. Acta Biochim. Biophys. Sin. 2021, 53, 683–696. [Google Scholar] [CrossRef]
- Luo, T.; Jia, X.; Feng, W.-D.; Wang, J.-Y.; Xie, F.; Kong, L.-D.; Wang, X.-J.; Lian, R.; Liu, X.; Chu, Y.-J.; et al. Bergapten inhibits NLRP3 inflammasome activation and pyroptosis via promoting mitophagy. Acta Pharmacol. Sin. 2023, 44, 1867–1878. [Google Scholar] [CrossRef]
- Liao, R.; Sun, Z.-C.; Wang, L.; Xian, C.; Lin, R.; Zhuo, G.; Wang, H.; Fang, Y.; Liu, Y.; Yang, R.; et al. Inhalable and bioactive lipid-nanomedicine based on bergapten for targeted acute lung injury therapy via orchestrating macrophage polarization. Bioact. Mater. 2024, 43, 406–422. [Google Scholar] [CrossRef]
- Rao, T.; Yang, W.; Ma, X.; Jiang, X.; Jiang, S.; Xu, S. Bergapten attenuates hemorrhagic shock induced multi-organ injury by inhibiting NLRP3 inflammasome activation and pyroptosis. Int. Immunopharmacol. 2024, 140, 112839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cheng, L.; Chen, T.; Liu, X.; Zhang, C.; Aji, A.; Guo, W.; Zhu, J.; Chu, Y.; Guo, D.; et al. Bergapten Ameliorates Psoriatic Skin Lesions and IL-17A-Induced Activation of the NF-κB Signaling Pathway via the Downregulation of CYP1B1. Phytother. Res. 2024, 39, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zou, X.; Wang, Z.; Shu, X.; Cao, X.; Xia, S.; Shao, P.; Bao, X.; Yang, H.; Xu, Y.; et al. Bergapten attenuates microglia-mediated neuroinflammation and ischemic brain injury by targeting Kv1.3 and Carbonyl reductase 1. Eur. J. Pharmacol. 2022, 933, 175242. [Google Scholar] [CrossRef] [PubMed]
- Adakudugu, E.A.; Ameyaw, E.O.; Obese, E.; Biney, R.P.; Henneh, I.T.; Aidoo, D.B.; Oge, E.N.; Attah, I.Y.; Obiri, D.D. Protective effect of bergapten in acetic acid-induced colitis in rats. Heliyon 2020, 6, e04710. [Google Scholar] [CrossRef]
- He, Y.; Zisan, Z.; Lu, Z.; Zheng, L.; Zhao, J. Bergapten alleviates osteoarthritis by regulating the ANP32A/ATM signaling pathway. FEBS Open Bio 2019, 9, 1144–1152. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, A.; Kaur, J.; Bhatti, M.S.; Singh, P.; Bhatti, R. Bergapten inhibits chemically induced nociceptive behavior and inflammation in mice by decreasing the expression of spinal PARP, iNOS, COX-2 and inflammatory cytokines. Inflammopharmacology 2019, 27, 749–760. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Yang, W.; Qi, X.; Lan, L.; Luo, L.; Yin, Z. Bergapten prevents lipopolysaccharide-induced inflammation in RAW264.7 cells through suppressing JAK/STAT activation and ROS production and increases the survival rate of mice after LPS challenge. Int. Immunopharmacol. 2017, 48, 159–168. [Google Scholar] [CrossRef]
- Liu, W.-X.; Jia, F.-L.; He, Y.-Y.; Zhang, B.-X. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J. Gastroenterol. 2012, 18, 2197–2202. [Google Scholar] [CrossRef]
- Li, L.; Cai, W.; Zhang, H.; Tang, J.; Yang, Y.; Huang, Y.; Xi, Q.; Zhang, R. Bergapten Ameliorates Renal Fibrosis by Inhibiting Ferroptosis. Phytother. Res. 2025, 39, 1355–1371. [Google Scholar] [CrossRef]
- Shen, Q.; Huang, W.; Qiu, Y.; Wang, S.; Zhang, B.; Sun, N.; Zhou, Q. Bergapten exerts a chondroprotective effect in temporomandibular joint osteoarthritis by combining intestinal flora alteration and reactive oxygen species reduction. Biomed. Pharmacother. 2023, 167, 115525. [Google Scholar] [CrossRef] [PubMed]
- Latif, K.; Khan, A.-U.; Haque, M.I.U.; Naeem, K. Bergapten Attenuates Nitroglycerin-Induced Migraine Headaches through Inhibition of Oxidative Stress and Inflammatory Mediators. ACS Chem. Neurosci. 2021, 12, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hussain, S.A.; Daddam, J.R.; Sun, M. Bergapten attenuates human papillary thyroid cancer cell proliferation by triggering apoptosis and the GSK-3β, P13K and AKT pathways. Adv. Clin. Exp. Med. 2024, 34, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, S.P.; Bose, P.; Sunita, P.; Siddique, M.U.M.; Lapenna, A. Bergapten inhibits liver carcinogenesis by modulating LXR/PI3K/Akt and IDOL/LDLR pathways. Biomed. Pharmacother. 2018, 108, 297–308. [Google Scholar] [CrossRef]
- De Amicis, F.; Aquila, S.; Morelli, C.; Guido, C.; Santoro, M.; Perrotta, I.; Mauro, L.; Giordano, F.; Nigro, A.; Andò, S.; et al. Bergapten drives autophagy through the up-regulation of PTEN expression in breast cancer cells. Mol. Cancer 2015, 14, 130. [Google Scholar] [CrossRef]
- Chiang, S.; Lin, C.; Lin, H.; Shieh, P.; Kao, S. Bergapten induces G1 arrest of non-small cell lung cancer cells, associated with the p53-mediated cascade. Mol. Med. Rep. 2019, 19, 1972–1978. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Kurach, Ł.; Boguszewska-Czubara, A.; Skalicka-Woźniak, K.; Kruk-Słomka, M.; Kurzepa, J.; Wydrzynska-Kuźma, M.; Biała, G.; Skiba, A.; Budzyńska, B. Bergapten Improves Scopolamine-Induced Memory Impairment in Mice via Cholinergic and Antioxidative Mechanisms. Front. Neurosci. 2020, 14, 730. [Google Scholar] [CrossRef]
- Budzynska, B.; Skalicka-Wozniak, K.; Kruk-Slomka, M.; Wydrzynska-Kuzma, M.; Biala, G. In vivo modulation of the behavioral effects of nicotine by the coumarins xanthotoxin, bergapten, and umbelliferone. Psychopharmacology 2016, 233, 2289–2300. [Google Scholar] [CrossRef]
- Salem, M.A.; Budzyńska, B.; Kowalczyk, J.; El Sayed, N.S.; Mansour, S.M. Tadalafil and bergapten mitigate streptozotocin-induced sporadic Alzheimer’s disease in mice via modulating neuroinflammation, PI3K/Akt, Wnt/β-catenin, AMPK/mTOR signaling pathways. Toxicol. Appl. Pharmacol. 2021, 429, 115697. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Li, X.; Wang, X.; Yang, S. Bergapten inhibits airway inflammation and MRGPRX2-mediated mast cells activation by targeting NR4A1. Int. Immunopharmacol. 2024, 130, 111798. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2007, 1, 848–858. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Q.; Hong, Y.; Gao, Y.; Hu, J.; Lang, M.; Zhang, H.; Zhou, Y.; Luo, H.; Zhang, X.; et al. β(2)-Microglobulin coaggregates with Aβ and contributes to amyloid pathology and cognitive deficits in Alzheimer’s disease model mice. Nat. Neurosci. 2023, 26, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, X.; Li, S.; Wu, Y.; Zhu, F.; Qin, C.; Zhang, Q.; Yang, Y. Naringenin ameliorates amyloid-β pathology and neuroinflammation in Alzheimer’s disease. Commun. Biol. 2024, 7, 912. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Bao, N.; Chen, J.; Tang, M.; Yang, L.; Yang, Y.; Zhang, H.; Han, J.; Yu, P.; Zhang, S.; et al. Neuromolecular and behavioral effects of cannabidiol on depressive-associated behaviors and neuropathic pain conditions in mice. Neuropharmacology 2024, 261, 110153. [Google Scholar] [CrossRef] [PubMed]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Moutinho, S. Women twice as likely to develop Alzheimer’s disease as men—But scientists do not know why. Nat. Med. 2025, 31, 704–707. [Google Scholar] [CrossRef]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of sex differences in Alzheimer’s disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef]
- Maarouf, C.L.; Kokjohn, T.A.; Whiteside, C.M.; Macias, M.P.; Kalback, W.M.; Sabbagh, M.N.; Beach, T.G.; Vassar, R.; Roher, A.E. Molecular Differences and Similarities between Alzheimer’s Disease and the 5×FAD Transgenic Mouse Model of Amyloidosis. Biochem. Insights 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A. Associations of the immune system in aggression traits and the role of microglia as mediators. Neuropharmacology 2024, 256, 110021. [Google Scholar] [CrossRef]
- Mary, A.; Mancuso, R.; Heneka, M.T. Immune Activation in Alzheimer Disease. Annu. Rev. Immunol. 2024, 42, 585–613. [Google Scholar] [CrossRef]
- Bellenguez, C.; Küçükali, F.; Jansen, I.E.; Kleineidam, L.; Moreno-Grau, S.; Amin, N.; Naj, A.C.; Campos-Martin, R.; Grenier-Boley, B.; Andrade, V.; et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022, 54, 412–436. [Google Scholar] [CrossRef]
- Wang, P.; Han, L.; Wang, L.; Tao, Q.; Guo, Z.; Luo, T.; He, Y.; Xu, Z.; Yu, J.; Liu, Y.; et al. Molecular pathways and diagnosis in spatially resolved Alzheimer’s hippocampal atlas. Neuron 2025, 113, 2123–2140.e9. [Google Scholar] [CrossRef]
- Kure, C.; Timmer, J.; Stough, C. The Immunomodulatory Effects of Plant Extracts and Plant Secondary Metabolites on Chronic Neuroinflammation and Cognitive Aging: A Mechanistic and Empirical Review. Front. Pharmacol. 2017, 8, 117. [Google Scholar] [CrossRef]
- Wang, M.; Jin, B.; Xu, J.; Wang, C. Avenanthramide-C Mitigates High-Fat Diet-Accelerated Alzheimer’s Pathologies via NOD1-Driven Neuroinflammation in 5×FAD Mice. Nutrients 2025, 17, 2679. [Google Scholar] [CrossRef]
- Shi, F.-D.; Yong, V.W. Neuroinflammation across neurological diseases. Science 2025, 388, eadx0043. [Google Scholar] [CrossRef]
- Singh, G.; Singh, A.; Singh, P.; Bhatti, R. Bergapten Ameliorates Vincristine-Induced Peripheral Neuropathy by Inhibition of Inflammatory Cytokines and NFκB Signaling. ACS Chem. Neurosci. 2019, 10, 3008–3017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Van Nguyen, T.; Jin, J.; Yu, Z.N.; Song, C.H.; Chai, O.H. Bergapten ameliorates combined allergic rhinitis and asthma syndrome after PM2.5 exposure by balancing Treg/Th17 expression and suppressing STAT3 and MAPK activation in a mouse model. Biomed. Pharmacother. 2023, 164, 114959. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, J.; Ge, X.; Deng, J.; Xu, X.; Zhao, Y.; Wang, H. Activation of ERK–Drp1 signaling promotes hypoxia-induced Aβ accumulation by upregulating mitochondrial fission and BACE1 activity. FEBS Open Bio 2021, 11, 2740–2755. [Google Scholar] [CrossRef]
- Almasoudi, S.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Eliwa, D.; Alexiou, A.; Papadakis, M.; Batiha, G.E.-S. Role of mitogen-activated protein kinase inhibitors in Alzheimer’s disease: Rouge of brain kinases. Brain Res. Bull. 2025, 224, 111296. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.-J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Ho, W.-C.; Hsu, C.-C.; Huang, H.-J.; Wang, H.-T.; Lin, A.M.-Y. Anti-inflammatory Effect of AZD6244 on Acrolein-Induced Neuroinflammation. Mol. Neurobiol. 2019, 57, 88–95. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, J. Natural Small-Molecule Bergapten Ameliorates Amyloid-β Pathology and Neuroinflammation in Alzheimer’s Disease. Nutrients 2025, 17, 3218. https://doi.org/10.3390/nu17203218
Zhang J, Zhang J. Natural Small-Molecule Bergapten Ameliorates Amyloid-β Pathology and Neuroinflammation in Alzheimer’s Disease. Nutrients. 2025; 17(20):3218. https://doi.org/10.3390/nu17203218
Chicago/Turabian StyleZhang, Jingyan, and Jing Zhang. 2025. "Natural Small-Molecule Bergapten Ameliorates Amyloid-β Pathology and Neuroinflammation in Alzheimer’s Disease" Nutrients 17, no. 20: 3218. https://doi.org/10.3390/nu17203218
APA StyleZhang, J., & Zhang, J. (2025). Natural Small-Molecule Bergapten Ameliorates Amyloid-β Pathology and Neuroinflammation in Alzheimer’s Disease. Nutrients, 17(20), 3218. https://doi.org/10.3390/nu17203218





