Egg Consumption and Mortality: A Prospective Cohort Study of Australian Community-Dwelling Older Adults
Abstract
1. Introduction
2. Materials and Methods
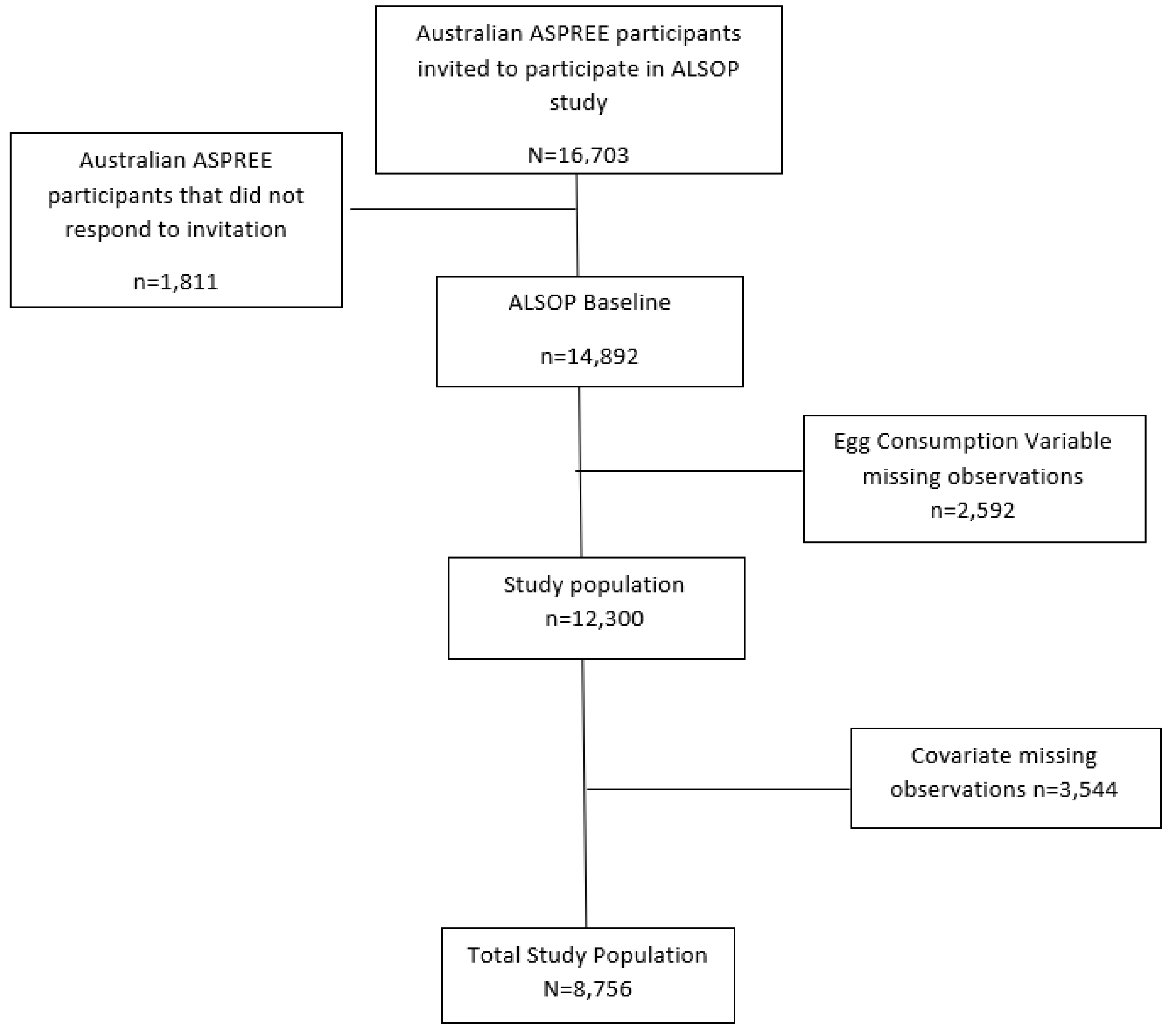
2.1. Study Population
2.2. Exposure Variable: Egg Consumption
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
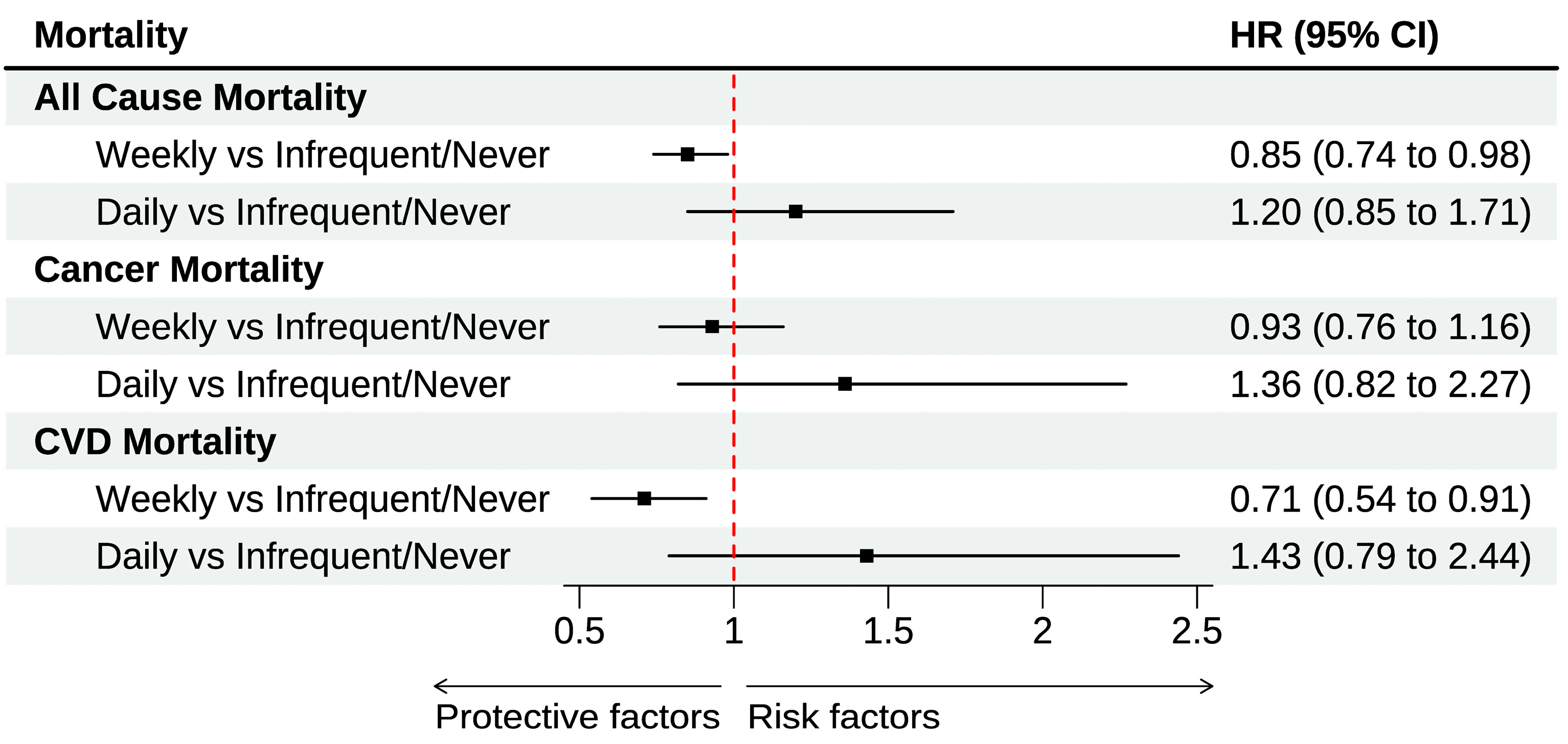
3.2. Egg Consumption and All-Cause and Cause-Specific Mortality
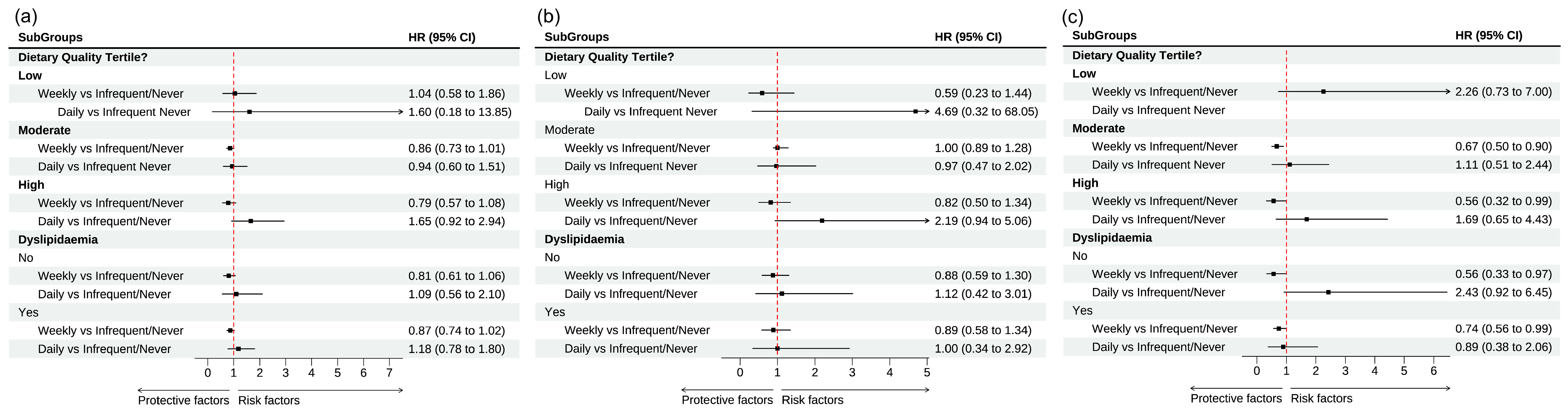
3.3. Egg Consumption and All-Cause and Cause-Specific Mortality: Subgroup Analysis
3.4. Sensitivity Analysis: Competing Risk Analysis
4. Discussion
4.1. Possible Implications for Dietary Guidelines
4.2. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Ageing and Health; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Coelho-Junior, H.J.; Calvani, R.; Azzolino, D.; Picca, A.; Tosato, M.; Landi, F.; Cesari, M.; Marzetti, E. Protein Intake and Sarcopenia in Older Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8718. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Júnior, H.J.; Calvani, R.; Tosato, M.; Landi, F.; Picca, A.; Marzetti, E. Protein Intake and Physical Function in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 81, 101731. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H.; Meguro, S.; Abe, Y.; Sasaki, T.; Asakura, K.; Arai, Y.; Itoh, H. Dietary Protein Intake and All-Cause Mortality: Results from The Kawasaki Aging and Wellbeing Project. BMC Geriatr. 2023, 23, 479. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. High Protein Intake Is Associated with Lower Risk of All-Cause Mortality in Community-Dwelling Chinese Older Men and Women. J. Nutr. Health Aging 2019, 23, 987–996. [Google Scholar] [CrossRef]
- Appleton, K.M. Barriers to and Facilitators of the Consumption of Animal-Based Protein-Rich Foods in Older Adults. Nutrients 2016, 8, 187. [Google Scholar] [CrossRef]
- Best, R.L.; Appleton, K.M. The Consumption of Protein-Rich Foods in Older Adults: An Exploratory Focus Group Study. J. Nutr. Educ. Behav. 2013, 45, 751–755. [Google Scholar] [CrossRef]
- Van den Heuvel, E.; Murphy, J.L.; Appleton, K.M. Towards a Food-Based Intervention to Increase Protein Intakes in Older Adults: Challenges to and Facilitators of Egg Consumption. Nutrients 2018, 10, 1409. [Google Scholar] [CrossRef]
- Drewnowski, A. The Nutrient Rich Foods Index Helps to Identify Healthy, Affordable Foods. Am. J. Clin. Nutr. 2010, 91, 1095S–1101S. [Google Scholar] [CrossRef]
- Rousset, S.; Jolivet, P. Discrepancy between the Expected and Actual Acceptability of Meat Products, Eggs and Fish: The Case of Older Consumers. J. Sens. Stud. 2002, 17, 61–75. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Andersen, C.J. Bioactive Egg Components and Inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gan, L.; Graubard, B.I.; Männistö, S.; Albanes, D.; Huang, J. Associations of Dietary Cholesterol, Serum Cholesterol, and Egg Consumption with Overall and Cause-Specific Mortality: Systematic Review and Updated Meta-Analysis. Circulation 2022, 145, 1506–1520. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K. What Eggsactly Are We Asking Here? Unscrambling the Epidemiology of Eggs, Cholesterol, and Mortality. Circulation 2022, 145, 1521–1523. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.-P.; Chen, S.; Li, Y.; Schwab, A.L.; Stampfer, M.J.; Sacks, F.M.; Rosner, B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Egg Consumption and Risk of Cardiovascular Disease: Three Large Prospective US Cohort Studies, Systematic Review, and Updated Meta-Analysis. BMJ 2020, 368, m513. [Google Scholar] [CrossRef]
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.M.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Horn, L.V. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory from the American Heart Association. Circulation 2020, 141, e39–e53. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Zhou, X.; Li, L. Egg Consumption and Risk of Cardiovascular Diseases and Diabetes: A Meta-Analysis. Atherosclerosis 2013, 229, 524–530. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Y.; Pan, L.; Wang, S.; Xie, K.; Deng, S.; Wang, R.; Guo, C.; Qin, P.; Wu, X.; et al. Association of Egg Consumption with Risk of All-Cause and Cardiovascular Disease Mortality: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. J. Nutr. 2022, 152, 2227–2237. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zargarzadeh, N.; Rigi, S.; Persad, E.; Pizarro, A.B.; Hasani-Ranjbar, S.; Larijani, B.; Willett, W.C.; Esmaillzadeh, A. Egg Consumption and Risk of All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2022, 13, 1762–1773. [Google Scholar] [CrossRef]
- Chen, G.-C.; Chen, L.-H.; Mossavar-Rahmani, Y.; Kamensky, V.; Shadyab, A.H.; Haring, B.; Wild, R.A.; Silver, B.; Kuller, L.H.; Sun, Y.; et al. Dietary Cholesterol and Egg Intake in Relation to Incident Cardiovascular Disease and All-Cause and Cause-Specific Mortality in Postmenopausal Women. Am. J. Clin. Nutr. 2021, 113, 948–959. [Google Scholar] [CrossRef]
- Zhuang, P.; Wu, F.; Mao, L.; Zhu, F.; Zhang, Y.; Chen, X.; Jiao, J.; Zhang, Y. Egg and Cholesterol Consumption and Mortality from Cardiovascular and Different Causes in the United States: A Population-Based Cohort Study. PLoS Med. 2021, 18, e1003508. [Google Scholar] [CrossRef]
- ASPREE Investigator Group. Study Design of ASPirin in Reducing Events in the Elderly (ASPREE): A Randomized, Controlled Trial. Contemp. Clin. Trials 2013, 36, 555–564. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef]
- McNeil, J.J.; Woods, R.L.; Ward, S.A.; Britt, C.J.; Lockery, J.E.; Beilin, L.J.; Owen, A.J. Cohort Profile: The ASPREE Longitudinal Study of Older Persons (ALSOP). Int. J. Epidemiol. 2019, 48, 1048–1049h. [Google Scholar] [CrossRef]
- Ernst, M.E.; Broder, J.C.; Wolfe, R.; Woods, R.L.; Nelson, M.R.; Ryan, J.; Shah, R.C.; Orchard, S.G.; Chan, A.T.; Espinoza, S.E.; et al. Health Characteristics and Aspirin Use in Participants at the Baseline of the ASPirin in Reducing Events in the Elderly—eXTension (ASPREE-XT) Observational Study. Contemp. Clin. Trials 2023, 130, 107231. [Google Scholar] [CrossRef]
- PROPOSAL P301; Production and Processing Standard for Egg and Egg Products—Overview of the Egg and Egg Product Industry in Australia. Food Standards Australia and New Zealand Primary: Wellington, New Zealand, 2009.
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. JNCI J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef]
- The Ryeson Index: Homepage. Available online: https://ryersonindex.org/ (accessed on 10 December 2024).
- 2033.0.55.001; Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA). Australian Bureau of Statistics: Canberra, Australia, 2011.
- NHMRC. Australian Guidelines to Reduce Health Risks from Drinking Alcohol; NHMRC: Canberra, Australia, 2020. [Google Scholar]
- Neumann, J.T.; Freak-Poli, R.; Orchard, S.G.; Wolfe, R.; Reid, C.M.; Tonkin, A.M.; Beilin, L.J.; McNeil, J.J.; Ryan, J.; Woods, R.L. Alcohol Consumption and Risks of Cardiovascular Disease and All-Cause Mortality in Healthy Older Adults. Eur. J. Prev. Cardiol. 2022, 29, e230–e232. [Google Scholar] [CrossRef]
- Govindaraju, T. Exploring Associations Between Diet and Quality of Life (QoL) Among Older Adults in the Context of Healthy Ageing. Ph.D. Thesis, Monash University, Clayton, Australia, 2023. [Google Scholar]
- Ryan, J.; Espinoza, S.; Ernst, M.E.; Ekram, A.R.M.S.; Wolfe, R.; Murray, A.M.; Shah, R.C.; Orchard, S.G.; Fitzgerald, S.; Beilin, L.J.; et al. Validation of a Deficit-Accumulation Frailty Index in the ASPirin in Reducing Events in the Elderly Study and Its Predictive Capacity for Disability-Free Survival. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 19–26. [Google Scholar] [CrossRef]
- Radloff, L.S. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. APM 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Ruggiero, E.; Di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; De Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M.; et al. Egg Consumption and Risk of All-Cause and Cause-Specific Mortality in an Italian Adult Population. Eur. J. Nutr. 2021, 60, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Kouvari, M.; Damigou, E.; Florentin, M.; Kosti, R.I.; Chrysohoou, C.; Pitsavos, C.S.; Panagiotakos, D.B. Egg Consumption, Cardiovascular Disease and Cardiometabolic Risk Factors: The Interaction with Saturated Fatty Acids. Results from the ATTICA Cohort Study (2002–2012). Nutrients 2022, 14, 5291. [Google Scholar] [CrossRef] [PubMed]
- StataCorp. Stata Statistical Software: Release 18; StataCorp LLC.: College Station, TX, USA, 2023. [Google Scholar]
- Wang, K.; Xiang, Q.; Hu, L.; Wang, L.; Zhang, Y. Frequency of Egg Intake Associated with Mortality in Chinese Adults: An 8-Year Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 14777. [Google Scholar] [CrossRef]
- Xu, L.; Lam, T.H.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Woo, J.; Cheng, K.K.; Thomas, G.N. Egg Consumption and the Risk of Cardiovascular Disease and All-Cause Mortality: Guangzhou Biobank Cohort Study and Meta-Analyses. Eur. J. Nutr. 2019, 58, 785–796. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Pencina, M.J.; Banach, M. Egg Consumption and Risk of Total and Cause-Specific Mortality: An Individual-Based Cohort Study and Pooling Prospective Studies on Behalf of the Lipid and Blood Pressure Meta-Analysis Collaboration (LBPMC) Group. J. Am. Coll. Nutr. 2019, 38, 552–563. [Google Scholar] [CrossRef]
- Darooghegi Mofrad, M.; Naghshi, S.; Lotfi, K.; Beyene, J.; Hypponen, E.; Pirouzi, A.; Sadeghi, O. Egg and Dietary Cholesterol Intake and Risk of All-Cause, Cardiovascular, and Cancer Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2022, 9, 878979. [Google Scholar] [CrossRef]
- Godos, J.; Micek, A.; Brzostek, T.; Toledo, E.; Iacoviello, L.; Astrup, A.; Franco, O.H.; Galvano, F.; Martinez-Gonzalez, M.A.; Grosso, G. Egg Consumption and Cardiovascular Risk: A Dose–Response Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2021, 60, 1833–1862. [Google Scholar] [CrossRef]
- Yang, P.-F.; Wang, C.-R.; Hao, F.-B.; Peng, Y.; Wu, J.-J.; Sun, W.-P.; Hu, J.-J.; Zhong, G.-C. Egg Consumption and Risks of All-Cause and Cause-Specific Mortality: A Dose–Response Meta-Analysis of Prospective Cohort Studies. Nutr. Rev. 2022, 80, 1739–1754. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.B.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Rosner, B.A.; Spiegelman, D.; Speizer, F.E.; Sacks, F.M.; et al. A Prospective Study of Egg Consumption and Risk of Cardiovascular Disease in Men and Women. JAMA 1999, 281, 1387–1394. [Google Scholar] [CrossRef]
- Xia, P.-F.; Pan, X.-F.; Chen, C.; Wang, Y.; Ye, Y.; Pan, A. Dietary Intakes of Eggs and Cholesterol in Relation to All-Cause and Heart Disease Mortality: A Prospective Cohort Study. J. Am. Heart Assoc. 2020, 9, e015743. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Fulgoni, V.L., III. Patterns of Egg Consumption Can Help Contribute to Nutrient Recommendations and Are Associated with Diet Quality and Shortfall Nutrient Intakes. Nutrients 2021, 13, 4094. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Zaw, J.J.T.; Wong, R.H.; Howe, P.R. LDL Cholesterol May Have Protective Properties for Brain Health in Older Age: Do We Need to Re-Think Current Guidelines? Alzheimer’s Dement. 2020, 16, e044364. [Google Scholar] [CrossRef]
- Richard, C.; Cristall, L.; Fleming, E.; Lewis, E.D.; Ricupero, M.; Jacobs, R.L.; Field, C.J. Impact of Egg Consumption on Cardiovascular Risk Factors in Individuals with Type 2 Diabetes and at Risk for Developing Diabetes: A Systematic Review of Randomized Nutritional Intervention Studies. Can. J. Diabetes 2017, 41, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Rouhani, M.H.; Rashidi-Pourfard, N.; Salehi-Abargouei, A.; Karimi, M.; Haghighatdoost, F. Effects of Egg Consumption on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Am. Coll. Nutr. 2018, 37, 99–110. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V. Omega-3 Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar]
- Mine, Y. Egg Proteins and Peptides in Human Health-Chemistry, Bioactivity and Production. Curr. Pharm. Des. 2007, 13, 875–884. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, M.; Yue, C.; Shi, Y.; Tan, Y.; Zha, L.; Zhang, J.; Chen, S. Association between Dietary Choline Intake and Cardiovascular Diseases: National Health and Nutrition Examination Survey 2011–2016. Nutrients 2023, 15, 4036. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australina Dietary Guidlines; National Health and Medical Research Council: Canberra, Australia, 2013. [Google Scholar]
- Chinese Nutrition Society. Dietary Guidelines for Chinese Residents 2016; Chinese Nutrition Society: Beijing, China, 2016. [Google Scholar]
- European Commission. European Commission Knowledge for Policy: Health Promotion and Disease Prevention Knowledge Gateway-Food-Based Dietary Guidelines in Europe: Summary of FBDG Recommendations for Eggs for the the EU, Iceland, Norway, Switzerland and the United Kingdom; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Sugihara, N.; Shirai, Y.; Imai, T.; Sezaki, A.; Abe, C.; Kawase, F.; Miyamoto, K.; Inden, A.; Kato, T.; Sanada, M.; et al. The Global Association between Egg Intake and the Incidence and Mortality of Ischemic Heart Disease—An Ecological Study. Int. J. Environ. Res. Public Health 2023, 20, 4138. [Google Scholar] [CrossRef]

| Covariates n (%) | Total (N = 8756) | Never/Infrequent Egg Consumption (n = 2119) 24.2% | Weekly Egg Consumption (n = 6414) 73.2% | Daily Egg Consumption (n = 223) 2.6% | |
|---|---|---|---|---|---|
| Sex | Female | 4725 (54.0) | 1102 (52.0) | 3515 (54.8) | 108 (48.4) |
| Age Median (IQR) | 76.9 (5.5) | 77.21 (5.7) | 76.8 (5.5) | 77.01 (5.5) | |
| IRSAD a | 1: Most Disadvantaged | 1335 (15.2) | 335 (16.0) | 966 (15.0) | 34 (15.2) |
| 2 | 1457 (16.6) | 361 (17.0) | 1054 (16.4) | 42 (18.8) | |
| 3 | 1605 (18.2) | 382 (18.0) | 1180 (18.4) | 43 (19.3) | |
| 4 | 1742 (20.0) | 418 (19.6) | 1280 (20.0) | 44 (19.7) | |
| 5: Least Disadvantaged | 2975 (30.0) | 623 (29.4) | 1934 (30.2) | 60 (27.0) | |
| Education | ≤12 years | 5057 (57.7) | 1282 (60.5) | 3659 (57.1) | 116 (52.0) |
| >12 years | 3699 (42.3) | 837 (39.5) | 2755 (42.9) | 107 (48.0) | |
| Physical Activity | Rarely/Never | 137 (1.5) | 46 (2.2) | 89 (1.4) | 2 (1.0) |
| Low to Moderate | 7432 (84.9) | 1824 (86.1) | 5429 (84.6) | 179 (80.2) | |
| Vigorous | 1187 (13.6) | 249 (11.7) | 896 (14.0) | 42 (18.8) | |
| Smoking | Never | 5770 (56.8) | 1180 (55.7) | 3669 (57.2) | 123 (55.2) |
| Former | 4197 (40.9) | 876 (41.3) | 2613 (40.7) | 94 (42.1) | |
| Current | 236 (2.3) | 63 (3.0) | 132 (2.1) | 6 (2.7) | |
| Alcohol | Non-Drinker | 2260 (25.2) | 596 (28.1) | 1561 (24.4) | 49 (22.0) |
| Within Guidelines | 3438 (39.3) | 766 (36.1) | 2587 (40.3) | 85 (38.1) | |
| Exceeds Guidelines | 3112 (35.5) | 757 (35.8) | 2266 (35.3) | 89 (39.9) | |
| Waist Circumference, cm [Mean (SD)] | 96 (12.6) | 95.9 (12.7) | 96.0 (12.7) | 97.9 (11.2) | |
| Hypertension | Yes | 7588 (86.7) | 1846 (87.1) | 5555 (86.6) | 187 (83.9) |
| Diabetes | Yes | 1005 (11.5) | 240 (11.3) | 737 (11.5) | 28 (12.6) |
| Dyslipidemia | Yes | 7060 (80.6) | 1691 (79.8) | 5198 (81.0) | 171 (76.7) |
| Polypharmacy | Yes | 2014 (23.0) | 505 (23.8) | 1458 (22.7) | 51 (22.9) |
| Frailty | Non-frail | 4573 (52.2) | 1069 (50.4) | 3392 (52.9) | 112 (50.2) |
| Score | Pre-Frail | 3280 (37.5) | 805 (38.0) | 3292 (37.3) | 83 (37.2) |
| Frail | 903 (10.3) | 245 (11.6) | 630 (9.8) | 28 (12.6) | |
| Self-reported | Poor | 83 (1.0) | 21 (1.0) | 62 (1.0) | 0 (0) |
| Oral Health | Fair/good | 3768 (43.0) | 934 (44.1) | 2730 (42.5) | 104 (46.6) |
| V. good/excellent | 4905 (56.0) | 1164 (54.9) | 4156 (56.5) | 119 (53.4) | |
| Depression b | None | 3705 (42.3) | 899 (42.4) | 2708 (42.2) | 98 (43.9) |
| Mild | 3677 (42.0) | 876 (41.3) | 2710 (24.3) | 91 (40.8) | |
| Moderate | 1374 (15.7) | 344 (16.3) | 996 (15.5) | 34 (15.3) | |
| Diet | T1—Low | 319 (3.6) | 119 (5.6) | 197 (3.1) | 3 (1.3) |
| Score | T2—Moderate | 5989 (68.4) | 1572 (74.2) | 4277 (66.7) | 140 (62.8) |
| T3—High | 2448 (28.0) | 428 (20.2) | 1940 (30.2) | 80 (35.9) | |
| Treatment Arm | |||||
| Placebo | 4355 (49.7) | 1040 (49.1) | 3192 (49.8) | 123 (55.2) | |
| Aspirin | 4401 (50.3) | 1079 (50.9) | 3222 (50.2) | 100 (44.8) | |
| Egg Consumption | All-Cause Mortality HR [95% CI] | Cancer Mortality HR [95% CI] | CVD Mortality HR [95% CI] |
|---|---|---|---|
| Crude | |||
| Never/infrequently | Ref | Ref | Ref |
| Weekly | 0.77 [0.67–0.89] | 0.89 [0.74–1.08] | 0.65 [0.50–0.83] |
| Daily | 1.20 [0.85–1.70] | 1.21 [0.75–1.95] | 1.29 [0.70–2.23] |
| Min. Adjusted a | |||
| Never/infrequently | Ref | Ref | Ref |
| Weekly | 0.82 [0.71–0.93] | 0.94 [0.78–1.13] | 0.67 [0.53–0.87] |
| Daily | 1.12 [0.79–1.58] | 1.19 [0.74–1.91] | 1.21 [0.66–2.22] |
| Fully Adjusted b | |||
| Never/infrequently | Ref | Ref | Ref |
| Weekly | 0.85 [0.74–0.98] | 0.93 [0.76–1.16] | 0.71 [0.54–0.91] |
| Daily | 1.20 [0.85–1.71] | 1.36 [0.82–02.27] | 1.43 [0.79–2.58] |
| Egg Consumption | All-Cause Mortality HR [95% CI] | Cancer Mortality HR [95% CI] | CVD Mortality HR [95% CI] |
|---|---|---|---|
| Diet Quality Score Tertile a | |||
| T1 | |||
| Infrequent/Never | Ref | Ref | Ref |
| Weekly | 1.04 [0.58–1.86] | 0.59 [0.23–1.44] | 2.26 [0.73–7.00] |
| Daily | 1.60 [0.18–13.85] | 4.69 [0.32–68.05] | ---- |
| T2 | |||
| Infrequent/Never | Ref | Ref | Ref |
| Weekly | 0.86 [0.73–1.01] | 1.00 [0.78–1.28] | 0.67 [0.50–0.90] |
| Daily | 0.94 [0.60–1.51] | 0.97 [0.47–2.02] | 1.11 [0.51–2.44] |
| T3 | |||
| Infrequent/Never | Ref | Ref | Ref |
| Weekly | 0.79 [0.57–1.08] | 0.82 [0.50–1.34] | 0.56 [0.32–0.99] |
| Daily | 1.65 [0.92–2.94] | 2.19 [0.94–5.06] | 1.69 [0.65–4.43] |
| Dyslipidemia b | |||
| No | |||
| Infrequent/Never | Ref | Ref | Ref |
| Weekly | 0.79 [0.60–1.04] | 0.85 [0.57–1.30] | 0.57 [0.33–0.97] |
| Daily | 1.14 [0.59–2.19] | 1.02 [0.35–2.96] | 2.43 [0.93–6.40] |
| Yes | |||
| Infrequent/Never | Ref | Ref | Ref |
| Weekly | 0.86 [0.74–1.01] | 0.96 [0.75–1.2448] | 0.73 [0.54–0.97] |
| Daily | 1.15 [0.76–1.80] | 1.48 [0.82–2.66] | 1.00 [0.46–2.20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wild, H.; Gasevic, D.; Woods, R.L.; Ryan, J.; Wolfe, R.; Chen, Y.; Govindaraju, T.; McNeil, J.J.; McCaffrey, T.; Beilin, L.J.; et al. Egg Consumption and Mortality: A Prospective Cohort Study of Australian Community-Dwelling Older Adults. Nutrients 2025, 17, 323. https://doi.org/10.3390/nu17020323
Wild H, Gasevic D, Woods RL, Ryan J, Wolfe R, Chen Y, Govindaraju T, McNeil JJ, McCaffrey T, Beilin LJ, et al. Egg Consumption and Mortality: A Prospective Cohort Study of Australian Community-Dwelling Older Adults. Nutrients. 2025; 17(2):323. https://doi.org/10.3390/nu17020323
Chicago/Turabian StyleWild, Holly, Danijela Gasevic, Robyn L. Woods, Joanne Ryan, Rory Wolfe, Yuquan Chen, Thara Govindaraju, John J. McNeil, Tracy McCaffrey, Lawrence J. Beilin, and et al. 2025. "Egg Consumption and Mortality: A Prospective Cohort Study of Australian Community-Dwelling Older Adults" Nutrients 17, no. 2: 323. https://doi.org/10.3390/nu17020323
APA StyleWild, H., Gasevic, D., Woods, R. L., Ryan, J., Wolfe, R., Chen, Y., Govindaraju, T., McNeil, J. J., McCaffrey, T., Beilin, L. J., Ilic, D., & Owen, A. J. (2025). Egg Consumption and Mortality: A Prospective Cohort Study of Australian Community-Dwelling Older Adults. Nutrients, 17(2), 323. https://doi.org/10.3390/nu17020323






