Adherence to the Mediterranean Diet and the Risk of Head and Neck Cancer: A Systematic Review and Meta-Analysis of Case–Control Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Included Studies
3.2. Quality Evaluation
3.3. Data Tabulation
3.4. Mediterranean Diet Adherence Evaluation
3.5. Adherence to the Mediterranean Diet and Head and Neck Cancer Risk
3.6. Head and Neck Cancer Survival
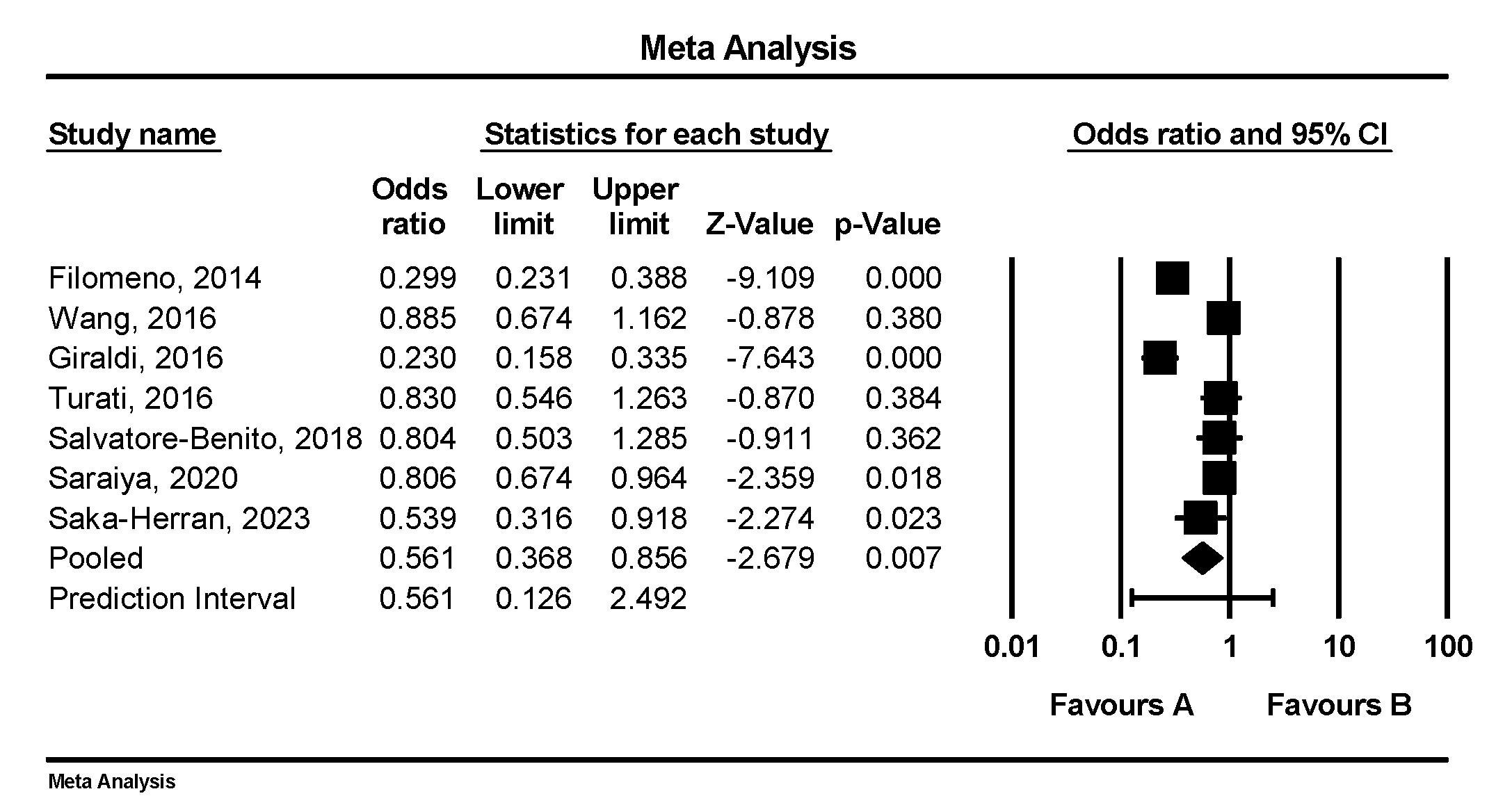
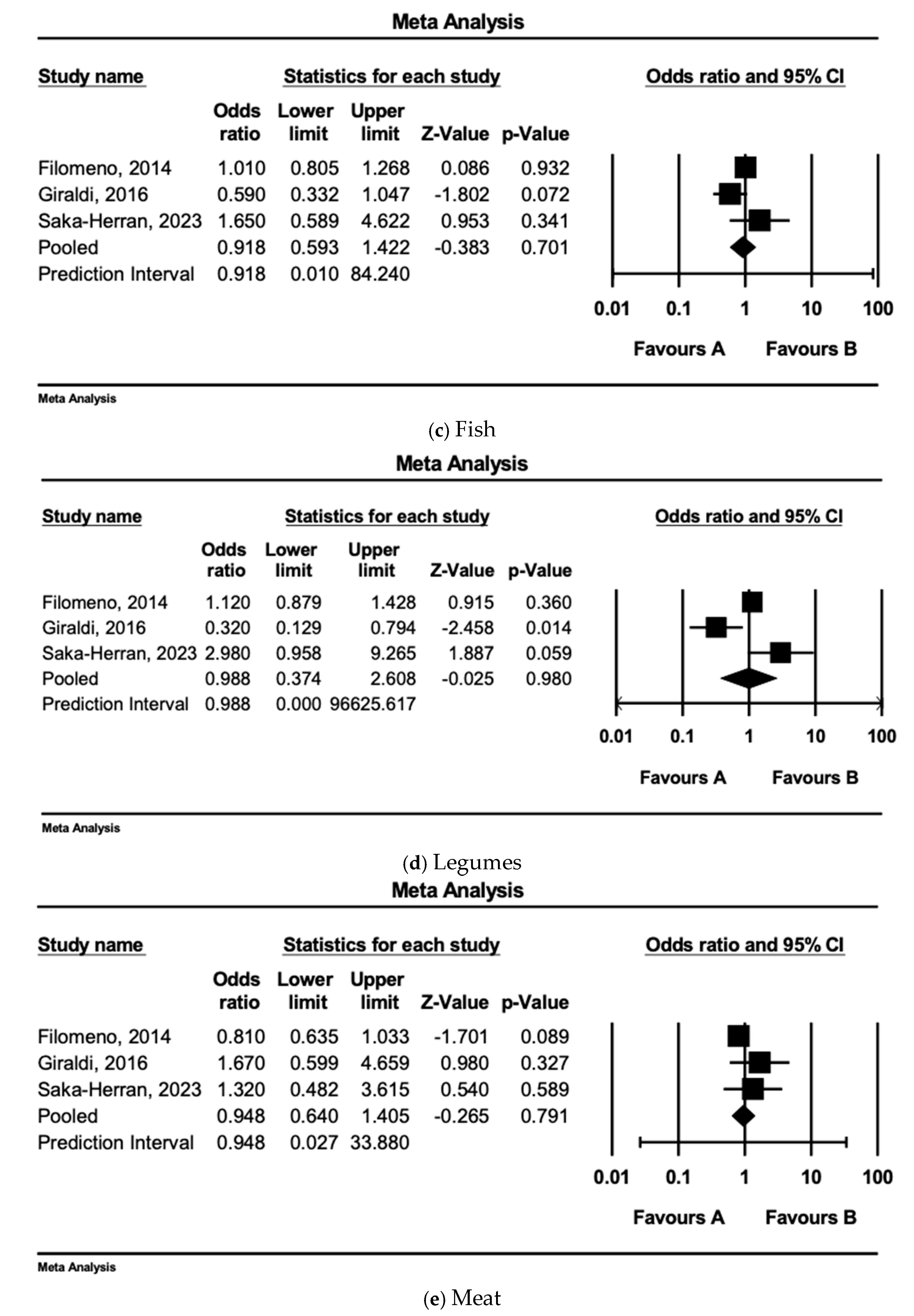
3.7. Individual Dietary Component/Food Group Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui VW, Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.-C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Lubin, J.H.; Muscat, J.; Gaudet, M.M.; Olshan, A.F.; Curado, M.P.; Maso, L.D.; Wünsch-Filho, V.; Sturgis, E.M.; Szeszenia-Dabrowska, N.; Castellsague, X.; et al. An examination of male and female odds ratios by BMI, cigarette smoking, and alcohol consumption for cancers of the oral cavity, pharynx, and larynx in pooled data from 15 case–control studies. Cancer Causes Control. 2011, 22, 1217–1231. [Google Scholar] [CrossRef]
- Chuang, S.-C.; Jenab, M.; Heck, J.E.; Bosetti, C.; Talamini, R.; Matsuo, K.; Castellsague, X.; Franceschi, S.; Herrero, R.; Winn, D.M.; et al. Diet and the risk of head and neck cancer: A pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012, 23, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Toorang, F.; Seyyedsalehi, M.S.; Sasanfar, B.; Rashidian, H.; Hadji, M.; Mohebbi, E.; Safari, R.; Najefi, F.; Naghibzadeh-Tahami, A.; Boffetta, P.; et al. Dietary total antioxidant capacity and head and neck cancer: A large case-control study in Iran. Front. Nutr. 2023, 10, 1226446. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Ko, S.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.; Elcarte, E.; et al. Disease burden, risk factors, and trends of lip, oral cavity, pharyngeal cancers: A global analysis. Cancer Med. 2023, 12, 18153–18164. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Milajerdi, A.; Namazi, N.; Larijani, B.; Azadbakht, L. The association of dietary quality indices and cancer mortality: A systematic review and meta-analysis of cohort studies. Nutr. Cancer 2018, 70, 1091–1105. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Mediterranean diet and survival among patients with coronary heart disease in Greece. Arch. Intern. Med. 2005, 165, 929–935. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-De-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; Van Der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef] [PubMed]
- McClain, K.M.; Bradshaw, P.T.; Khankari, N.K.; Gammon, M.D.; Olshan, A.F. Fish/shellfish intake and the risk of head and neck cancer. Eur. J. Cancer Prev. 2019, 28, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 polyunsaturated fatty acids and cancer: Lessons learned from clinical trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar]
- Prasad, R.; Katiyar, S.K. Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS ONE 2012, 7, e46404. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Taha, H.M.; Rozek, L.S.; Chen, X.; Li, Z.; Zarins, K.R.; Slade, A.N.; Wolf, G.T.; E Arthur, A. Risk of disease recurrence and mortality varies by type of fat consumed before cancer treatment in a longitudinal cohort of head and neck squamous cell carcinoma patients. J. Nutr. 2022, 152, 1298–1305. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- American Lung Association. Overall Smoking Trends. American Lung Association. Available online: https://www.lung.org/research/trends-in-lung-disease/tobacco-trends-brief/overall-smoking-trends (accessed on 3 January 2025).
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic noncommunicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Phelps, N.H.; Singleton, R.K.; Zhou, B.; Heap, R.A.; Mishra, A.; Bennett, J.E.; Paciorek, C.J.; Lhoste, V.P.F.; Carrillo-Larco, R.M.; Stevens, G.A.; et al. Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M. Comprehensive meta-analysis software. In Systematic Reviews in Health Research: Meta-Analysis in Context; Egger, M., Higgins, J.P.T., Davey Smith, G., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 535–548. [Google Scholar]
- Jpt, H. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Cochrane: Alberta, ON, Canada, 2022. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Lagiou, A.; Nikolopoulos, E.; Lagogiannis, G.; Barbouni, A.; Lefantzis, D.; Trichopoulos, D.; Brennan, P.; Lagiou, P. Mediterranean diet and upper aerodigestive tract cancer: The Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study. Br. J. Nutr. 2010, 104, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, V.; Bradshaw, P.T.; Meyer, K.; Lund, J.; Slade, G.D.; Olshan, A.F. The association between the Mediterranean Diet Score and death from cancer of the head and neck. Cancer Causes Control. 2024, 35, 77–92. [Google Scholar] [CrossRef]
- Benito, A.S.; Zanuy, M.V.; Cano, M.A.; Alonso, A.R.; Bravo, I.A.; Blanco, E.R.; Jiménez, M.M.; Sanz, M.L. Adherence to Mediterranean diet: A comparison of patients with head and neck cancer and healthy population. Endocrinol. Diabetes Nutr. 2019, 66, 417–424. [Google Scholar]
- Turati, F.; Bravi, F.; Polesel, J.; Bosetti, C.; Negri, E.; Garavello, W.; Taborelli, M.; Serraino, D.; Libra, M.; Montella, M.; et al. Adherence to the Mediterranean diet and nasopharyngeal cancer risk in Italy. Cancer Causes Control. 2017, 28, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, X.-L.; Fan, Y.-Y.; Liu, Y.-T.; Zhang, X.-L.; Lu, Y.-K.; Xu, C.-H.; Chen, Y.-M. Diet quality scores and risk of nasopharyngeal carcinoma in Chinese adults: A case-control study. Nutrients 2016, 8, 112. [Google Scholar] [CrossRef]
- Saka-Herrán, C.; Pereira-Riveros, T.; Jané-Salas, E.; López-López, J. Association between the mediterranean diet and vitamin C and the risk of head and neck cancer. Nutrients 2023, 15, 2846. [Google Scholar] [CrossRef] [PubMed]
- Saraiya, V.; Bradshaw, P.; Meyer, K.; Gammon, M.; Slade, G.; Brennan, P.; Abedi-Ardekani, B.; Olshan, A. The association between diet quality and cancer incidence of the head and neck. Cancer Causes Control. 2020, 31, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Giraldi, L.; Panic, N.; Cadoni, G.; Boccia, S.; Leoncini, E. Association between Mediterranean diet and head and neck cancer: Results of a large case–control study in Italy. Eur. J. Cancer Prev. 2017, 26, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Filomeno, M.; Bosetti, C.; Garavello, W.; Levi, F.; Galeone, C.; Negri, E.; La Vecchia, C. The role of a Mediterranean diet on the risk of oral and pharyngeal cancer. Br. J. Cancer 2014, 111, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.; Russo, A.; Tagliabue, G.; Berrino, F. Tobacco and diet as determinants of survival in male laryngeal cancer patients. Int. J. Cancer 1996, 65, 308–313. [Google Scholar] [CrossRef]
- Bosetti, C.; Gallus, S.; Trichopoulou, A.; Talamini, R.; Franceschi, S.; Negri, E.; La Vecchia, C. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1091–1094. [Google Scholar]
- Franceschi, S.; Favero, A.; Conti, E.; Talamini, R.; Volpe, R.; Negri, E.; Barzan, L.; La Vecchia, C. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br. J. Cancer 1999, 80, 614–620. [Google Scholar] [CrossRef]
- Bosetti, C.; La Vecchia, C.; Talamini, R.; Negri, E.; Levi, F.; Maso, L.D.; Franceschi, S. Food groups and laryngeal cancer risk: A case-control study from Italy and Switzerland. Int. J. Cancer 2002, 100, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rexrode, K.M.; Mantzoros, C.S.; Manson, J.E.; Willett, W.C.; Hu, F.B. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Martínez, J.A.; De Irala, J.; Martínez-González, M.A. Determinants of the adherence to an “a priori” defined Mediterranean dietary pattern. Eur. J. Nutr. 2002, 41, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Alberti-Fidanza, A.; Fidanza, F. Mediterranean adequacy index of Italian diets. Public Health Nutr. 2004, 7, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Kargın, D.; Tomaino, L.; Serra-Majem, L. Experimental outcomes of the mediterranean diet: Lessons learned from the predimed randomized controlled trial. Nutrients 2019, 11, 2991. [Google Scholar] [CrossRef]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean diet and breast cancer risk. Nutrients 2018, 10, 326. [Google Scholar] [CrossRef]
- La Torre, G.; De Carlo, I.; Sestili, C.; Cocchiara, R.A.; Lia, L.; Di Bella, O.; Cianfanelli, S.; D’Egidio, V.; Mancino, M.; Palmeri, V.; et al. Non-adherence to Mediterranean diet and synergy with lifestyle habits in the occurrence of breast cancer: A case-control study in Italy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4535–4539. [Google Scholar] [PubMed]
- Du, H.; Cao, T.; Lu, X.; Zhang, T.; Luo, B.; Li, Z. Mediterranean diet patterns in relation to lung cancer risk: A meta-analysis. Front. Nutr. 2022, 9, 844382. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Khalesi, S.; Makiabadi, E.; Alibeyk, S.; Hajigholam-Saryazdi, M.; Hejazi, E. Adherence to the Mediterranean diet and the risk of lung cancer: A systematic review and dose-response meta-analysis of observational studies. Nutr. Rev. 2022, 80, 1118–1128. [Google Scholar] [CrossRef]
- Bai, X.; Li, X.; Ding, S.; Dai, D. Adherence to the Mediterranean Diet and Risk of Gastric Cancer: A systematic review and Meta-analysis. Nutrients 2023, 15, 3826. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, J.; Giraldi, L.; Arzani, D.; Pastorino, R.; Biondi, A.; Persiani, R.; Boccia, S.; Leoncini, E. Adherence to Mediterranean diet and risk of gastric cancer: Results of a case–control study in Italy. Eur. J. Cancer Prev. 2017, 26, 491–496. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Nardi, M.; Cinnirella, A.; Campagnoli, E.; Maffeo, M.; Perrone, P.M.; Shishmintseva, V.; Grosso, F.M.; Castrofino, A.; Castaldi, S.; et al. Adherence to Mediterranean diet and risk of pancreatic cancer: Systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2023, 20, 2403. [Google Scholar] [CrossRef]
- Schneider, L.; Su, L.J.; Arab, L.; Bensen, J.T.; Farnan, L.; Fontham, E.T.H.; Song, L.; Hussey, J.; Merchant, A.T.; Mohler, J.L.; et al. Dietary patterns based on the Mediterranean diet and DASH diet are inversely associated with high aggressive prostate cancer in PCaP. Ann. Epidemiol. 2019, 29, 16–22.e1. [Google Scholar] [CrossRef] [PubMed]
- Lander, D.P.; Kallogjeri, D.; Piccirillo, J.F. Smoking, Drinking, and Dietary Risk Factors for Head and Neck Cancer in Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial Participants. JAMA Otolaryngol.–Head Neck Surg. 2024, 150, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Morales-Berstein, F.; Biessy, C.; Viallon, V.; Goncalves-Soares, A.; Casagrande, C.; Hémon, B.; Kliemann, N.; Cairat, M.; Lopez, J.B.; Al Nahas, A.; et al. Ultra-processed foods, adiposity and risk of head and neck cancer and oesophageal adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition study: A mediation analysis. Eur. J. Nutr. 2024, 63, 377–396. [Google Scholar] [CrossRef]
- Li, W.-Q.; Park, Y.; Wu, J.W.; Goldstein, A.M.; Taylor, P.R.; Hollenbeck, A.R.; Freedman, N.D.; Abnet, C.C. Index-based dietary patterns and risk of head and neck cancer in a large prospective study. Am. J. Clin. Nutr. 2014, 99, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Muñoz, D.; Schröder, H.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; López-Sabater, M.C.; et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. Eur. J. Clin. Nutr. 2008, 62, 570–574. [Google Scholar] [CrossRef]
- Machowetz, A.; Poulsen, H.E.; Gruendel, S.; Weimann, A.; Fitó, M.; Marrugat, J.; Torre, R.; Salonen, J.T.; Nyyssönen, K.; Mursu, J.; et al. Effect of olive oils on biomarkers of oxidative DNA stress in Northern and Southern Europeans. FASEB J. 2007, 21, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Bertuccio, P.; Bosetti, C.; Turati, F.; Ferraroni, M.; La Vecchia, C. Adherence to the Mediterranean diet and gastric cancer risk in Italy. Int. J. Cancer 2014, 134, 2935–2941. [Google Scholar] [CrossRef]
- Rossi, M.; Turati, F.; Lagiou, P.; Trichopoulos, D.; Augustin, L.S.; La Vecchia, C.; Trichopoulou, A. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: Results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia 2013, 56, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Tzima, N.; Chrysohoou, C.; Economou, M.; Zampelas, A.; Stefanadis, C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: The ATTICA study. Am. J. Clin. Nutr. 2005, 82, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Saura-Calixto, F. Contribution of macromolecular antioxidants to dietary antioxidant capacity: A study in the Spanish Mediterranean diet. Plant Foods Hum. Nutr. 2015, 70, 365–370. [Google Scholar] [CrossRef]
- Castelló, A.; Boldo, E.; Pérez-Gómez, B.; Lope, V.; Altzibar, J.M.; Martín, V.; Castaño-Vinyals, G.; Guevara, M.; Dierssen-Sotos, T.; Tardón, A.; et al. Adherence to the Western, Prudent and Mediterranean dietary patterns and breast cancer risk: MCC-Spain study. Maturitas 2017, 103, 8–15. [Google Scholar] [CrossRef]
- Li, F.; An, S.; Hou, L.; Chen, P.; Lei, C.; Tan, W. Red and processed meat intake and risk of bladder cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 2100. [Google Scholar]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Mediterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13, S14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Meng, X.; Gan, R.-Y.; Zhang, J.-J.; Li, H.-B. Dietary natural products for prevention and treatment of breast cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef]
- Amor, S.; Châlons, P.; Aires, V.; Delmas, D. Polyphenol extracts from red wine and grapevine: Potential effects on cancers. Diseases 2018, 6, 106. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health benefits of the Mediterranean diet: Metabolic and molecular mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Country | Type of Study | Cases/ | Age (Years ± SD or IQR) | Sex (Males/Females) | Year of Diagnosis | Mediterranean Diet Adherence Score | Type of Cancer | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls (n) | |||||||||||
| Cases | Controls | Cases | Controls | ||||||||
| Crosignani et al., 1996 [36] | Italy | Case–control, Population-based | 220/0 | 59 (32–75) | - | 220/0 | No control | Registry | Tertiles of intake of various food groups | Laryngeal cancer | All-cause mortality |
| Bosetti et al., 2003 [37,38,39] | Italy | Case–control | 598/1492 | 58 (22–77) | 58 (20–78) | 512/87 | 1008/484 | 1992–1998 | An MD score was defined on the basis of eight characteristics of the traditional MD. Adherence was stratified as <3, 3, 4, 5, and ≥6 | Cancers of the oral cavity and pharynx | OCP cancer risk |
| Italy and Switzerland | Case–control | 457/1087 | 61 (30–79) | 61 (31–79) | 478/49 | 1052/245 | 1992–2000 | Cancer of the larynx | Laryngeal cancer risk | ||
| Samoli et al., 2010 [27] | Greece | Case–control | 239/194 | 61.3 ± 0.8 | 60.6 ± 1.0 | 192/47 | 143/51 | 2002–2005 | MDS (two- or three-unit increase) | Cancers of the oral cavity, pharynx (excluding nasopharynx), larynx and esophagus | Upper aerodigestive tract cancer risk |
| Filomeno et al., 2014 [35] | Italy and Switzerland | Case–control | 768/2078 | 58 (22–79) | 59 (19–79) | 593/175 | 1368/710 | 1997–2009 | MDS (≤2 vs. ≥6 | Cancers of the oral cavity and pharynx (OCP) | OCP cancer risk |
| MDP (≤57.9 vs. ≥66.2) | |||||||||||
| MAI (≤0.92 vs. ≥2.1) | |||||||||||
| Giraldi et al., 2016 [34] | Italy | Case–control | 500/433 | 63.1 ± NA | 58.8 ± NA | 402/98 | 254/179 | 2002–2014 | MDS (Continuous) | Cancers of the oral cavity, oropharynx, hypopharynx, and larynx | Head and neck cancer risk |
| Turati et al., 2016 [30] | Italy | Case–control | 198/594 | 52 (18–76) | 52 (19–76) | 157/41 | 471/123 | 1992–2008 | MDS (≤4 vs. 5 and ≤4 vs. ≥6) | Nasopharyngeal cancer | NPC risk |
| Wang et al., 2016 [31] | China | Case–control | 600/600 | 47.4 ± 9.0 | 47.4 ± 9.0 | 448/152 | 448/152 | 2009–2011 | aMed (≤2 vs. ≥6) | Nasopharyngeal cancer | NPC risk |
| Salvatore-Benito et al., 2018 [29] | Spain | Case–control | 68/100 | 64.9 ± 9.7 | 59.7 ± 17.9 | 58/10 | 44/56 | 2018 | MEDAS [≤7 (poor adherence) vs. 8–9 (moderate adherence) vs. ≥10 (good adherence)] | Head and neck cancers | HNC risk |
| Saraiya et al., 2020 [33] | USA | Case–control | 1170/1303 | No mean/median reported | No mean/median reported | 899/271 | 904/399 | 2002–2006 | MDS, 1 SD unit change (SD = 1.7) | Oral cavity, pharynx, and larynx | HNC risk |
| MDS-HNC, 1 SD unit change (SD = 2.2) | |||||||||||
| Saka-Herran et al., 2023 [32] | Spain | Case–control | 101/101 | 66 ± 10.2 | 65.8 ± 9.9 | 69/32 | 69/32 | 2018–2022 | MD questionnaire that was adapted from the Spanish Society of Atherosclerosis [unhealthy (≤4) vs. healthy (10–14) vs. regular (5–9)] | Head and Neck | HNC risk |
| Saraiya et al., 2024 [28] | USA | Case–control, Population-based, cases linked to the National Death Index, 1:1 | 1184/0 | No mean/median reported | - | 912/272 | No control | 2002–2006 | MDS highest versus lowest quintile | Oral cavity, pharynx, and larynx | All-cause and HNC-specific mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalaquett, N.; Lidoriki, I.; Lampou, M.; Saab, J.; Hadkhale, K.; Christophi, C.; Kales, S.N. Adherence to the Mediterranean Diet and the Risk of Head and Neck Cancer: A Systematic Review and Meta-Analysis of Case–Control Studies. Nutrients 2025, 17, 287. https://doi.org/10.3390/nu17020287
Zalaquett N, Lidoriki I, Lampou M, Saab J, Hadkhale K, Christophi C, Kales SN. Adherence to the Mediterranean Diet and the Risk of Head and Neck Cancer: A Systematic Review and Meta-Analysis of Case–Control Studies. Nutrients. 2025; 17(2):287. https://doi.org/10.3390/nu17020287
Chicago/Turabian StyleZalaquett, Nader, Irene Lidoriki, Maria Lampou, Jad Saab, Kishor Hadkhale, Costas Christophi, and Stefanos N. Kales. 2025. "Adherence to the Mediterranean Diet and the Risk of Head and Neck Cancer: A Systematic Review and Meta-Analysis of Case–Control Studies" Nutrients 17, no. 2: 287. https://doi.org/10.3390/nu17020287
APA StyleZalaquett, N., Lidoriki, I., Lampou, M., Saab, J., Hadkhale, K., Christophi, C., & Kales, S. N. (2025). Adherence to the Mediterranean Diet and the Risk of Head and Neck Cancer: A Systematic Review and Meta-Analysis of Case–Control Studies. Nutrients, 17(2), 287. https://doi.org/10.3390/nu17020287







