Dietary Modification with Food Order and Divided Carbohydrate Intake Improves Glycemic Excursions in Healthy Young Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects of Experiment
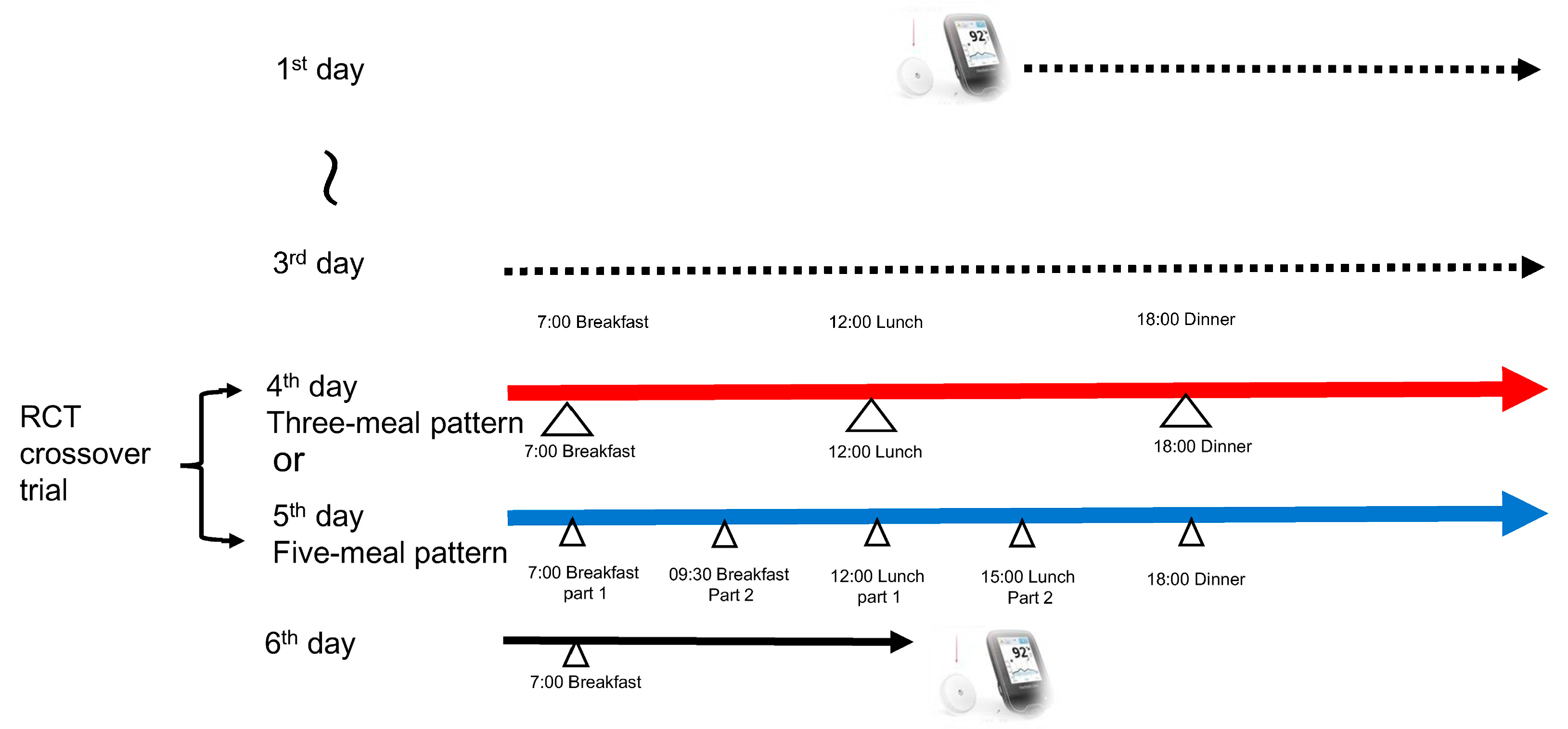
2.2. Study Design
- Breakfast (07:00–07:15): 120 g of white bread, a boiled egg, broccoli, tomato, strawberry jam (sugar-free), and milk
- Lunch (12:00–12:15): 200 g of boiled white rice, grilled salmon, tomato, boiled spinach, and sautéed-vegetable
- Dinner (18:00–18:15): 200 g of boiled white rice, hamburger steak, boiled spinach, tomato, sautéed-vegetable, and simmered turnip greens
- All meal components were consumed simultaneously within 15 min.
- Breakfast, Part 1 (07:00–07:15): 60 g of white bread, a boiled egg, broccoli, tomato, strawberry jam (sugar-free), and milk
- Breakfast, Part 2 (09:30–09:45): 60 g of white bread, tomato, broccoli, and strawberry jam (sugar-free)
- Lunch, Part 1 (12:00–12:15): 150 g of boiled white rice, grilled salmon, sautéed-vegetable, tomato, and boiled spinach
- Lunch, Part 2 (15:00–15:15): 100 g of boiled white rice, sautéed-vegetable, and tomato
- Dinner (18:00–18:15): 150 g of boiled white rice, hamburger steak, sautéed-vegetable, tomato, and simmered turnip greens
- All meal components were consumed the food order (vegetables → protein → carbohydrates) within 15 min.
2.3. Sample Size and Statistical Analysis
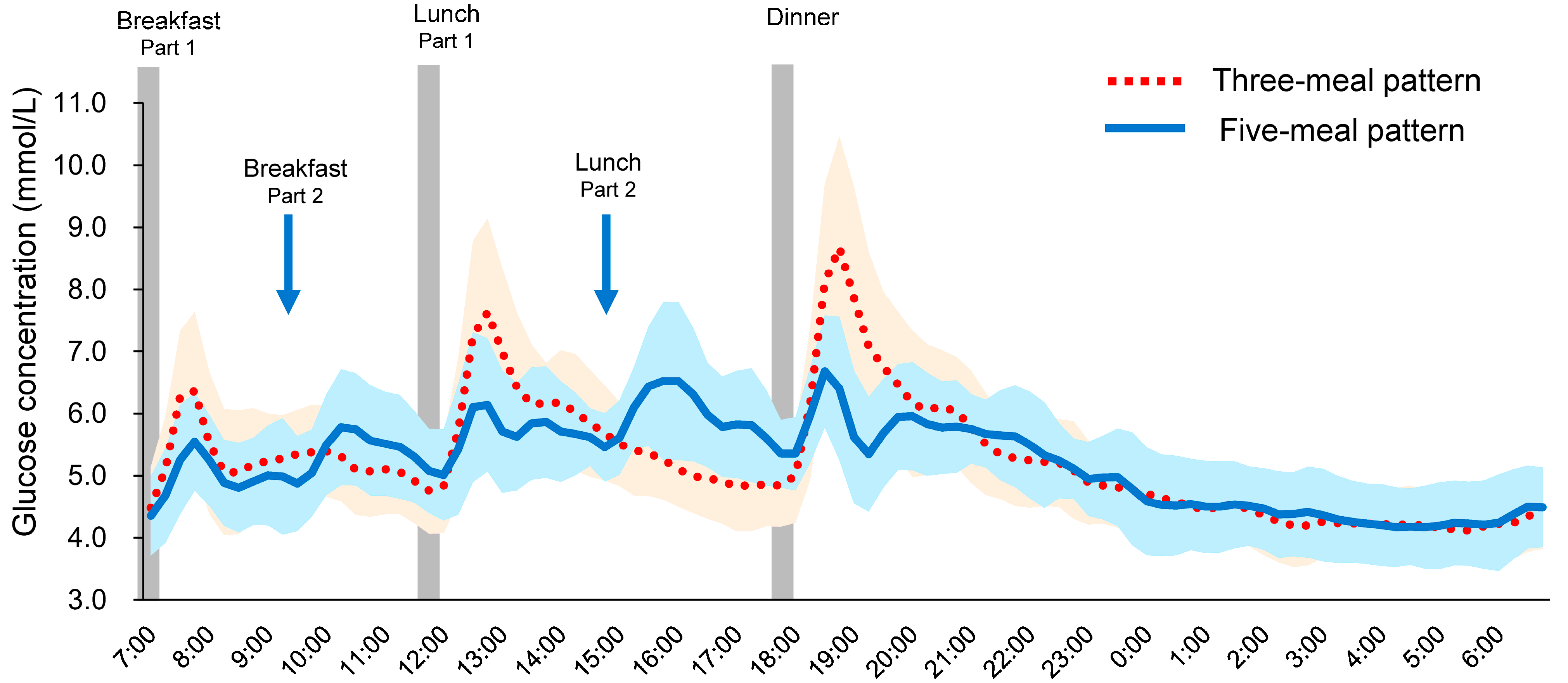
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibib, L.; Al-Qaisi, M.; Guess, N.; Miras, A.D.; Greenwald, S.E.; Pelling, M.; Ahmed, A. Manipulation of Post-Prandial Hyperglycaemia in Type 2 Diabetes: An Update for Practitioners. Diabetes Metab. Syndr. Obes. 2024, 17, 3111–3130. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S306–S320. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Society of Diabetes and Pregnancy. Revised 3rd Edition Glucose Metabolism Abnormalities in Pregnant Women Medical and Management Manual; Medical View Co., Ltd.: Tokyo, Japan, 2022; pp. 116–117. [Google Scholar]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef]
- Imai, S.; Fukui, M.; Kajiyama, S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J. Clin. Biochem. Nutr. 2014, 54, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.P.; Iliescu, R.G.; Thomas, C.E.; Aronne, L.J. Food Order Has a Significant Impact on Postprandial Glucose and Insulin Levels. Diabetes Care 2015, 38, e98–e99. [Google Scholar] [CrossRef]
- Nitta, A.; Imai, S.; Kajiayama, S.; Matsuda, M.; Miyawaki, T.; Matsumoto, S.; Kajiyama, S.; Hashimoto, Y.; Ozasa, N.; Fukui, M. Impact of Dietitian-Led Nutrition Therapy of Food Order on 5-Year Glycemic Control in Outpatients with Type 2 Diabetes at Primary Care Clinic: Retrospective Cohort Study. Nutrients 2022, 14, 2865. [Google Scholar] [CrossRef]
- Shukla, A.P.; Dickison, M.; Coughlin, N.; Karan, A.; Mauer, E.; Truong, W.; Casper, A.; Emiliano, A.B.; Kumar, R.B.; Saunders, K.H.; et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes. Metab. 2019, 21, 377–381. [Google Scholar] [CrossRef]
- Kubota, S.; Liu, Y.; Iizuka, K.; Kuwata, H.; Seino, Y.; Yabe, D. A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes. Nutrients 2020, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, S.; Imai, S.; Hashimoto, Y.; Yamane, C.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Divided consumption of late-night-dinner improves glucose excursions in young healthy women: A randomized cross-over clinical trial. Diabetes Res. Clin. Pract. 2018, 136, 78–84. [Google Scholar] [CrossRef]
- Imai, S.; Saito, Y.; Kajiyama, S.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, T.; Fukui, M. Late-night-dinner deteriorates postprandial glucose and insulin whereas consuming dinner dividedly ameliorates them in patients with type 2 diabetes: A randomized crossover clinical trial. Asia Pac. J. Clin. Nutr. 2020, 29, 68–76. [Google Scholar] [CrossRef]
- Imai, S.; Kajiyama, S.; Hashimoto, Y.; Nitta, A.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Tanaka, M.; Kajiyama, S.; Fukui, M. Consuming snacks mid-afternoon compared with just after lunch improves mean amplitude of glycaemic excursions in patients with type 2 diabetes: A randomized crossover clinical trial. Diabetes Metab. 2018, 44, 482–487. [Google Scholar] [CrossRef]
- Nitta, A.; Imai, S.; Kajiyama, S.; Miyawaki, T.; Matsumoto, S.; Ozasa, N.; Kajiyama, S.; Hashimoto, Y.; Tanaka, M.; Fukui, M. Impact of different timing of consuming sweet snack on postprandial glucose excursions in healthy women. Diabetes Metab. 2019, 45, 369–374. [Google Scholar] [CrossRef]
- Miller, V.; Jenkins, D.A.; Dehghan, M.; Srichaikul, K.; Rangarajan, S.; Mente, A.; Mohan, V.; Swaminathan, S.; Ismail, R.; Luz Diaz, M.; et al. Prospective Urban and Rural Epidemiology (PURE) study investigators. Associations of the glycaemic index and the glycaemic load with risk of type 2 diabetes in 127 594 people from 20 countries (PURE): A prospective cohort study. Lancet Diabetes Endocrinol. 2024, 12, 330–338. [Google Scholar] [CrossRef]
- Rodbard, D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol. Ther. 2009, 11, 551–565. [Google Scholar] [CrossRef]
- Dandona, P. Minimizing Glycemic Fluctuations in Patients with Type 2 Diabetes: Approaches and Importance. Diabetes Technol. Ther. 2017, 19, 498–506. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Pereira, M.A.; Ebbeling, C.B.; Aronica, L.; Ludwig, D.S. Evidence for the carbohydrate-insulin model in a reanalysis of the Diet Intervention Examining the Factors Interacting with Treatment Success (DIETFITS) trial. Am. J. Clin. Nutr. 2023, 117, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, S.; Muscelli, E.; Gastaldelli, A.; Barsotti, E.; Astiarraga, B.D.; Holst, J.J.; Mari, A.; Ferrannini, E. Improved tolerance to sequential glucose loading (Staub-Traugott effect): Size and mechanisms. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E532–E537. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Landau, Z.; Bar-Dayan, Y.; Froy, O. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: A randomized clinical trial. Diabetes Care 2015, 38, 1820–1826. [Google Scholar] [CrossRef]
- Lee, S.H.; Tura, A.; Mari, A.; Ko, S.H.; Kwon, H.S.; Song, K.H.; Yoon, K.H.; Lee, K.W.; Ahn, Y.B. Potentiation of the early-phase insulin response by a prior meal contributes to the second-meal phenomenon in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E984–E990. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Gardiner, J.; McBurney, M.I.; Anderson, S.; Weatherspoon, L.J.; Henry, D.N.; Hord, N.G. Effects of breakfast meal composition on second meal metabolic responses in adults with Type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2006, 60, 1122–1129. [Google Scholar] [CrossRef]
- Morris, C.J.; Garcia, J.I.; Myers, S.; Yang, J.N.; Trienekens, N.; Scheer, F.A. The Human Circadian System Has a Dominating Role in Causing the Morning/Evening Difference in Diet-Induced Thermogenesis. Obesity 2015, 23, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Almeda-Valdes, P.; Patterson, B.W.; Okunade, A.L.; Imai, S.; Mittendorfer, B.; Klein, S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J. Clin. Endocrinol. Metab. 2014, 99, E1666–E1670. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, O.; Mari, A.; Deacon, C.F.; Carr, R.D.; Winzell, M.S.; Vikman, J.; Ahrén, B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metab. 2009, 94, 2887–2892. [Google Scholar] [CrossRef]
- Morris, C.J.; Yang, J.N.; Scheer, F.A.J.L. The impact of the circadian timing system on cardiovascular and metabolic function. Prog. Brain Res. 2012, 199, 337–358. [Google Scholar] [CrossRef]
- Wong, J.M.; Jenkins, D.J. Carbohydrate digestibility and metabolic effects. J. Nutr. 2007, 137, 2539S–2546S. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef]
- Kuwata, H.; Iwasaki, M.; Shimizu, S.; Minami, K.; Maeda, H.; Seino, S.; Nakada, K.; Nosaka, C.; Murotani, K.; Kurose, T.; et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia 2016, 59, 453–461. [Google Scholar] [CrossRef]
- Nauck, M.A.; Müller, T.D. Incretin hormones and type 2 diabetes. Diabetologia 2023, 66, 1780–1795. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–924. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.T.; Wan, S.R.; Ma, X.M.; Long, Y.; Xu, Y.; Jiang, Z.Z. The role of oxidative stress in diabetes mellitus-induced vascular endothelial dysfunction. Cardiovasc. Diabetol. 2023, 22, 237. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.E.B.F.; Moreira, N.C.S.; Sakamoto-Hojo, E.T. Mechanisms underlying the pathophysiology of type 2 diabetes: From risk factors to oxidative stress, metabolic dysfunction, and hyperglycemia. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2022, 874–875, 503437. [Google Scholar] [CrossRef] [PubMed]
| Three-Meal Pattern | Five-Meal Pattern | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) | Fiber (g) | Detail Content | Energy (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) | Fiber (g) | Detail Content | ||
| Breakfast | 568 | 28.2 | 19.2 | 84.2 | 9.1 | White bread (120 g), tomato, broccoli, a boiled egg, milk, strawberry jam (sugar free) | Breakfast Part 1 | 385 | 21.1 | 16.5 | 47.0 | 4.6 | White bread (60 g), tomato, broccoli, milk, a boiled egg, strawberry jam (sugar free) |
| Breakfast Part 2 | 183 | 7.1 | 2.7 | 37.2 | 4.5 | White bread (60 g), tomato, broccoli, strawberry jam (sugar free) | |||||||
| Lunch | 529 | 22.0 | 9.9 | 93.5 | 8.6 | Boiled white rice (200 g), grilled salmon, sautéed-vegetable, tomato, boiled spinach | Lunch Part 1 | 426 | 20.0 | 9.3 | 69.7 | 7.0 | Boiled white rice (150 g), grilled salmon, sautéed-vegetable, tomato, boiled spinach |
| Lunch Part 2 | 205 | 4.0 | 1.0 | 47.3 | 3.3 | Boiled white rice (100 g), sautéed-vegetable, tomato | |||||||
| Dinner | 725 | 27.6 | 23.6 | 106.8 | 9.5 | Boiled white rice (200 g), hamburger steak, sautéed-vegetable, tomato, simmered turnip greens | Dinner | 623 | 25.6 | 23.1 | 83.3 | 7.8 | Boiled white rice (150 g), hamburger steak, sautéed-vegetable, tomato, simmered turnip greens |
| Total | 1822 | 77.8 | 52.6 | 284.5 | 27.2 | Total | 1822 | 77.8 | 52.6 | 284.5 | 27.2 | ||
| Three-Meal Pattern | Five-Meal Pattern | |
|---|---|---|
| MBG (mmol/L) | 5.25 ± 0.14 | 5.20 ± 0.14 |
| SD (mmol/L) | 1.07 ± 0.08 | 0.84 ± 0.04 **† |
| MAGE (mmol/L) | 3.49 ± 0.32 | 2.56 ± 0.13 **† |
| MAX (mmol/L) | 8.96 ± 0.40 | 7.35 ± 0.25 ***†† |
| MIN (mmol/L) | 3.85 ± 0.15 | 3.92 ± 0.17 |
| LAGE (mmol/L) | 5.11 ± 0.41 | 3.43 ± 0.18 ***†† |
| %CV (%) | 1.14 ± 0.09 | 0.91 ± 0.06 ***† |
| Breakfast GP (mmol/L) | 6.60 ± 0.27 | 5.88 ± 0.19 **† |
| Lunch GP (mmol/L) | 7.92 ± 0.32 | 6.68 ± 0.21 ***†† |
| Dinner GP (mmol/L) | 8.93 ± 0.40 | 7.04 ± 0.20 ***††† |
| Breakfast | ||
| IAUC 60 min (mmol/L × min) | 72 ± 8 | 44 ± 6 **†† |
| IAUC 120 min (mmol/L × min) | 115 ± 13 | 80 ± 9 **† |
| IAUC 180 min (mmol/L × min) | 167 ± 17 | 125 ± 13 * |
| IAUC 240 min (mmol/L × min) | 211 ± 18 | 200 ± 16 |
| IAUC 300 min (mmol/L × min) | 241 ± 21 | 257 ± 21 |
| Lunch | ||
| IAUC 60 min (mmol/L × min) | 108 ± 13 | 46 ± 7 ***††† |
| IAUC 120 min (mmol/L × min) | 198 ± 24 | 93 ± 14 ***†† |
| IAUC 180 min (mmol/L × min) | 259 ± 25 | 134 ± 18 ***††† |
| IAUC 240 min (mmol/L × min) | 290 ± 25 | 212 ± 28 ** |
| IAUC 300 min (mmol/L × min) | 303 ± 27 | 275 ± 36 |
| Dinner | ||
| IAUC 60 min (mmol/L × min) | 141 ± 14 | 50 ± 7 ***††† |
| IAUC 120 min (mmol/L × min) | 250 ± 29 | 79 ± 12 ***††† |
| IAUC 180 min (mmol/L × min) | 314 ± 35 | 114 ± 19 ***††† |
| IAUC 240 min (mmol/L × min) | 344 ± 36 | 138 ± 20 ***††† |
| IAUC 300 min (mmol/L × min) | 361 ± 37 | 148 ± 24 ***††† |
| 24 h IAUC (mmol/L × min) | 1236 ± 97 | 1319 ± 99 |
| TIR (%) 3.9–7.8 mmol/L | 86.9 ± 4.6 | 90.3 ± 3.6 |
| TBR (%) < 3.9 mmol/L | 8.9 ± 4.7 | 8.3 ± 3.7 |
| TAR (%) > 7.8 mmol/L | 4.2 ± 1.0 | 1.4 ± 0.6 **† |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, Y.; Miyawaki, T.; Kajiyama, S.; Kitta, K.; Kajiyama, S.; Hashimoto, Y.; Fukui, M.; Imai, S. Dietary Modification with Food Order and Divided Carbohydrate Intake Improves Glycemic Excursions in Healthy Young Women. Nutrients 2025, 17, 3194. https://doi.org/10.3390/nu17203194
Higuchi Y, Miyawaki T, Kajiyama S, Kitta K, Kajiyama S, Hashimoto Y, Fukui M, Imai S. Dietary Modification with Food Order and Divided Carbohydrate Intake Improves Glycemic Excursions in Healthy Young Women. Nutrients. 2025; 17(20):3194. https://doi.org/10.3390/nu17203194
Chicago/Turabian StyleHiguchi, Yuki, Takashi Miyawaki, Shizuo Kajiyama, Kaoru Kitta, Shintaro Kajiyama, Yoshitaka Hashimoto, Michiaki Fukui, and Saeko Imai. 2025. "Dietary Modification with Food Order and Divided Carbohydrate Intake Improves Glycemic Excursions in Healthy Young Women" Nutrients 17, no. 20: 3194. https://doi.org/10.3390/nu17203194
APA StyleHiguchi, Y., Miyawaki, T., Kajiyama, S., Kitta, K., Kajiyama, S., Hashimoto, Y., Fukui, M., & Imai, S. (2025). Dietary Modification with Food Order and Divided Carbohydrate Intake Improves Glycemic Excursions in Healthy Young Women. Nutrients, 17(20), 3194. https://doi.org/10.3390/nu17203194









