1. Introduction
Osteoporosis is a prevalent, debilitating, and often silent skeletal disease characterized by a loss of bone mass, compromised bone strength, and an increased risk of fracture [
1]. It results from an imbalance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. In addition to conventional pharmaceutical treatment such as bisphosphonates and teriparatide, which are associated with significant side effects, nutrient supplements have gained increasing public attention as potential therapies for osteoporosis.
The gut microbiota is in proximity to various cell types and influences multiple physiological systems, including the intestinal nervous and immune systems, enteroendocrine function, and enterocyte permeability. It also contributes to the production of vitamins and secondary bile salts, competes with pathogenic microorganisms to prevent their invasion, and stimulates immune responses [
2,
3]. The gut microbiota comprises diverse microorganisms, including probiotics, which are live and non-pathogenic microorganisms that, when administered in adequate amounts, confer health benefits to the host [
4].
Mounting evidence suggests that probiotics may have preventive and therapeutic effects on osteoporosis. A randomized, double-blind, placebo-controlled clinical trial involving 50 patients aged 50 to 72 with bone loss demonstrated that supplementation with multiple probiotics positively affected bone health in menopausal women by reducing the rate of bone turnover [
5]. A recent study showed that
Lactobacillus reuteri could ameliorate osteoporosis induced by type 1 diabetes in a mouse model by preventing TNF-α-mediated inhibition of Wnt10b and markers of osteoblast maturation [
6]. Additionally, McCabe et al. reported that supplementation with
L. reuteri ATCC PTA 6475 improved trabecular bone parameters of the femur and lumbar vertebrae in male mice with intestinal inflammation [
7]. Collectively, current evidence supports the potential of probiotics to treat secondary osteoporosis associated with postmenopausal estrogen deficiency, type 1 diabetes, and intestinal inflammation. However, the effects of
Lactobacillus on secondary osteoporosis following long-term steroid treatment remain under investigation [
8].
In this study, deoxycorticosterone acetate (DOCA), an adrenocortical hormone and corticosteroid used clinically to treat primary adrenal insufficiency and inflammation, was employed as a model to induce hypertension [
9,
10]. Notably, steroid use is a recognized causative factor contributing to osteoporosis and has a high incidence among individuals with this condition [
8]. Many chronic inflammatory diseases require steroids for treatment, yet long-term steroid use or short-term use of high-dose steroids may result in bone loss. We utilized a strain of
Lacticaseibacillus rhamnosus isolated from a fecal sample from a healthy infant from Hong Kong, which was previously applied in hypertension treatment, to investigate whether supplementation with this strain can ameliorate DOCA-salt-induced osteoporosis and its associated bone alterations.
2. Materials and Methods
2.1. Bacterial Culture
LR-AC1 was isolated from the fecal sample of a healthy infant from Hong Kong [
11]. LR-AC1 was cultured in De Man, Rogosa and Sharpe (MRS) Broth, and its identity was confirmed by 16S ribosomal RNA (rRNA) sequencing.
2.2. Animal Experiment
Sprague Dawley (SD) rats were bred by the Centralized Animal Facilities (CAFs) at The Hong Kong Polytechnic University. To establish a DOCA-salt-induced rat model of osteoporosis, male rats aged 6 weeks and weighing between 180 and 200 g were used. The animal experiment was approved by the Animal Subjects Ethics Committee of The Hong Kong Polytechnic University (ASESC No: 17-18/50-ABCT-R-HMRF) [
9,
12]. All experimental procedures and licenses were approved and granted by the Hong Kong Department of Health. The rats were housed at the CAFs under controlled conditions at a temperature of 21 ± 2 °C, relative humidity of 55% ± 10%, and a 12 h light/dark cycle. Standard diet and water were provided ad libitum. Prior to the start of the experiment, all animals were acclimated to the experimental environment for 2 weeks. Cage randomization and treatment administration were blinded to reduce bias.
To investigate the effect of LR-AC1 on healthy rats, the SD rats were orally administered an LR-AC1 suspension in 0.85% NaCl daily at dosages of 10
8, 10
9, or 10
10 CFU/kg b.w., according to which they were grouped as LR-D1, LR-D2, or LR-D3, respectively. The dose and duration of LR-AC1 administration followed a previous study in
Lacticaseibacillus rhamnosus [
13,
14]. The SHAM group received tap water. The treatment period lasted for 5 weeks (
Figure 1).
Hypertension was induced by intraperitoneal injection of DOCA (20 mg/kg b.w., Sigma, D7000, St. Louis, Missouri, USA) twice weekly, in combination with 1.0% NaCl and 0.2% KCl administered in drinking water, while the SHAM group received tap water. The treatment period lasted for 14 weeks.
To investigate the effect of LR-AC1 on the rat model of DOCA-salt-induced osteoporosis, 10
10 CFU/kg b.w. of LR-AC1 suspension in 0.85% NaCl was administered every day by oral gavage to the rats that had undergone DOCA treatment for 9 weeks. The probiotic treatment lasted for 5 weeks (
Figure 1). At the end of the experiment, the rats were anesthetized with CO
2 for subsequent analyses. Both the left and right legs were stored in 75% ethanol after fixation for 48 h. Fecal samples were collected weekly and immediately following anesthesia and stored at −80 °C.
2.3. 16S rRNA Sequencing
Total genomic DNA was extracted from the collected fecal samples using the TIANamp Stool DNA Kit (TIANGEN, Beijing, China). The V3–V4 region of the 16S rRNA gene was amplified using the 16S-338F forward primer and 16S-806R reverse primer [
9]. Sequencing of the 16S rRNA gene was performed by Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China), using the Illumina HiSeq 2000 platform (Illumina, Inc., San Diego, CA, USA). For post-sequencing analysis, primer sequences were trimmed, and raw data were processed using QIIME 2 with high-performance computing resources provided by the Information Technology Services Office, The Hong Kong Polytechnic University. Paired-end sequences with high-quality reads were denoised using the DADA2 plugin, and taxonomy was assigned based on the SILVA database. Alpha diversity indices and beta-diversity weighted UniFrac principal coordinate analysis (PCoA) plots were generated using QIIME 2 pipelines.
2.4. High-Resolution Micro-CT Scanning and Analysis
At the end of the treatment, all rats were euthanized, and their legs were harvested for further analyses. Micro-CT scanning was conducted at the Department of Orthopedics and Traumatology, The University of Hong Kong. The right tibias were scanned using an in vivo X-ray microtomograph Skyscan 1076 (SkyScan n.v., Aartselaar, Belgium). Images were acquired with an X-ray tube voltage of 88 kV, a current of 100 µA, and a Al1.0 mm filter. The exposure time was 560 ms, with a rotation step size of 0.600 degrees. The micro-CT pixel size was 9 µm. Image reconstruction was performed using NRecon software.
The primary spongiosa of the right tibia was analyzed by Micro-CT 3D.SUITE software (BRUKER, Billerica, MA, USA), which includes DATAVIEW and CTAN. The images were saved as Recon files and opened with Dataview (version 1.4.4.0), with the position adjusted to ensure they all had the same direction. A single volume of interest, which covered all the parts to be analyzed, was selected. CTAN was used to further analyze the images. In brief, the volume of interest saved from Dataview was applied. The top and bottom of the selection were chosen. For the primary spongiosa, the top of the selection was the first image in which the trabecular bone of the primary spongiosa appeared in full. A total of 200 images were counted, and the 200th image was the bottom of the selection. The region of interest was drawn, and the dataset of the region of interest was saved to create a 3D model. Finally, 3D analysis was performed in Morphometry preview to determine the percent bone volume, trabecular thickness, trabecular number, and trabecular separation, which were saved.
2.5. Tissue Processing
The bone samples were fixed in 4% Paraformaldehyde (PFA) solution for 48 h, followed by storage in 75% alcohol. A 10% Ethylene Diamine Tetraacetic Acid (EDTA) solution was used for decalcification, and the solution was changed every four days until the bone samples softened. The bone samples were then placed in a Leica TP1020 tissue processor (Nussloch, Germany) for processing. They were embedded in paraffin using a Leica ARCADIA H (Nussloch, Germany) until they cooled down. The embedded samples were then cut into 5 µm thick sections for staining.
2.6. Immunohistochemistry for Osterix and CD31+
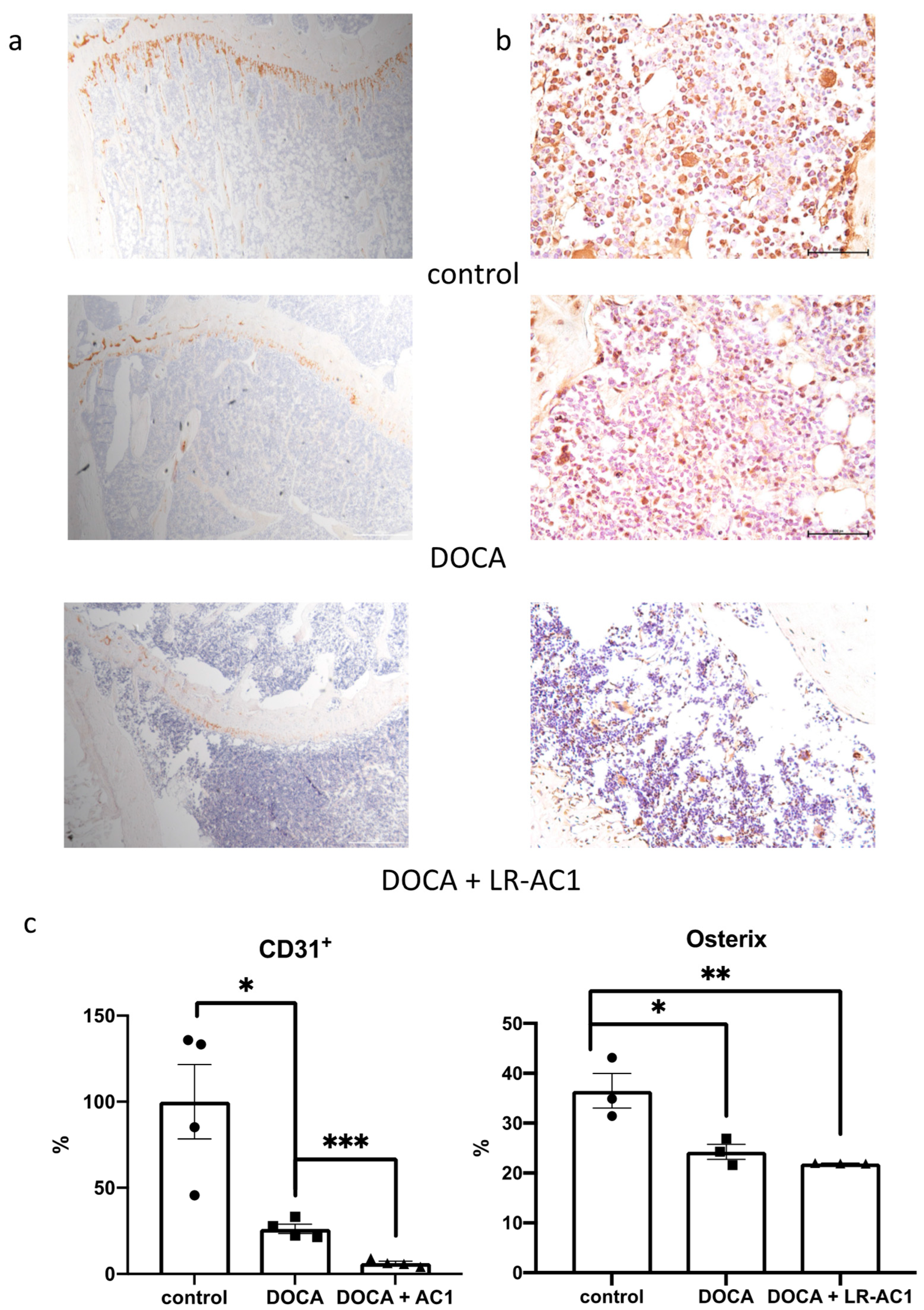
Immunohistological staining for Osterix (Sp7) and CD31+ was performed using the sections of embedded tissue described previously. After deparaffinization and rehydration, the slices were placed in a citrate buffer at 90 °C for 10 min to retrieve the antigen for Osterix staining or placed in a Tris/EDTA buffer at a pH of 9.0 and 90 °C–95 °C for 3 min for CD31+ staining. Then, the sections were treated with 3% hydrogen peroxide for 10 min in the dark, followed by blocking with 10% horse serums. The primary antibodies Sp7 (Cat# ab209484, Abcam, Cambridge, UK) (1/1000) and CD31+ (Cat# ab182981, Abcam) (1/1000) were co-incubated with the sections at 4 °C overnight, following the manufacturer’s instructions. At the same time, the negative control samples were incubated with PBS. A Vectastain ABC kit and a DAB peroxidase substrate kit (Vector Labs, Newark, CA, USA) were used to stain the targeted antigen for 3,3′-Diaminobenzidine (DAB). The sections were then subjected to hematoxylin staining and dehydration. Finally, DPX was used as a mounting medium to mount the slides. Compared to the negative control, the positive signals appeared brown. ImageJ 1.53j (National Institutes of Health, Bethesda, MD, USA) was used to analyze the DAB staining results. For CD31+, the area fraction was calculated, and the osterix result was the ratio of nuclei with positive signals to the total number of nuclei.
2.7. Preparation of LR-AC1 Cell-Free Conditioned Supernatant (CCS)
A single colony of LR-AC1 was inoculated in MRS broth at 37 °C for 18 h, diluted to 10%, and subcultured in fresh MRS until the OD reached 0.3. The culture was centrifuged at 4000×
g rpm for 10 min. The cell pellet was collected, washed twice with sterile phosphate-buffered saline (PBS), resuspended in Dulbecco’s modified Eagle medium (DMEM) with an OD600 of 3.0, and incubated at 37 °C for 3 h on a shaker of 60 rpm. The supernatant was collected and passed through a 0.22 µm filter. The fluid was fractionated to include only the fraction < 10 kDa, using a centrifugal Amicon filter unit (Merck Millipore Ltd., Cork, Ireland). The LR-AC1 CCS was pipetted into 96-well plates in 250 µL aliquots, lyophilized, and stored at −80 °C [
15].
2.8. Treatment of LR-AC1 CCS on Osteoclasts
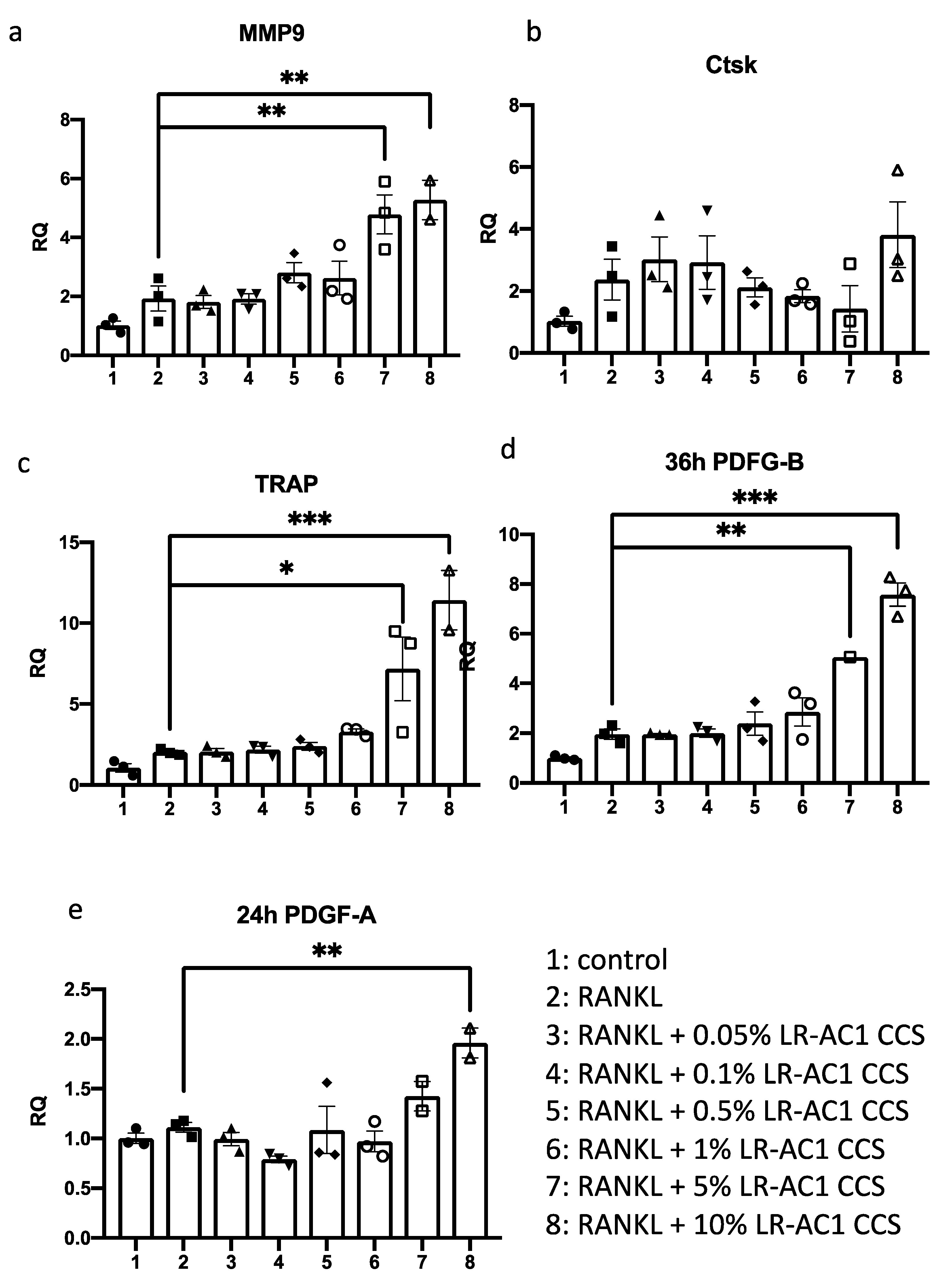
RAW264.7 TIB-1TM cells were cultured with DMEM containing 10% fetal bovine serum (FBS) in an incubator with a CO2 content of 5% at 37 °C. RAW264.7 cells were passaged using a cell scraper and seeded in a 24-well plate at a density of 2 × 104 cells/well for 24 h before treatment. Receptor activator of nuclear factor kappa-B ligand (RANKL), 30 ng/mL, was used to induce osteoclast differentiation. Final concentrations of 0.05%, 0.1%, 0.5%, 1%, 5%, and 10% LR-AC1 CCS were used to treat the induced cells for 24 h for the detection of osteoclast biomarkers, including Matrix metallopeptidase 9 (MMP-9), Cathepsin K (Ctsk), and Tartrate-resistant acid phosphatase (TRAP), and the angiogenesis biomarker Platelet-derived growth factor A (PDGF-A). The same treatment procedure was used to detect the biomarkers of angiogenesis Platelet-derived growth factor B (PDGF-B), except the treatment time with LR-AC1 CCS was 36 h. The cells were collected for RNA extraction at the end of the experiment. There were three biological and technical replicates each.
2.9. Reverse Transcriptase Quantitative Polymerase Chain Reaction (RT-qPCR)
After RNA extraction, cDNA synthesis was performed using PrimeScript RT Master Mix (TaKaRa Cat# RR036A, Shiga, Japan). The mixtures were incubated first at 37 °C for 15 min for reverse transcription, then at 85 °C for 5 sec to inactivate the reverse transcriptase, and then cooled down to 4 °C. The cDNA samples were stored at −20 °C for future use. The qPCR was performed using the QuantStudio™ 7 Flex Real-Time PCR System (Waltham, MA, USA). The primer sequences for the targets, including PDGF-A, PDGF-B, MMP-9, Ctsk, and TRAP, are shown in
Table 1.
2.10. Wound Healing Assay of BMSCs Co-Cultured with RAW264.7
Transwell was used to perform the wound healing assay. BMSC PCS-500-012
TM (6 × 10
4 cells/well) cells were seeded in a 24-well cell culture plate, while RAW264.7 (1 × 10
4 cells/well) cells were seeded in the transwell insert for incubation at 37 °C for 24 h. A 1000 µL pipette tip was used to create a gap in the BMSC culture layer. The culture medium was removed, and treatment mediums (
Table 2) were added to both the transwell and insert. Pictures were taken before and after treatment under a microscope (OLYMPUS CKX53, Tokyo, Japan). Image J (National Institutes of Health, Bethesda, MD, USA) was used to measure the gap area. There were three biological and technical replicates each.
2.11. Statistical Analyses
GraphPad Prism 8 was used for all statistical analyses. All the results are presented as means ± SEM. One-way analysis of variance (ANOVA) with Tukey’s test was used to compare multiple values from different groups. p values less than 0.05 were considered significant. Pearson correlation coefficients were used for correlation analysis.
4. Discussion
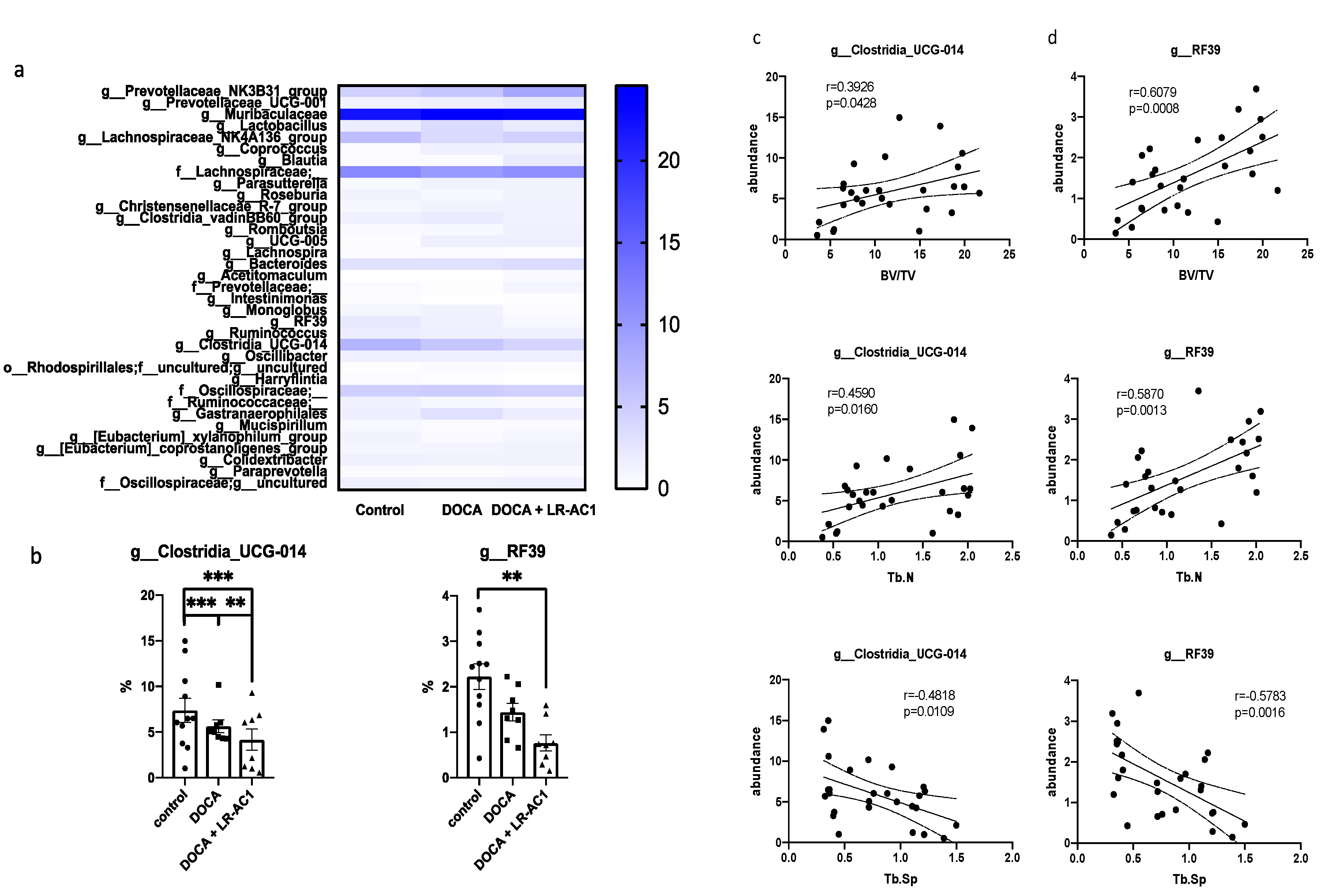
In this study, DOCA-salt treatment resulted in bone loss characterized by a reduction in trabecular bone, a decrease in osteoblasts, and impaired angiogenesis. LR-AC1 CCS increased the expression of osteoclastogenic and angiogenic markers, suggesting that LR-AC1 can enhance bone resorption and induce angiogenesis in vitro. The interaction between LR-AC1 CCS treatment on RAW264.7 cells and DOCA treatment on BMSCs inhibited BMSC migration. In vivo, live LR-AC1 aggravated bone loss in rats with DOCA salt induced osteoporosis by reducing osteogenesis and inhibiting angiogenesis but had no effect on healthy rats. Changes in gut microbiota were associated with DOCA-salt-induced bone loss, with the relative abundances of the genera g_RF39 and g_Clostridia_UCG-014 showing trends consistent with bone loss and correlating with the bone parameters.
In this study, we demonstrated that DOCA salt induced both osteoporosis and hypertension. DOCA, a glucocorticoid, is associated with significant adverse effects, including bone loss. The possible mechanisms through which glucocorticoids induce osteoporosis are attributed to promoting osteoblast and osteocyte apoptosis, altering the gut microbiota composition, and undermining the intestinal barrier [
18]. Glucocorticoids reduce bone mass by affecting bone formation and bone resorption and calcium deficiency. Furthermore, glucocorticoids exert stress on the endocrine system, leading to irregular hormone regulation, including decreased osteoblast synthesis, reduced androgen secretion, and diminished growth hormone levels, all of which inhibit bone formation [
19]. Our findings indicate that DOCA salt treatment reduced the expression of osterix, impacting osteoblast differentiation and consequently inhibiting bone formation. Additionally, glucocorticoids increase parathyroid hormone secretion and enhance osteoclast activity, further promoting bone resorption [
19]. We performed TRAP staining to investigate the effects of DOCA salt treatment on osteoclasts; however, the results were inconclusive. Calcium absorption in the gut could be affected by glucocorticoid treatment, which leads to calcium deficiency. Since calcium absorption in the gut was not measured in this study, the impact of DOCA on calcium absorption remains unknown. Based on previously published findings, steroid-induced osteoporosis could potentially be treated by modulating bone metabolism and supplementing calcium [
19].
Supplementation with probiotics, including
Lactobacillus spp., is considered a preventive strategy or even therapeutic option for osteoporosis. The
Lactobacillus spp. used in this study was a strain of
L. rhamnosus. Previous studies demonstrated that certain strains of
L. rhamnosus can attenuate osteoporosis. For example, Leena Sapra et al. showed that treatment with
L. rhamnosus enhanced the expression of anti-osteoclastogenic cytokines while reducing the expression of osteoclastogenic cytokines in an ovariectomy (OVX)-induced mouse model of osteoporosis. Additionally, in a postmenopausal mouse model, bone loss was mitigated by
L. rhamnosus, as observed following various analyses. The inhibition of osteoclastogenesis by
L. rhamnosus also influenced the differentiation of Treg-Th17 cells. Furthermore,
L. rhamnosus treatment reduced the percentage of osteoclastogenic CD4
+Rorγt
+Th17 cells and increased the percentage of anti-osteoclastogenic CD4
+Foxp3
+Tregs and CD8
+Foxp3
+Tregs at distinct immunological sites [
20]. Jau-Yi et al. utilized gonadotropin-releasing hormone (GnRH) agonists, specifically Lupron Depot, to induce a sex-steroid-deficiency osteoporosis model in both conventional and germ-free mice. The researchers reported that this induction led to increased gut permeability and promoted the expression of osteoclastogenic cytokines. Gut microbiota appeared to be an important factor driving bone loss in their study. Interestingly, another
L. rhamnosus strain GG (LGG) was shown to enhance gut barrier integrity, thereby protecting bone from sex steroid deficiency [
21]. Zhou’s group further observed that LGG had a positive effect on tenofovir disoproxil fumarate (TDF)-induced bone loss, demonstrating that LGG could modulate the gut microbiota, improve intestinal integrity, and inhibit the inflammatory response associated with TDF treatment [
22]. Overall,
L. rhamnosus has been shown to exert beneficial effects on various types of osteoporosis.
Similarly to previous studies involving other
L. rhamnosus strains, this study utilized
L. rhamnosus strain AC1, isolated in Hong Kong, for the treatment of osteoporosis, followed by micro-CT analysis and H&E staining to observe the bone phenotype. In contrast to the work of Leena Sapra et al., which focused on both cortical and trabecular bone in lumbar vertebrae-5, the tibia, and the femur, our study concentrated solely on the trabecular bone of the tibia [
20]. Unexpectedly, LR-AC1 not only failed to improve bone loss induced by DOCA but also aggravated bone loss. Unlike the positive effects reported in previous studies using other
L. rhamnosus species in OVX-, sex-steroid-deficiency-, and TDF-induced osteoporosis models, LR-AC1 appeared to negatively impact DOCA-induced osteoporosis [
20,
21,
22]. Various studies have demonstrated that probiotic supplementation has positive effects on bone loss induced by OVX surgery. However, some findings suggest that these effects may be strain-specific, for instance, treatment with
L. plantarum AR237,
L. paracasei, or a mixture of
Lactobacillus species showed no effect on femur bone mineral density (BMD) [
23,
24]. We conducted staining of bone tissues and in cellulo experiments to investigate osteoclastogenesis and osteoblastogenesis. Both sets of results suggested that LR-AC1 treatment promoted osteoclastogenesis, although its effect on osteoblastogenesis remains undetermined. As angiogenesis plays a critical role in bone regeneration, we also examined the status of osteoblastogenesis and angiogenesis instead of osteoclastogenesis in our samples. Coupling of angiogenesis and osteogenesis contributes to bone development and bone repair [
25]. Type H vessels, which are in proximity to the growth plate, exhibit high levels of expression of CD31
+ and regulate the proliferation and differentiation of osteoblasts and osteoclasts. Our study revealed a reduction in CD31
+ expression following osteoporosis induction with DOCA, which was further diminished by live LR-AC1 treatment. Intriguingly, when RAW264.7 cells were induced to differentiate into osteoclast, the LR-AC1 CCS treatment increased the expression of PDGF, indicating that LR-AC1 CCS may promote angiogenesis. PDGF-BB, which is secreted by preosteoclasts, contributes to the migration and differentiation of mesenchymal stem cells and endothelial progenitor cells, promoting angiogenesis and osteogenesis. It has been established that glucocorticoids inhibit PDGF-BB secretion, which suppresses angiogenesis and reduces osteogenesis [
26]. The results of our wound healing assay indicated that the interaction between DOCA and LR-AC1 CCS suppressed the migration of BMSCs in a co-culture with RAW264.7 cells. Live LR-AC1 treatment in rats with DOCA salt-induced osteoporosis might impair the migration of BMSCs which, in turn, suppresses angiogenesis and eventually aggravates bone loss.
We also explored the effect of LR-AC1 treatment on the alteration of gut microbiota. In this study,
Clostridia-UCG-014 exhibited a significant positive association with the phenotype of bone loss. A previous study demonstrated that
Clostridia-UCG-014 is an important genus associated with tryptophan metabolism [
27]. Two pathways of tryptophan metabolism, namely the kynurenine pathway and serotonin pathway, have been reported to be associated with bone biology [
28]. The kynurenine pathway has complex effects on osteoblastogenesis. For instance, 3-hydroxykynurenine (3-HKYN), a known metabolite from the kynurenine pathway, reduces the activity of osteoblasts. On the other hand, the end products of this pathway, such as 3-hydroxyAA, xanthurenic acid, picolinic acid, quinolinic acid, and NAD
+, play positive roles in bone formation. Serotonin also has varying impacts on osteogenesis [
29,
30]. Gut-derived serotonin inhibits bone formation by reducing osteoblastogenesis and increasing bone resorption. However, brain-derived serotonin enhances bone formation and inhibits bone resorption, positively affecting bone mass [
31]. In our results, the relative abundance of
Clostridia-UCG-014 followed a trend consistent with bone loss. Therefore, treatment with live LR-AC1 in DOCA-salt-induced osteoporosis altered the composition of the gut microbiota and reduced the relative abundance of
Clostridia-UCG-014, which may, in turn, have affected tryptophan metabolism and disturbed bone homeostasis.
A culture supernatant of LGG was utilized to improve calcium deficiency by promoting the intestinal absorption of vitamin D in senile osteoporosis. The LGG supernatant upregulated vitamin D transporters, enhancing the absorption of cholecalciferol in the intestine and increasing the levels of 25OHD3 in vivo and in vitro [
32]. Although vitamin D absorption was not measured in this study, the results indicated that LR-AC1 treatment aggravated bone loss, suggesting LR-AC1 has limited or no effect on bone loss, regardless of calcium deficiency. Overall, these findings suggest that bone formation and resorption may be the critical factors in osteoporosis in our study.
Several considerations and limitations of this study should be acknowledged. First, this study was conducted exclusively in male rats using a DOCA-salt-induced osteoporosis model, and the observation period was limited to the duration of the intervention without extended follow-up assessment. The use of CCS may not perfectly reproduce the in-vivo metabolites of LR-AC1 in the rats. Additionally, the bone analysis focused specifically on the trabecular bone tissue of the tibia, and cortical bone parameters and other skeletal sites, including the spine and femur, were not evaluated in this investigation. Furthermore, the potential relationships between alive LR-AC1 administration and gut health parameters, particularly gut barrier integrity and its possible influence on bone metabolism, require additional investigation to better understand the mechanistic pathways involved. Last but not least, although this study identified associations between live LR-AC1 treatment and tryptophan metabolism, the detailed mechanisms underlying these metabolic changes and their subsequent effects on bone homeostasis remain to be fully elucidated in future research.