Self-Reported Non-Celiac Wheat Sensitivity and Other Food Sensitivities in Patients with Primary Sjögren’s Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion
- Age ≥18 and ≤75 years;
- Diagnosis of pSS according to the 2016 ACR/EULAR criteria [40];
- Written consent to participate in the study.
- Age ≥18 and ≤75 years;
- Absence of the minimal criteria for pSS [40], or no diagnosis of other autoimmune disorders (excluding Hashimoto’s thyroiditis), in accordance with the current international criteria;
- Written consent to participate in the study.
- Diagnosis of CeD, according to current diagnostic criteria [43];
- Self-exclusion of wheat from the diet and refusal to reintroduce it for diagnostic purposes before entering the study (whenever required to exclude CeD);
- Drug and/or alcohol abuse (>30 g/day for men and >20 g/day for women);
- Treatment with systemic steroids and/or non-steroidal anti-inflammatory and/or immunosuppressive drugs in the 2 weeks before duodenal biopsy (whenever required to exclude CeD);
- Pregnancy or breastfeeding;
- Diagnosis of chronic inflammatory bowel disease or other organic pathologies affecting the digestive system (e.g., WA, microscopic colitis, diverticulitis, segmental colitis associated with diverticulosis, etc.), neurological diseases, major psychiatric disorders, infectious diseases, immunological deficiencies, and impairment limiting physical activity;
- Incomplete medical records;
- Lack of a clinical follow-up of at least 12 months after diagnosis with >2 outpatient visits during the follow-up period.
2.2. Outcomes
- Primary outcome: prevalence of self-reported NCWS and/or other food sensitivities in pSS patients
- Secondary outcome: evaluation of the demographic, clinical and immunological characteristics of pSS patients with or without self-reported NCWS
- -
- Anti-nuclear antibody (ANA) titers and patterns;
- -
- Anti-SSA/Ro antibodies (Sjögren’s-syndrome-related antigen A);
- -
- Anti-SSB/La antibodies (Sjögren’s-syndrome-related antigen B);
- -
- Rheumatoid factor (RF);
- -
- C3 and C4 complement component;
- -
- γ-globulinemia;
- -
- Monoclonal component of the γ-globulin fraction.
2.3. Statistical Analysis
3. Results
3.1. Primary Outcome: Prevalence of Self-Reported NCWS and/or Sensitivities/Intolerance to Other Foods
3.2. Secondary Outcome: Evaluation of the Demographic, Clinical and Immunological Features of pSS Patients With or Without Self-Reported NCWS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| ANA | Anti-nuclear antibodies |
| anti-SSA/Ro | anti-Sjögren’s-syndrome-related antigen A |
| anti-SSB/La | anti-Sjögren’s-syndrome-related antigen B |
| ATIs | Amylase-trypsin inhibitors |
| CeD | Celiac disease |
| DBPCWC | Double-blind placebo-controlled wheat challenge |
| DM | Type 2 diabetes mellitus |
| ESSDAI | EULAR Sjögren’s syndrome disease activity index |
| ESSPRI | EULAR Sjögren’s syndrome patient-reported index |
| EULAR | European League Against Rheumatism (now European Alliance of Associations for Rheumatology) |
| GFD | Gluten-free diet |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| IP | Intestinal permeability |
| MFS | Multiple food sensitivity |
| NCWS | Non-celiac wheat sensitivity |
| NO | Nitric oxide |
| pSS | Primary Sjögren’s syndrome |
| RF | Rheumatoid factor |
| SRMI | Self-reported milk intolerance |
| TLR-4 | Toll-like receptor-4 |
| TNF-α | Tumor necrosis factor-α |
| WA | Wheat allergy |
| WFD | Wheat-free diet |
References
- Fox, R.I.; Stern, M.; Michelson, P. Update in Sjögren Syndrome. Curr. Opin. Rheumatol. 2000, 12, 391–398. [Google Scholar] [CrossRef]
- Maciel, G.; Crowson, C.S.; Matteson, E.L.; Cornec, D. Prevalence of Primary Sjögren’s Syndrome in a US Population-Based Cohort. Arthritis Care Res. 2017, 69, 1612–1616. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zerón, P.; Theander, E.; Baldini, C.; Seror, R.; Retamozo, S.; Quartuccio, L.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.-E.; et al. Early Diagnosis of Primary Sjögren’s Syndrome: EULAR-SS Task Force Clinical Recommendations. Expert Rev. Clin. Immunol. 2016, 12, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Moutsopoulos, H.M. The Geoepidemiology of Sjögren’s Syndrome. Autoimmun. Rev. 2010, 9, A305–A310. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zeron, P.; Siso-Almirall, A.; Bosch, X. Primary Sjogren Syndrome. BMJ 2012, 344, e3821. [Google Scholar] [CrossRef]
- Carsons, S.E.; Blum, M.A. Sjogren Syndrome; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Negrini, S.; Emmi, G.; Greco, M.; Borro, M.; Sardanelli, F.; Murdaca, G.; Indiveri, F.; Puppo, F. Sjögren’s Syndrome: A Systemic Autoimmune Disease. Clin. Exp. Med. 2022, 22, 9–25. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; Seror, R.; Bootsma, H.; Bowman, S.J.; Dörner, T.; Gottenberg, J.-E.; Mariette, X.; Theander, E.; Bombardieri, S.; et al. Characterization of Systemic Disease in Primary Sjögren’s Syndrome: EULAR-SS Task Force Recommendations for Articular, Cutaneous, Pulmonary and Renal Involvements. Rheumatology 2015, 54, 2230–2238. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T Cells in Primary Sjögren’s Syndrome: Targets for Early Intervention. Rheumatology 2021, 60, 3088–3098. [Google Scholar] [CrossRef]
- Nocturne, G.; Cornec, D.; Seror, R.; Mariette, X. Use of Biologics in Sjögren’s Syndrome. Rheum. Dis. Clin. N. Am. 2016, 42, 407–417. [Google Scholar] [CrossRef]
- Retamozo, S.; Flores-Chavez, A.; Consuegra-Fernández, M.; Lozano, F.; Ramos-Casals, M.; Brito-Zerón, P. Cytokines as Therapeutic Targets in Primary Sjögren Syndrome. Pharmacol. Ther. 2018, 184, 81–97. [Google Scholar] [CrossRef]
- Wang, X.; Lin, X.; Su, Y.; Wang, H. Systematic Review with Meta-Analysis: Efficacy and Safety of Biological Treatment on Salivary Gland Function in Primary Sjögren’s Syndrome. Front. Pharmacol. 2023, 14, 1093924. [Google Scholar] [CrossRef]
- Lendrem, D.; Mitchell, S.; McMeekin, P.; Bowman, S.; Price, E.; Pease, C.T.; Emery, P.; Andrews, J.; Lanyon, P.; Hunter, J.; et al. Health-Related Utility Values of Patients with Primary Sjögren’s Syndrome and Its Predictors. Ann. Rheum. Dis. 2014, 73, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Innovative Medicines Initiative New Subgroups of Sjögren’s Patients Mean Better Treatments Possible. Available online: https://www.ihi.europa.eu/news-events/newsroom/new-subgroups-sjogrens-patients-mean-better-treatments-possible (accessed on 3 October 2025).
- Wang, X.; Bootsma, H.; de Koning, J.; Kroese, F.G.M.; Pringle, S. Novel Approaches for Rescuing Function of the Salivary Gland Epithelium in Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 261–270. [Google Scholar] [PubMed]
- Mignogna, M.D.; Fedele, S.; Russo, L.L.; Muzio, L.L.; Wolff, A. Sjögren’s Syndrome: The Diagnostic Potential of Early Oral Manifestations Preceding Hyposalivation/Xerostomia. J. Oral Pathol. Med. 2005, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.C.; Alunno, A.; Cafaro, G.; Valentini, V.; Marcucci, E.; Bartoloni, E.; Gerli, R. The Clinical Spectrum of Primary Sjögren’s Syndrome: Beyond Exocrine Glands. Reumatismo 2017, 69, 93. [Google Scholar] [CrossRef]
- Baldini, C.; Pepe, P.; Quartuccio, L.; Priori, R.; Bartoloni, E.; Alunno, A.; Gattamelata, A.; Maset, M.; Modesti, M.; Tavoni, A.; et al. Primary Sjögren’s Syndrome as a Multi-Organ Disease: Impact of the Serological Profile on the Clinical Presentation of the Disease in a Large Cohort of Italian Patients. Rheumatology 2014, 53, 839–844. [Google Scholar] [CrossRef]
- Machowicz, A.; Hall, I.; de Pablo, P.; Rauz, S.; Richards, A.; Higham, J.; Poveda-Gallego, A.; Imamura, F.; Bowman, S.J.; Barone, F.; et al. Mediterranean Diet and Risk of Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 216–221. [Google Scholar]
- Lidén, M.; Kristjánsson, G.; Valtysdottir, S.; Venge, P.; Hällgren, R. Cow’s Milk Protein Sensitivity Assessed by the Mucosal Patch Technique Is Related to Irritable Bowel Syndrome in Patients with Primary Sjögren’s Syndrome. Clin. Exp. Allergy 2008, 38, 929–935. [Google Scholar] [CrossRef]
- Lidén, M.; Kristjánsson, G.; Valtýsdóttir, S.; Hällgren, R. Gluten Sensitivity in Patients with Primary Sjögren’s Syndrome. Scand. J. Gastroenterol. 2007, 42, 962–967. [Google Scholar] [CrossRef]
- Patinen, P.; Aine, L.; Collin, P.; Hietanen, J.; Korpela, M.; Enckell, G.; Kautiainen, H.; Konttinen, Y.; Reunala, T. Oral Findings in Coeliac Disease and Sjögren’s Syndrome. Oral Dis. 2004, 10, 330–334. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; Iacono, G.; Soresi, M.; D’Alcamo, A.; Cavataio, F.; Brusca, I.; Florena, A.M.; Ambrosiano, G.; Seidita, A.; et al. Non-Celiac Wheat Sensitivity Diagnosed by Double-Blind Placebo-Controlled Challenge: Exploring a New Clinical Entity. Am. J. Gastroenterol. 2012, 107, 1898–1906. [Google Scholar] [CrossRef]
- Manza, F.; Lungaro, L.; Costanzini, A.; Caputo, F.; Carroccio, A.; Mansueto, P.; Seidita, A.; Raju, S.A.; Volta, U.; De Giorgio, R.; et al. Non-Celiac Gluten/Wheat Sensitivity—State of the Art: A Five-Year Narrative Review. Nutrients 2025, 17, 220. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113.e12. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal Cell Damage and Systemic Immune Activation in Individuals Reporting Sensitivity to Wheat in the Absence of Coeliac Disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef]
- Di Liberto, D.; Mansueto, P.; D’Alcamo, A.; Pizzo, M.L.; Presti, E.L.; Geraci, G.; Fayer, F.; Guggino, G.; Iacono, G.; Dieli, F.; et al. Predominance of Type 1 Innate Lymphoid Cells in the Rectal Mucosa of Patients with Non-Celiac Wheat Sensitivity: Reversal After a Wheat-Free Diet. Clin. Transl. Gastroenterol. 2016, 7, e178. [Google Scholar] [CrossRef]
- Mansueto, P.; Di Liberto, D.; Fayer, F.; Soresi, M.; Geraci, G.; Giannone, A.G.; Seidita, A.; D’Alcamo, A.; La Blasca, F.; Lo Pizzo, M.; et al. TNF-α, IL-17, and IL-22 Production in the Rectal Mucosa of Nonceliac Wheat Sensitivity Patients: Role of Adaptive Immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G281–G288. [Google Scholar] [CrossRef]
- Volta, U.; De Giorgio, R.; Caio, G.; Uhde, M.; Manfredini, R.; Alaedini, A. Nonceliac Wheat Sensitivity. Gastroenterol. Clin. N. Am. 2019, 48, 165–182. [Google Scholar] [CrossRef]
- Seidita, A.; Giuliano, A.; Soresi, M.; Chiavetta, M.; Nardi, E.; Mogavero, G.; Giannone, G.; Carroccio, A.; Mansueto, P. Fecal Calprotectin Levels in Patients with Non-Celiac Wheat Sensitivity: A Proof of Concept. Intern. Emerg. Med. 2024, 19, 1255–1266. [Google Scholar] [CrossRef]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-Intestinal Manifestations of Non-Celiac Gluten Sensitivity: An Expanding Paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised Clinical Trial: Gluten May Cause Depression in Subjects with Non-coeliac Gluten Sensitivity—An Exploratory Clinical Study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef]
- Bonciolini, V.; Bianchi, B.; Del Bianco, E.; Verdelli, A.; Caproni, M. Cutaneous Manifestations of Non-Celiac Gluten Sensitivity: Clinical Histological and Immunopathological Features. Nutrients 2015, 7, 7798–7805. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Grunewald, R.A.; Kandler, R.H.; Chattopadhyay, A.K.; Jarratt, J.A.; Sanders, D.S.; Sharrack, B.; Wharton, S.B.; Davies-Jones, G.A.B. Neuropathy Associated with Gluten Sensitivity. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, P.; Seidita, A.; Soresi, M.; Giuliano, A.; Riccio, G.; Volta, U.; Caio, G.; La Blasca, F.; Disclafani, R.; De Giorgio, R.; et al. Anemia in Non-Celiac Wheat Sensitivity: Prevalence and Associated Clinical and Laboratory Features. Dig. Liver Dis. 2023, 55, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Isasi, C.; Tejerina, E.; Morán, L.M. Non-Celiac Gluten Sensitivity and Rheumatic Diseases. Reumatol. Clin. 2016, 12, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; D’Alcamo, A.; Cavataio, F.; Soresi, M.; Seidita, A.; Sciumè, C.; Geraci, G.; Iacono, G.; Mansueto, P. High Proportions of People with Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology 2015, 149, 596–603.e1. [Google Scholar] [CrossRef]
- Mansueto, P.; Soresi, M.; Candore, G.; Garlisi, C.; Fayer, F.; Gambino, C.M.; La Blasca, F.; Seidita, A.; D’Alcamo, A.; Lo Sasso, B.; et al. Autoimmunity Features in Patients With Non-Celiac Wheat Sensitivity. Am. J. Gastroenterol. 2021, 116, 1015–1023. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Bruemmer, D.; Collins, B.S.; Ekhlaspour, L.; Gaglia, J.L.; Hilliard, M.E.; Johnson, E.L.; Khunti, K.; et al. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- de Candia, P.; Prattichizzo, F.; Garavelli, S.; De Rosa, V.; Galgani, M.; Di Rella, F.; Spagnuolo, M.I.; Colamatteo, A.; Fusco, C.; Micillo, T.; et al. Type 2 Diabetes: How Much of an Autoimmune Disease? Front. Endocrinol. 2019, 10, 451. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef]
- Carroccio, A.; Giambalvo, O.; Blasca, F.; Iacobucci, R.; D’Alcamo, A.; Mansueto, P. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 2017, 9, 771. [Google Scholar] [CrossRef]
- Seror, R.; Ravaud, P.; Mariette, X.; Bootsma, H.; Theander, E.; Hansen, A.; Ramos-Casals, M.; Dörner, T.; Bombardieri, S.; Hachulla, E.; et al. EULAR Sjögren’s Syndrome Patient Reported Index (ESSPRI): Development of a Consensus Patient Index for Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2011, 70, 968–972. [Google Scholar] [CrossRef]
- Seror, R.; Bowman, S.J.; Brito-Zeron, P.; Theander, E.; Bootsma, H.; Tzioufas, A.; Gottenberg, J.-E.; Ramos-Casals, M.; Dörner, T.; Ravaud, P.; et al. EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI): A User Guide. RMD Open 2015, 1, e000022. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dörner, T.; Fisher, B.A.; Gottenberg, J.-E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR Recommendations for the Management of Sjögren’s Syndrome with Topical and Systemic Therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Xiao, X.; Wang, Y.; Huang, B.; Chen, J.; Cheng, G.; Jin, Y. Metabolic Impact of Low Dose IL-2 Therapy for Primary Sjögren’s Syndrome in a Double-Blind, Randomized Clinical Trial. Clin. Rheumatol. 2024, 43, 3789–3798. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, J.; Miao, M.; Zhang, R.; Cheng, G.; Wang, Y.; Feng, R.; Huang, B.; Luan, H.; Jia, Y.; et al. Efficacy and Safety of Low-Dose Interleukin 2 for Primary Sjögren Syndrome. JAMA Netw. Open 2022, 5, e2241451. [Google Scholar] [CrossRef]
- Seth, N.P.; Xu, R.; DuPrie, M.; Choudhury, A.; Sihapong, S.; Tyler, S.; Meador, J.; Avery, W.; Cochran, E.; Daly, T.; et al. Nipocalimab, an Immunoselective FcRn Blocker That Lowers IgG and Has Unique Molecular Properties. mAbs 2025, 17, 2461191. [Google Scholar] [CrossRef]
- Hubbard, J.; Campbell, K.; Sivils, K.; Hoffman, R.; Lo, K.H.; Leu, J.H.; Bowman, S.; Liva, S.; Zuraw, Q.; Stevens, A.M.; et al. POS1130 Designing of A Phase 2, Multicenter, Randomized, Placebo-Controlled, Double-Blind Study to Assess the Efficacy and Safety of Nipocalimab, an FcRn Inhibitor, in Adults With Primary Sjögren’s Syndrome. Ann. Rheum. Dis. 2023, 82, 892–893. [Google Scholar] [CrossRef]
- Dörner, T.; Posch, M.G.; Li, Y.; Petricoul, O.; Cabanski, M.; Milojevic, J.M.; Kamphausen, E.; Valentin, M.-A.; Simonett, C.; Mooney, L.; et al. Treatment of Primary Sjögren’s Syndrome with Ianalumab (VAY736) Targeting B Cells by BAFF Receptor Blockade Coupled with Enhanced, Antibody-Dependent Cellular Cytotoxicity. Ann. Rheum. Dis. 2019, 78, 641–647. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Mai, F.; Mercuri, A.; Centorame, D.; Cipollone, J.; Mariani, F.M.; Rossi, M.; Bartoloni, E.; Grassi, D.; et al. Adherence to the Mediterranean Diet and the Impact on Clinical Features in Primary Sjögren’s Syndrome. Clin. Exp. Rheumatol. 2021, 39, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liang, Y.; Liu, Y.; Li, Y.; Shen, L.; Shi, G. High-Fat Diet-Induced Intestinal Dysbiosis Is Associated with the Exacerbation of Sjogren’s Syndrome. Front. Microbiol. 2022, 13, 916089. [Google Scholar] [CrossRef] [PubMed]
- Haupt-Jorgensen, M.; Groule, V.; Reibel, J.; Buschard, K.; Pedersen, A.M.L. Gluten-free Diet Modulates Inflammation in Salivary Glands and Pancreatic Islets. Oral Dis. 2022, 28, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Carroccio, A.; Mansueto, P.; D’Alcamo, A.; Iacono, G. Non-Celiac Wheat Sensitivity as an Allergic Condition: Personal Experience and Narrative Review. Am. J. Gastroenterol. 2013, 108, 1845–1852. [Google Scholar] [CrossRef]
- Zhang, S.; Sicherer, S.; Berin, M.C.; Agyemang, A. Pathophysiology of Non-IgE-Mediated Food Allergy. Immunotargets Ther. 2021, 10, 431–446. [Google Scholar] [CrossRef]
- Venter, C.; Brown, T.; Meyer, R.; Walsh, J.; Shah, N.; Nowak-Węgrzyn, A.; Chen, T.-X.; Fleischer, D.M.; Heine, R.G.; Levin, M.; et al. Better Recognition, Diagnosis and Management of Non-IgE-Mediated Cow’s Milk Allergy in Infancy: IMAP—An International Interpretation of the MAP (Milk Allergy in Primary Care) Guideline. Clin. Transl. Allergy 2017, 7, 26. [Google Scholar] [CrossRef]
- Veenbergen, S.; Kozmar, A.; van Daele, P.L.A.; Schreurs, M.W.J. Autoantibodies in Sjögren’s Syndrome and Its Classification Criteria. J. Transl. Autoimmun. 2022, 5, 100138. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Claude Mbanya, J.; et al. Erratum to “IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045” [Diabetes Res. Clin. Pract. 183 (2022) 109119]. Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, Prevalence, and Co-Occurrence of Autoimmune Disorders over Time and by Age, Sex, and Socioeconomic Status: A Population-Based Cohort Study of 22 Million Individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Kim-Lee, C.; Suresh, L.; Ambrus, J.L. Gastrointestinal Disease in Sjogren’s Syndrome: Related to Food Hypersensitivities. SpringerPlus 2015, 4, 766. [Google Scholar] [CrossRef] [PubMed]
- Parreau, S.; Jacques, J.; Dumonteil, S.; Palat, S.; Geyl, S.; Gondran, G.; Bezanahary, H.; Liozon, E.; Azaïs, J.; Colombie, S.; et al. Abdominal Symptoms during Sjogren’s Syndrome: A Pilot Study. Adv. Rheumatol. 2021, 61, 5. [Google Scholar] [CrossRef] [PubMed]
- Erbasan, F.; Çekin, Y.; Turgut Çoban, D.; Karasu, U.; Süren, D.; Çekin, A.H. The Frequency of Primary Sjogren’s Syndrome and Fibromyalgia in Irritable Bowel Syndrome. Pak. J. Med. Sci. 2017, 33, 137. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Walker, M.M.; Jones, M.P.; Koloski, N.A.; Keely, S.; Talley, N.J. Wheat Intolerance and Chronic Gastrointestinal Symptoms in an Australian Population-Based Study: Association Between Wheat Sensitivity, Celiac Disease and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2018, 113, 1036–1044. [Google Scholar] [CrossRef]
- Potter, M.D.; Jones, M.P.; Walker, M.M.; Koloski, N.A.; Keely, S.; Holtmann, G.; Talley AC, N.J. Incidence and Prevalence of Self-reported Non-coeliac Wheat Sensitivity and Gluten Avoidance in Australia. Med. J. Aust. 2020, 212, 126–131. [Google Scholar] [CrossRef]
- Ontiveros, N.; Real-Delor, R.E.; Mora-Melgem, J.A.; Beltrán-Cárdenas, C.E.; Figueroa-Salcido, O.G.; Vergara-Jiménez, M.d.J.; Cárdenas-Torres, F.I.; Flores-Mendoza, L.K.; Arámburo-Gálvez, J.G.; Cabrera-Chávez, F. Prevalence of Wheat/Gluten-Related Disorders and Gluten-Free Diet in Paraguay: An Online Survey-Based Study. Nutrients 2021, 13, 396. [Google Scholar] [CrossRef]
- Shahbazkhani, B.; Fanaeian, M.M.; Farahvash, M.J.; Aletaha, N.; Alborzi, F.; Elli, L.; Shahbazkhani, A.; Zebardast, J.; Rostami-Nejad, M. Prevalence of Non-Celiac Gluten Sensitivity in Patients with Refractory Functional Dyspepsia: A Randomized Double-Blind Placebo Controlled Trial. Sci. Rep. 2020, 10, 2401. [Google Scholar] [CrossRef]
- Dhoble, P.; Abraham, P.; Desai, D.; Joshi, A.; Gupta, T.; Doctor, S.; Deshpande, A.; Basavanna, R. Self-Reported Wheat Sensitivity in Irritable Bowel Syndrome and Healthy Subjects: Prevalence of Celiac Markers and Response to Wheat-Free Diet. J. Neurogastroenterol. Motil. 2021, 27, 596–601. [Google Scholar] [CrossRef]
- Cha, R.R.; Kim, J.H.; Koo, H.S.; Jung, K.W.; Min, Y.W.; Choi, C.H.; Ryu, H.S.; Kwon, Y.H.; Cho, D.H.; Kwon, J.G.; et al. Self-Reported Non-Celiac Gluten Sensitivity in the Korean Population: Demographic and Clinical Characteristics. J. Neurogastroenterol. Motil. 2022, 28, 283–290. [Google Scholar] [CrossRef]
- Aziz, I.; Lewis, N.R.; Hadjivassiliou, M.; Winfield, S.N.; Rugg, N.; Kelsall, A.; Newrick, L.; Sanders, D.S. A UK Study Assessing the Population Prevalence of Self-Reported Gluten Sensitivity and Referral Characteristics to Secondary Care. Eur. J. Gastroenterol. Hepatol. 2014, 26, 33–39. [Google Scholar] [CrossRef]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of Gluten-Related Disorders: Consensus on New Nomenclature and Classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef]
- Carroccio, A.; Soresi, M.; Chiavetta, M.; La Blasca, F.; Compagnoni, S.; Giuliano, A.; Fayer, F.; Mandreucci, F.; Castellucci, D.; Seidita, A.; et al. Frequency and Clinical Aspects of Neurological and Psychiatric Symptoms in Patients with Non-Celiac Wheat Sensitivity. Nutrients 2021, 13, 1971. [Google Scholar] [CrossRef] [PubMed]
- Rastmanesh, R.; Isacco, C.G.; Vellingiri, B.; Pepoyan, A.; Marotta, F.; Tekin, I.; Catanzaro, R. Potassium-Rich, Gluten-Free Diets for Patients with Sjögren’s Syndrome: A Hypothesis. Endocr. Metab. Immune Disord. Drug Targets 2025, 25. [Google Scholar] [CrossRef] [PubMed]
- Hollon, J.; Puppa, E.; Greenwald, B.; Goldberg, E.; Guerrerio, A.; Fasano, A. Effect of Gliadin on Permeability of Intestinal Biopsy Explants from Celiac Disease Patients and Patients with Non-Celiac Gluten Sensitivity. Nutrients 2015, 7, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Pickert, G.; Ashfaq-Khan, M.; Zevallos, V. Non-Celiac Wheat Sensitivity: Differential Diagnosis, Triggers and Implications. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 469–476. [Google Scholar] [CrossRef]
| pSS (N = 82) | Controls (N = 161) | p | |
|---|---|---|---|
| Age (years) (mean ± SD) | 62.5 ± 11.1 | 63.8 ± 12.7 | NS |
| Gender (n, %) | |||
| 1. Male | 4 (4.9) | 20 (12.4) | NS |
| 2. Female | 78 (91.0) | 141 (87.6) | |
| Ethnicity (n, %) | |||
| 1. Caucasian | 82 (100.0) | 146 (90.7) | 0.03 |
| 2. African | 0 (0.0) | 15 (9.3) | NS |
| 3. Asian | 0 (0.0) | 0 (0.0) | NS |
| 4. Middle Eastern | 0 (0.0) | 0 (0.0) | NS |
| Marital status (n, %) | |||
| 1. Single | 11 (13.4) | 12 (7.5) | NS |
| 2. Married | 58 (70.7) | 125 (77.6) | NS |
| 3. Divorced | 3 (3.7) | 9 (5.6) | NS |
| 4. Widowed | 10 (12.2) | 15 (9.3) | NS |
| Qualifications (n, %) | |||
| 1. None | 3 (3.7) | 6 (3.7) | NS |
| 2. Elementary school diploma | 8 (9.8) | 51 (31.7) | 0.0003 |
| 3. Middle school diploma | 32 (39.0) | 86 (53.4) | 0.047 |
| 4. High school diploma | 29 (35.4) | 12 (7.5) | <0.0001 |
| 5. University degree | 10 (12.2) | 6 (3.7) | 0.025 |
| Employment status (n, %) | |||
| 1. Employee | 16 (19.5) | 36 (22.4) | NS |
| 2. Freelance/Professional | 5 (6.1) | 6 (3.7) | NS |
| 3. Laborer/unskilled worker, craftsperson | 2 (2.4) | 0 (0.0) | NS |
| 4. Unemployed | 43 (52.5) | 110 (68.3) | 0.022 |
| 5. Looking for work | 2 (2.4) | 3 (1.9) | NS |
| 6. Unable to work | 0 (0.0) | 0 (0.0) | NS |
| 7. Other | 14 (17.1) | 6 (3.7) | 0.0009 |
| pSS (N = 82) | Controls (N = 161) | p | |
|---|---|---|---|
| Episodes of abdominal pain or heaviness or discomfort in the last 12 months (n, %) | 56 (68.3) | 69 (42.9) | 0.0003 |
| Frequency of episodes of abdominal pain or heaviness or discomfort (n, %) | N = 56 | N = 69 | |
| 1. One day a month | 9 (16.1) | 9 (13) | NS |
| 2. Two days a month | 5 (8.9) | 12 (17.4) | NS |
| 3. Three days a month | 8 (14.3) | 12 (17.4) | NS |
| 4. Four days a month | 7 (12.5) | 15 (21.7) | NS |
| 5. 5–10 days per month | 9 (16.1) | 15 (21.7) | NS |
| 6. More than 10 days a month | 18 (32.1) | 6 (8.7) | 0.0021 |
| Reduction in abdominal pain or heaviness or discomfort after having a bowel movement (n, %) | N = 56 | N = 69 | |
| 41 (73.2) | 48 (69.6) | NS | |
| Abdominal swelling in the last 12 months (n, %) | 50 (60.9) | 39 (24.2) | <0.0001 |
| Reduction in abdominal swelling after having a bowel movement (n, %) | N = 50 | N = 39 | |
| 37 (74.0) | 28 (71.8) | NS | |
| Self-reported association between intestinal disorders and a change in usual bowel moments (n, %) | N = 56 | N = 69 | |
| 42 (75.0) | 39 (56.5) | 0.049 | |
| Type of modification in usual bowel movements (n, %) | N = 42 | N = 39 | |
| 1. Diarrhea | 10 (23.8) | 5 (12.8) | NS |
| 2. Constipation | 13 (31.0) | 17 (43.6) | NS |
| 3. Mixed bowel movements | 11 (26.2) | 8 (20.5) | NS |
| 4. Other | 8 (19.0) | 9 (23.1) | NS |
| Association between intestinal disorders and episodes of psychophysical stress (n, %) | N = 56 | N = 69 | |
| 42 (75.0) | 39 (56.5) | 0.049 | |
| Associated comorbidities (n, %) | |||
| 1. Anxiety | 45 (54.9) | 51 (31.7) | 0.0008 |
| 2. Depression | 19 (23.2) | 24 (14.9) | NS |
| 3. Bipolar disorder | 2 (2.4) | 0 (0.0) | NS |
| 4. Schizophrenia | 0 (0.0) | 0 (0.0) | NS |
| 5. Thyroid diseases | 27 (32.9) | 15 (9.3) | <0.0001 |
| 6. Diabetes mellitus | 5 (6.1) | 161 (100.0) | <0.0001 |
| 7. Pernicious anemia (vitamin B12 deficiency) | 2 (2.4) | 3 (1.9) | NS |
| 8. Chronic fatigue | 27 (32.9) | 48 (29.8) | NS |
| 9. Fibromyalgia | 24 (29.3) | 0 (0.0) | <0.0001 |
| 10. Chronic intestinal inflammatory diseases | 0 (0.0) | 0 (0.0) | NS |
| 11. Chronic headache | 16 (19.5) | 9 (5.6) | 0.001 |
| 12. Irritable bowel syndrome | 33 (40.2) | 9 (5.6) | <0.0001 |
| 13. Celiac disease | 0 (0.0) | 0 (0.0) | NS |
| 14. Gastroesophageal reflux | 42 (51.2) | 66 (41.0) | NS |
| 15. Other autoimmune diseases | 19 (23.1) | 0 (0.0) | <0.0001 |
| 16. Other non-autoimmune diseases | 8 (9.8) | 81 (50.3) | <0.0001 |
| pSS (n = 82) | Controls (n = 161) | p | |
|---|---|---|---|
| Worsening of symptoms related to pSS after eating wheat-containing foods (n, %) | 18 (21.9) | NA | NA |
| Clinical manifestations worsened following wheat-containing food intake (n, %) | N = 18 | ||
| 1. Xerostomia | 8 (44.4) | ||
| 2. Xerophthalmia | 2 (11.2) | NA | NA |
| 3. Xerostomia and xerophthalmia | 8 (44.4) | ||
| Degree of symptom worsening (n, %) | N = 18 | ||
| 1. Mild | 6 (33.3) | ||
| 2. Moderate | 9 (50.0) | NA | NA |
| 3. Severe | 3 (16.7) | ||
| Improvement in one or more of the symptoms related to pSS by eliminating wheat-containing foods from the diet (n, %) | N = 18 | ||
| 11 (61.1) | NA | NA | |
| Clinical manifestations improved following wheat-containing food elimination (n, %) | N = 11 | ||
| 1. Xerostomia | 3 (27.3) | ||
| 2. Xerophthalmia | 1 (9.1) | NA | NA |
| 3. Xerostomia and xerophthalmia | 7 (63.6) | ||
| Degree of symptom improvement (n, %) | N = 11 | ||
| 1. Very slight improvement | 0 (0.0) | ||
| 2. Slight improvement | 2 (18.2) | ||
| 3. Satisfactory improvement | 5 (45.4) | NA | NA |
| 4. Clear improvement which permitted a reduction in therapy | 4 (36.4) | ||
| 5. Very clear improvement which led to the disappearance of symptoms and permitted therapy to be suspended | 0 (0.0) | ||
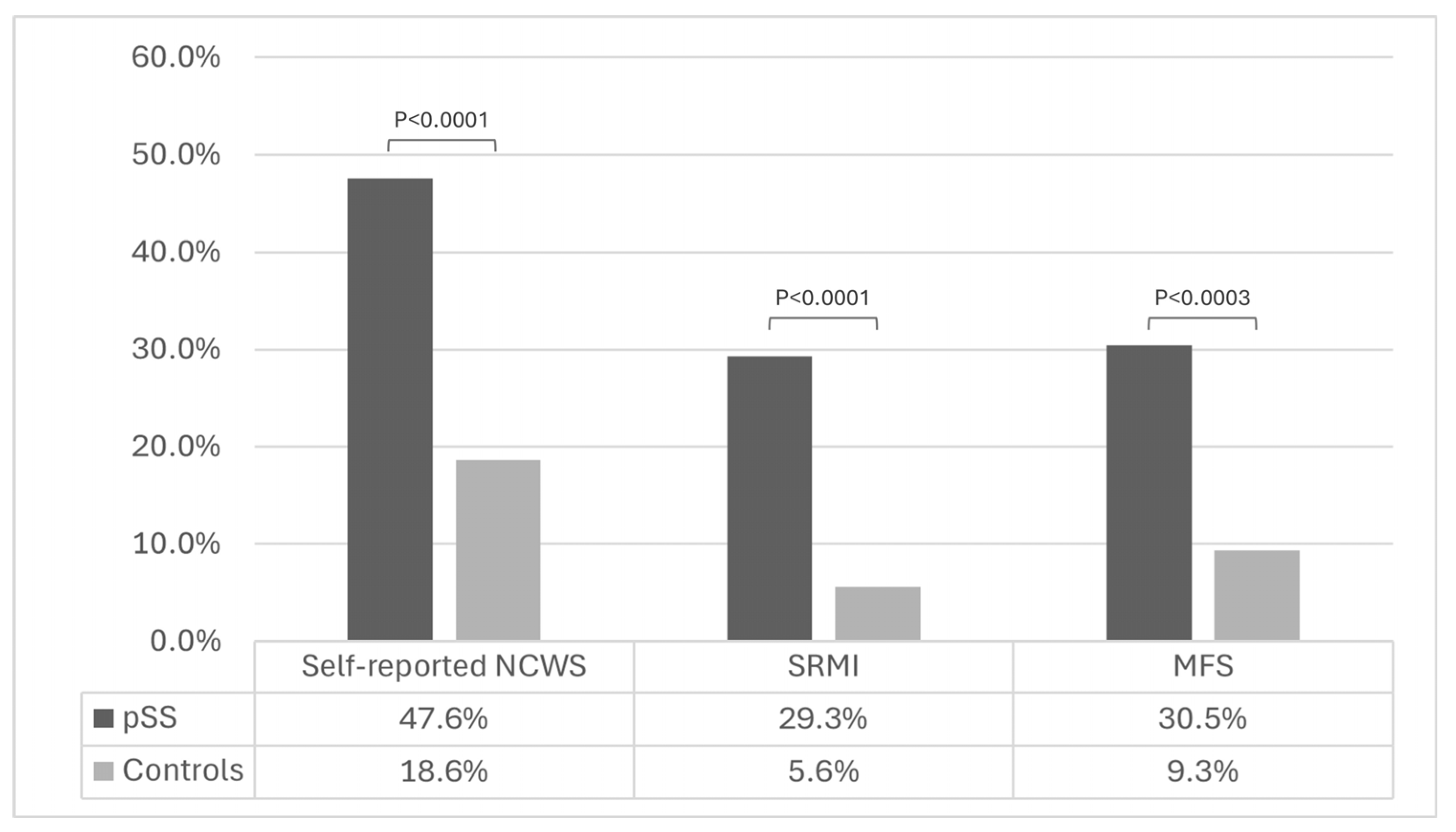
| Symptoms unrelated to pSS after eating wheat-containing products (n, %) | 39 (47.6) | 30 (18.6) | <0.0001 |
| Symptoms referred after eating wheat-containing products (n, %) | N = 39 | N = 30 | |
| 1. Intestinal bloating | 28 (71.8) | 18 (60.0) | NS |
| 2. Abdominal pain | 26 (66.7) | 8 (26.7) | 0.002 |
| 3. Abdominal heaviness | 23 (59.0) | 15 (50.0) | NS |
| 4. Diarrhea | 14 (35.9) | 3 (10.0) | 0.028 |
| 5. Constipation | 20 (51.3) | 9 (30.0) | NS |
| 6. Asthenia | 19 (48.7) | 6 (20.0) | 0.027 |
| 7. Belching | 10 (25.6) | 6 (20.0) | NS |
| 8. Flatulence | 20 (51.3) | 1 (3.3) | <0.0001 |
| 9. Nausea and/or vomiting | 12 (30.8) | 9 (30.0) | NS |
| 10. Headache | 13 (33.3) | 1 (3.3) | 0.006 |
| 11. Poor motor coordination | 7 (17.9) | 0 (0.0) | NS |
| 12. Numbness or prickling sensation on the skin | 9 (23.1) | 1 (3.3) | 0.049 |
| 13. Anemia | 6 (15.4) | 0 (0.0) | NS |
| 14. Redness of the skin | 3 (7.7) | 0 (0.0) | NS |
| 15. Joint pain | 18 (46.2) | 6 (20.0) | 0.045 |
| 16. Other (indicate) | 0 (0.0) | 0 (0.0) | NS |
| Frequency of symptoms after eating wheat-containing foods (n, %) | N = 39 | N = 30 | |
| 1. Always | 16 (41.0) | 0 (0.0) | <0.0001 |
| 2. Often (≥3 days/week) | 11 (28.2) | 24 (80.0) | <0.0001 |
| 3. A few days a week (<3 days/week) | 6 (15.4) | 0 (0.0) | NS |
| 4. A few times a month (once or more a month) | 5 (12.8) | 6 (20.0) | NS |
| 5. A few times a year (less than once a month) | 1 (2.6) | 0 (0.0) | NS |
| 6. Less than once a year | 0 (0.0) | 0 (0.0) | NS |
| Timing of symptom onset after wheat-containing food intake (n, %) | N = 39 | N = 30 | |
| 1. Almost immediately (less than an hour) | 16 (41.0) | 9 (30.0) | NS |
| 2. 1–6 h | 21 (53.8) | 18 (60.0) | NS |
| 3. 6–24 h | 0 (0.0) | 3 (10.0) | NS |
| 4. The next day | 1 (2.6) | 0 (0.0) | NS |
| 5. A few days | 1 (2.6) | 0 (0.0) | NS |
| Duration of symptoms (n, %) | N = 39 | N = 30 | |
| 1. A few minutes | 4 (10.3) | 12 (40.0) | 0.009 |
| 2. A few hours | 21 (53.8) | 15 (50.0) | NS |
| 3. A few days | 11 (28.2) | 3 (10.0) | NS |
| 4. A few weeks | 2 (5.1) | 0 (0.0) | NS |
| 5. A few months | 1 (2.6) | 0 (0.0) | NS |
| Wheat-containing foods associated with symptom onset (n, %) | N = 39 | N = 30 | |
| 1. Cereals | 5 (12.8) | 0 (0.0) | NS |
| 2. Bread | 20 (51.3) | 15 (50.0) | NS |
| 3. Pasta | 26 (66.7) | 3 (10.0) | <0.0001 |
| 4. Pizza | 19 (48.7) | 15 (50.0) | NS |
| 5. Biscuits | 8 (20.5) | 0 (0.0) | 0.008 |
| 6. Sweets/Candies | 12 (30.8) | 0 (0.0) | 0.001 |
| 7. Other (specify) | 0 (0.0) | 0 (0.0) | NS |
| Period elapsed since the first episodes of wheat intolerance (months) [Median (IQR)] | 120 (48–217) | 5.5 (2–63) | 0.0001 |
| Subjects who had consulted a doctor/dietician/health care specialist for wheat-intake-related disorders (n, %) | N = 39 | N = 30 | |
| 26 (66.7) | 18 (60.0) | NS | |
| Professional consulted (multiple answers possible) (n, %) | N = 26 | N = 18 | |
| 1. Gastroenterologist | 19 (73.1) | 6 (33.3) | 0.021 |
| 2. General practitioner | 12 (46.2) | 9 (50.0) | NS |
| 3. Dietitian | 6 (15.4) | 6 (33.3) | NS |
| 4. Allergologist | 0 (0.0) | 18 (100.0) | <0.0001 |
| 5. Hematologist | 1 (2.6) | 0 (0.0) | NS |
| 6. Rheumatologist | 1 (2.6) | 0 (0.0) | NS |
| Testing carried out (n, %) | N = 26 | N = 18 | |
| 1. Celiac disease serology | 16 (61.5) | 3 (16.7) | 0.008 |
| 2. Prick test for food allergy | 7 (17.9) | 14 (77.8) | 0.003 |
| 3. Esophagogastroduodenoscopy | 16 (61.5) | 3 (16.7) | 0.008 |
| 4. Abdomen ultrasound examination | 1 (3.8) | 3 (16.7) | NS |
| 5. Patch test for nickel allergy | 1 (3.8) | 4 (22.2) | NS |
| 6. Genetic (HLA) typing | 1 (3.8) | 0 (0.0) | NS |
| Possible explanation for referred symptoms given by specialists (n, %) | N = 26 | N = 18 | |
| 1. Celiac disease | 8 (30.8) | 3 (16.7) | NS |
| 2. Wheat allergy | 0 (0.0) | 0 (0.0) | NS |
| 3. Irritable bowel syndrome | 9 (34.6) | 1 (5.6) | NS |
| 4. NCWS | 3 (11.5) | 0 (0.0) | NS |
| 5. Gastritis | 1 (3.8) | 0 (0.0) | NS |
| 6. Diverticulosis | 1 (3.8) | 3 (16.7) | NS |
| 7. No explanation | 5 (19.2) | 12 (66.7) | 0.004 |
| Subjects who had undergone a period of wheat-containing product elimination | N = 39 | N = 30 | |
| (n, %) | 20 (51.3) | 0 (0.0) | <0.0001 |
| Improvement in one or more of the symptoms by eliminating wheat-containing foods from the diet (n, %) | N = 20 | ||
| 19 (95.0) | NA | NA | |
| Subjects following a wheat-free diet at the time of recruitment (n, %) | N = 20 | ||
| 15 (75.0) | NA | NA | |
| Intake of products containing ancient grains during wheat-free diet (n, %) | N = 20 | ||
| 10 (50.0) | NA | NA | |
| Lack of symptom onset when taking ancient grains (compared to modern grains) (n, %) | N = 10 | ||
| 8 (80.0) | NA | NA |
| pSS (n = 82) | Controls (n = 161) | p | |
|---|---|---|---|
| Worsening of the symptoms related to pSS after eating non-wheat-containing foods (n, %) | 10 (12.2) | NA | NA |
| Clinical manifestations worsened following non-wheat-containing food intake (n, %) | N = 10 | ||
| 1. Xerostomia | 5 (50.0%) | ||
| 2. Xerophthalmia | 0 (0.0) | NA | NA |
| 3. Xerostomia and xerophthalmia | 5 (50.0%) | ||
| Degree of symptom worsening (n, %) | N = 10 | ||
| 1. Mild | 1 (10.0) | ||
| 2. Moderate | 7 (70.0) | NA | NA |
| 3. Severe | 2 (20.0) | ||
| Improvement in one or more of the symptoms related to pSS by eliminating non-wheat-containing foods from the diet (n, %) | N = 10 | ||
| 8 (80.0) | NA | NA | |
| Clinical manifestations improved following non-wheat-containing food elimination (n, %) | N = 8 | ||
| 1. Xerostomia | 0 (0.0) | ||
| 2. Xerophthalmia | 3 (37.5) | NA | NA |
| 3. Xerostomia and xerophthalmia | 5 (62.5) | ||
| Degree of symptom improvement (n, %) | N = 8 | ||
| 1. Very slight improvement | 0 (0.0) | ||
| 2. Slight improvement | 3 (37.5) | ||
| 3. Satisfactory improvement | 1 (12.5) | NA | NA |
| 4. Clear improvement permitting a reduction in therapy | 4 (50.0) | ||
| 5. Very clear improvement which led to the disappearance of symptoms and permitted therapy to be suspended | 0 (0.0) | ||
| Symptoms unrelated to pSS after eating non-wheat-containing products (n, %) | 35 (42.7) | 21 (13.0) | <0.0001 |
| Symptoms referred after eating non-wheat-containing products (n, %) | N = 35 | N = 21 | |
| 1. Intestinal bloating | 19 (54.3) | 9 (42.9) | NS |
| 2. Abdominal pain | 18 (51.4) | 6 (28.6) | NS |
| 3. Abdominal heaviness | 18 (51.4) | 6 (28.6) | NS |
| 4. Diarrhea | 14 (40.0) | 1 (4.8) | 0.01 |
| 5. Constipation | 11 (31.4) | 12 (57.1) | NS |
| 6. Asthenia | 12 (34.2) | 3 (14.3) | NS |
| 7. Belching | 12 (34.2) | 1 (4.8) | 0.027 |
| 8. Flatulence | 19 (54.3) | 3 (14.3) | 0.007 |
| 9. Nausea and/or vomiting | 12 (34.3) | 6 (28.6) | NS |
| 10. Headache | 9 (25.7) | 3 (14.3) | NS |
| 11. Poor motor coordination | 5 (14.3) | 0 (0.0) | NS |
| 12. Numbness or prickling sensation on the skin | 6 (17.1) | 0 (0.0) | NS |
| 13. Anemia | 3 (8.6) | 0 (0.0) | NS |
| 14. Redness of the skin | 6 (17.1) | 0 (0.0) | NS |
| 15. Joint pain | 7 (20.0) | 0 (0.0) | NS |
| 16. Other (specify) | 0 (0.0) | 0 (0.0) | NS |
| Frequency of symptoms after eating non-wheat-containing foods (n, %) | N = 35 | N = 21 | |
| 1. Always | 14 (40.0) | 15 (71.4) | 0.045 |
| 2. Often (≥3 days/week) | 14 (40.0) | 6 (28.6) | NS |
| 3. A few days a week (<3 days/week) | 2 (5.7) | 0 (0.0) | NS |
| 4. A few times a month (once or more a month) | 4 (11.4) | 0 (0.0) | NS |
| 5. A few times a year (less than once a month) | 1 (2.9) | 0 (0.0) | NS |
| 6. Less than once a year | 0 (0.0) | 0 (0.0) | NS |
| Timing of symptom onset after non-wheat-containing food intake (n, %) | N = 35 | N = 21 | |
| 1. Almost immediately (less than an hour) | 14 (40.0) | 6 (28.6) | NS |
| 2. 1–6 h | 18 (51.4) | 12 (57.1) | NS |
| 3. 6–24 h | 3 (8.6) | 0 (0,0) | NS |
| 4. The next day | 0 (0.0) | 3 (14.3) | NS |
| 5. A few days | 0 (0.0) | 0 (0.0) | NS |
| Duration of symptoms (n, %) | N = 35 | N = 21 | |
| 1. A few minutes | 7 (20.0) | 6 (28.6) | NS |
| 2. A few hours | 22 (62.9) | 15 (71.4) | NS |
| 3. A few days | 6 (17.1) | 0 (0.0) | NS |
| 4. A few weeks | 0 (0.0) | 0 (0.0) | NS |
| 5. A few months | 0 (0.0) | 0 (0.0) | NS |
| Period elapsed since the first episodes of wheat intolerance (months) [Median (IQR)] | 129 (60–240) | 120 (36–240) | NS |
| Subjects who had consulted a doctor/dietician/health care specialist for wheat-intake-related disorders (n, %) | N = 35 | N = 21 | |
| 28 (80.0) | 3 (14.3) | <0.0001 | |
| Professional consulted (multiple answers possible) (n, %) | N = 28 | N = 3 | |
| 1. Gastroenterologist | 12 (42.9) | 2 (66.7) | NS |
| 2. General practitioner | 17 (60.7) | 2 (66.7) | NS |
| 3. Dietitian | 3 (10.7) | 0 (0.0) | NS |
| 4. Allergologist | 2 (7.1) | 0 (0.0) | NS |
| 5. Hematologist | 1 (3.6) | 0 (0.0) | NS |
| 6. Rheumatologist | 1 (3.6) | 0 (0.0) | NS |
| Testing carried out (n, %) | N = 28 | N = 3 | |
| 1. Patch test for nickel allergy | 6 (21.4) | 1 (33.3) | NS |
| 2. Prick test for food allergy | 9 (32.1) | 1 (33.3) | NS |
| 3. Lactose breath test | 11 (39.3) | 1 (33.3) | NS |
| 4. Esophagogastroduodenoscopy | 5 (17.9) | 2 (66.7) | NS |
| 5. Search for food-specific IgE | 1 (3.6) | 0 (0.0) | NS |
| Possible explanation for referred symptoms given by specialists (n, %) | N = 28 | N = 3 | |
| 1. Nickel allergy | 4 (14.3) | 0 (0.0) | NS |
| 2. Food allergy | 5 (17.9) | 0 (0.0) | NS |
| 3. Lactose intolerance | 11 (39.3) | 1 (33.3) | NS |
| 4. Irritable bowel syndrome | 10 (35.7) | 2 (66.7) | NS |
| 5. No explanation | 6 (21.4) | 1 (33.3) | NS |
| OR | CI 95% | p | |
|---|---|---|---|
| Self-reported NCWS | 3.96 | 2.2–7.13 | <0.0001 |
| SRMI | 6.99 | 3.07–15.93 | <0.0001 |
| MFS | 4.27 | 2.1–8.68 | 0.0017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidita, A.; Mansueto, P.; Soresi, M.; Di Liberto, D.; De Carlo, G.; Bisso, G.; Cosenza, S.; Pistone, M.; Giuliano, A.; Spagnuolo, G.; et al. Self-Reported Non-Celiac Wheat Sensitivity and Other Food Sensitivities in Patients with Primary Sjögren’s Syndrome. Nutrients 2025, 17, 3172. https://doi.org/10.3390/nu17193172
Seidita A, Mansueto P, Soresi M, Di Liberto D, De Carlo G, Bisso G, Cosenza S, Pistone M, Giuliano A, Spagnuolo G, et al. Self-Reported Non-Celiac Wheat Sensitivity and Other Food Sensitivities in Patients with Primary Sjögren’s Syndrome. Nutrients. 2025; 17(19):3172. https://doi.org/10.3390/nu17193172
Chicago/Turabian StyleSeidita, Aurelio, Pasquale Mansueto, Maurizio Soresi, Diana Di Liberto, Gabriele De Carlo, Gianluca Bisso, Salvatore Cosenza, Mirco Pistone, Alessandra Giuliano, Gabriele Spagnuolo, and et al. 2025. "Self-Reported Non-Celiac Wheat Sensitivity and Other Food Sensitivities in Patients with Primary Sjögren’s Syndrome" Nutrients 17, no. 19: 3172. https://doi.org/10.3390/nu17193172
APA StyleSeidita, A., Mansueto, P., Soresi, M., Di Liberto, D., De Carlo, G., Bisso, G., Cosenza, S., Pistone, M., Giuliano, A., Spagnuolo, G., Bertolino, C., Bellanti, C., Citarrella, R., La Barbera, L., Guggino, G., & Carroccio, A. (2025). Self-Reported Non-Celiac Wheat Sensitivity and Other Food Sensitivities in Patients with Primary Sjögren’s Syndrome. Nutrients, 17(19), 3172. https://doi.org/10.3390/nu17193172







