Preventing Sepsis in Preterm Infants with Bovine Lactoferrin: A Randomized Trial Exploring Immune and Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Randomization and Bias Control
2.4. Intervention
2.5. General Characteristics and Clinical History
2.6. Outcome Measures
2.6.1. LOS
2.6.2. Neonatal Complications, Overall Mortality, and Mortality Attributable to LOS
2.6.3. Biochemical, Inflammation, and Antioxidant Markers
2.7. Sample Size
2.8. Statistical Analysis
3. Results
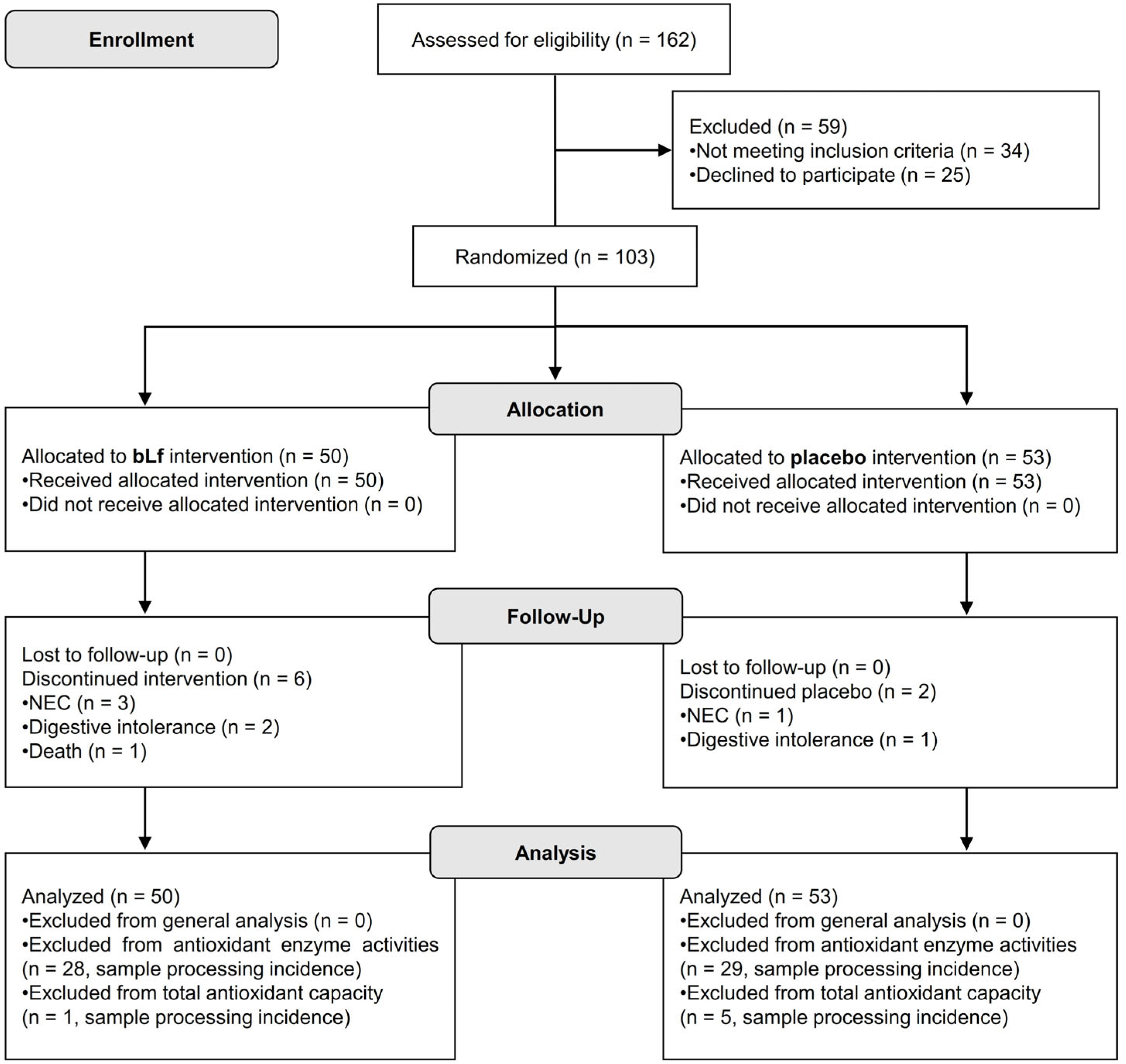
3.1. Patient Recruitment
3.2. Maternal and Newborn Characteristics
3.3. Incidence of LOS and Comorbidities Associated with Prematurity
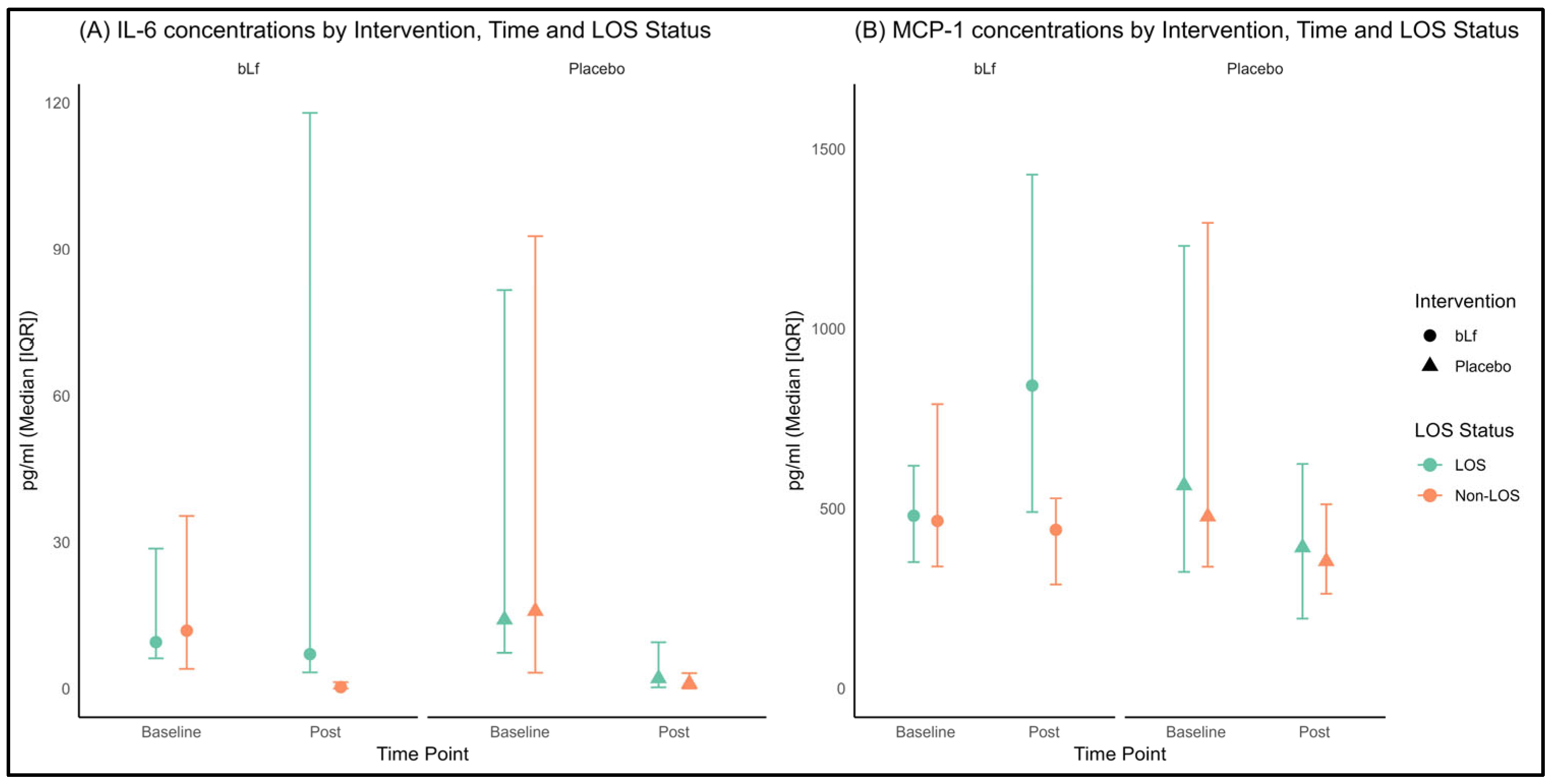
3.4. Plasma Cytokines
3.5. Antioxidant Enzyme Activities and TAC
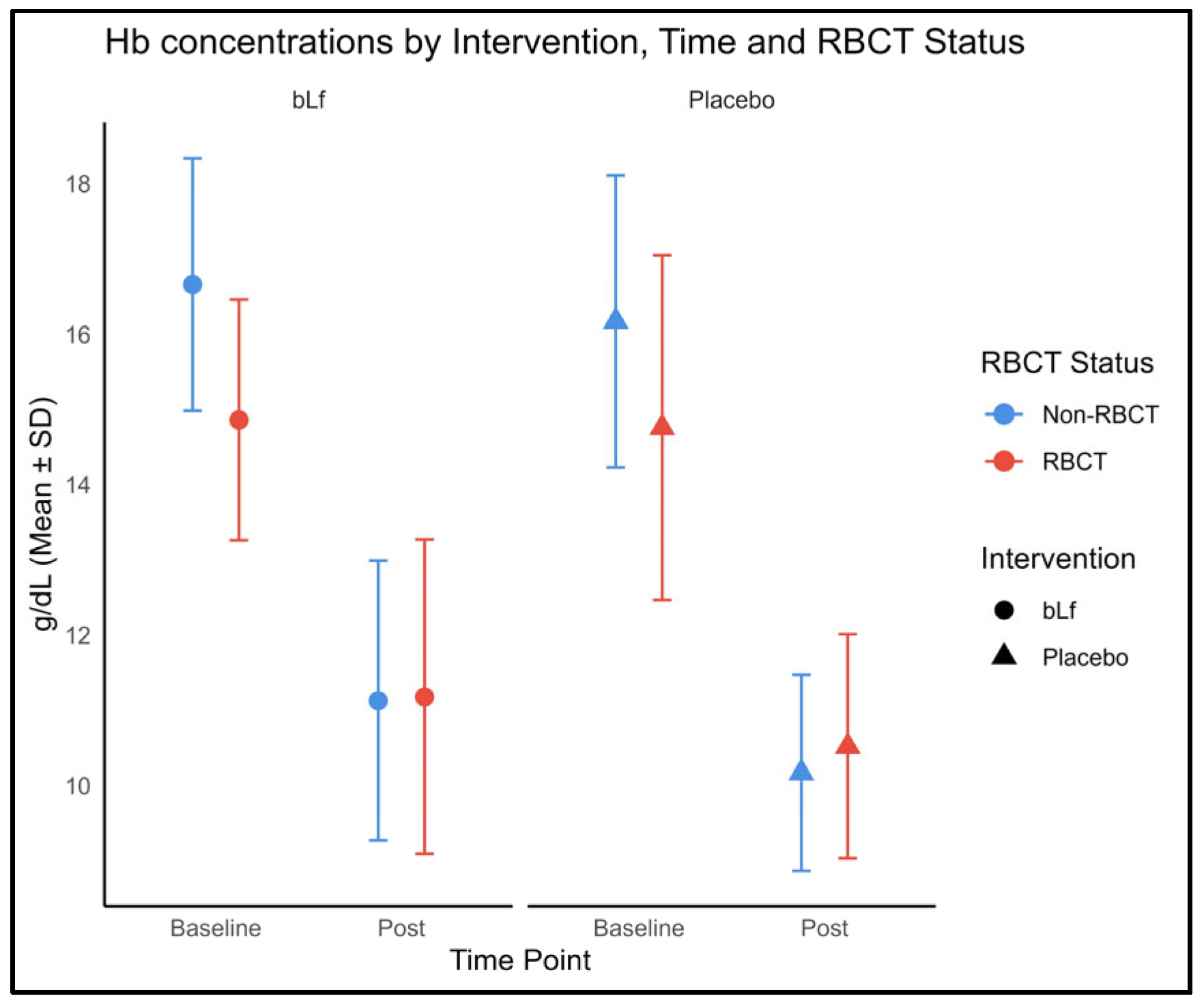
3.6. Hemoglobin Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aRR | Adjusted risk ratio |
| ANCOVA | Analysis of covariance |
| ANOVA | Analysis of variance |
| bLf | Bovine lactoferrin |
| BPD | Bronchopulmonary dysplasia |
| CAT | Catalase |
| CI | Confidence interval |
| CONSORT | Consolidated Standards of Reporting Trials statement |
| ELBW | Extremely low birth weight |
| GA | Gestational age |
| hsPDA | Hemodynamically significant patent ductus arteriosus |
| Hb | Hemoglobin |
| hLf | Human lactoferrin |
| ITT | Intention-to-treat |
| IFN-γ | Interferon-gamma |
| IL | Interleukin (IL-1β, IL-6, IL-8, IL-10) |
| IQR | Interquartile range |
| LGG | Lactobacillus rhamnosus |
| Lf | Lactoferrin |
| LOS | Late-onset sepsis |
| LPS | Lipopolysaccharide |
| mmol Trolox/L | millimolar Trolox equivalents |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NEC | Necrotizing enterocolitis |
| NICU | Neonatal Intensive Care Unit |
| NNT | Number needed to treat |
| PPIs | Proton pump inhibitors |
| RBCT | Red blood cell transfusion |
| RR | Risk ratio |
| ROP | Retinopathy of prematurity |
| SD | Standard deviation |
| TAC | Total antioxidant capacity |
| TNF-α | Tumor necrosis factor-α |
| VLBW | Very-low-birth-weight |
| χ2 | Chi-square |
References
- Fernandez Colomer, B.; Cernada Badia, M.; Coto Cotallo, D.; Lopez Sastre, J. The Spanish National Network Grupo Castrillo: 22 Years of Nationwide Neonatal Infection Surveillance. Am. J. Perinatol. 2020, 37 (Suppl. 02), S71–S75. [Google Scholar] [CrossRef] [PubMed]
- Kurul, S.; Fiebig, K.; Flint, R.B.; Reiss, I.K.M.; Küster, H.; Simons, S.H.P.; Voller, S.; Taal, H.R. Knowledge gaps in late-onset neonatal sepsis in preterm neonates: A roadmap for future research. Pediatr. Res. 2022, 91, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Tamayo, T. Analysis of Morbidity and Mortality Data Results 2019–2021 [Internet]. Available online: www.seneo.es (accessed on 3 September 2024).
- Fernández Colomer, B.; Coto Cotallo, G.D. Sepsis. Medidas de control. Profilaxis antifúngica. In Experto Universitario en Neonatología: Atención del Recién Nacido Prematuro, 2nd ed.; Editorial Médica Panamericana: Madrid, Spain, 2020. [Google Scholar]
- Chong, H.-Y.; Tan, L.T.-H.; Law, J.W.-F.; Hong, K.-W.; Ratnasingam, V.; Ab Mutalib, N.-S.; Lee, L.-H.; Letchumanan, V. Exploring the Potential of Human Milk and Formula Milk on Infants’ Gut and Health. Nutrients 2022, 14, 3554. [Google Scholar] [CrossRef] [PubMed]
- Gruden, Š.; Poklar Ulrih, N. Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef] [PubMed]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.H.; Rekdal, Ø.; Svendsen, J.S.; Gutteberg, T.J. Lactoferricin of Bovine Origin is More Active than Lactoferricins of Human, Murine and Caprine Origin. Scand. J. Infect. Dis. 1998, 30, 513–517. [Google Scholar] [CrossRef]
- Wakabayashi, H.; Yamauchi, K.; Abe, F. Quality control of commercial bovine lactoferrin. Biometals 2018, 31, 313–319. [Google Scholar] [CrossRef]
- Manzoni, P.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Stolfi, I.; Decembrino, L.; Laforgia, N.; Vagnarelli, F.; et al. Bovine Lactoferrin Supplementation for Prevention of Late-Onset Sepsis in Very Low-Birth-Weight Neonates: A Randomized Trial. JAMA. 2009, 302, 1421–1428. [Google Scholar] [CrossRef]
- Barrington, K.J.; Assaad, M.-A.; Janvier, A. The Lacuna Trial: A double-blind randomized controlled pilot trial of lactoferrin supplementation in the very preterm infant. J. Perinatol. 2016, 36, 666–669. [Google Scholar] [CrossRef]
- Kaur, G.; Gathwala, G. Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: A randomized placebo-controlled clinical trial. J. Trop. Pediatr. 2015, 61, 370–376. [Google Scholar] [CrossRef]
- Griffiths, J.; Jenkins, P.; Vargova, M.; Bowler, U.; Juszczak, E.; King, A.; Linsell, L.; Murray, D.; Partlett, C.; Patel, M.; et al. Enteral lactoferrin supplementation for very preterm infants: A randomised placebo-controlled trial (ELFIN). Lancet 2019, 393, 423–433. [Google Scholar] [CrossRef]
- Tarnow-Mordi, W.O.; Abdel-Latif, M.E.; Martin, A.; Pammi, M.; Robledo, K.; Manzoni, P.; Osborn, D.; Lui, K.; Keech, A.; Hague, W.; et al. The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): A multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc. Health 2020, 4, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Zegarra, J.; Cam, L.; Llanos, R.; Pezo, A.; Cruz, K.; Zea-Vera, A.; Cárcamo, C.; Campos, M.; Bellomo, S. Randomized Controlled Trial of Lactoferrin for Prevention of Sepsis in Peruvian Neonates Less than 2500 g. Pediatr. Infect Dis. J. 2015, 34, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Zegarra, J.; Bellomo, S.; Carcamo, C.P.; Cam, L.; Castañeda, A.; Villavicencio, A.; Gonzales, J.; Rueda, M.S.; Turin, C.G.; et al. Randomized Controlled Trial of Bovine Lactoferrin for Prevention of Sepsis and Neurodevelopment Impairment in Infants Weighing Less Than 2000 Grams. J. Pediatr. 2020, 219, 118–125.e5. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.P.; Adamkin, D.H.; Niklas, V.; Radmacher, P.; Sherman, J.; Wertheimer, F.; Petrak, K. Randomized Controlled Trial of Talactoferrin Oral Solution in Preterm Infants. J. Pediatr. 2016, 173, 68–73.e3. [Google Scholar] [CrossRef]
- Manzoni, P.; Meyer, M.; Stolfi, I.; Rinaldi, M.; Cattani, S.; Pugni, L.; Romeo, M.G.; Messner, H.; Decembrino, L.; Laforgia, N.; et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: A randomized clinical trial. Early Hum. Dev. 2014, 90, S60–S65. [Google Scholar] [CrossRef]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: A randomized controlled trial. Pediatrics 2012, 129, 116–123. [Google Scholar] [CrossRef]
- Akin, I.M.; Atasay, B.; Dogu, F.; Okulu, E.; Arsan, S.; Karatas, H.D.; Ikinciogullari, A.; Turmen, T. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am. J. Perinatol. 2014, 31, 1111–1120. [Google Scholar] [CrossRef]
- Pammi, M.; Suresh, G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2020, 3, CD007137. [Google Scholar] [CrossRef]
- Presti, S.; Manti, S.; Parisi, G.F.; Papale, M.; Barbagallo, I.A.; Volti, G.L.; Leonardi, S. Lactoferrin: Cytokine modulation and application in clinical practice. J. Clin. Med. 2021, 10, 5482. [Google Scholar] [CrossRef]
- Abd Elrahman, N.; El Dessouky, A.; Alshafei, M. Role of lactoferrin supplementation in prevention of late-onset sepsis in preterm neonates. Zagazig Univ. Med. J. 2020, 28, 261–268. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A critical player in neonatal host defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Dall’AGnola, A.; Tomé, D.; Kaufman, D.A.; Tavella, E.; Pieretto, M.; Messina, A.; De Luca, D.; Bellaiche, M.; Mosca, A.; Piloquet, H.; et al. Role of Lactoferrin in Neonates and Infants: An Update. Am. J. Perinatol. 2018, 35, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Raghuveer, T.S.; McGuire, E.M.; Martin, S.M.; A Wagner, B.; Rebouché, C.J.; Buettner, G.R.; A Widness, J. Lactoferrin in the Preterm Infants’ Diet Attenuates Iron-Induced Oxidation Products. Pediatr. Res. 2002, 52, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Falsaperla, R.; Lombardo, F.; Filosco, F.; Romano, C.; Saporito, M.A.N.; Puglisi, F.; Piro, E.; Ruggieri, M.; Pavone, P. Oxidative stress in preterm infants: Overview of current evidence and future prospects. Pharmaceuticals 2020, 13, 145. [Google Scholar] [CrossRef]
- Verani, J.R.; McGee, L.; Schrag, S.J. Prevention of perinatal group B streptococcal disease—Revised guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–36. [Google Scholar]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Cendrero, J.A.C.; Solé-Violán, J.; Benítez, A.B.; Catalán, J.N.; Fernández, J.A.; Santana, P.S.; de Castro, F.R. Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest 1999, 116, 462–470. [Google Scholar] [CrossRef]
- Rangelova, V.; Kevorkyan, A.; Raycheva, R.; Krasteva, M. Ventilator-Associated Pneumonia in the Neonatal Intensive Care Unit—Incidence and Strategies for Prevention. Diagnostics 2024, 14, 240. [Google Scholar] [CrossRef]
- Proyecto NeoKissEs. Evaluación de la Efectividad de un Sistema de Vigilancia sobre las Tasas de Infección Nosocomial en Recién Nacidos de Muy Bajo Peso. 2nd ed. Barakaldo (Bizkaia, Spain): Hospital Universitario Cruces, Biocruces Health Research Institute; 2015. Available online: http://www.redsamid.net/es/produccion/proyectos-interes/proyecto-neokisses.html (accessed on 30 September 2025).
- Walsh, M.C.; Kliegman, R.M. Necrotizing Enterocolitis: Treatment Based on Staging Criteria. Pediatr. Clin. N. Am. 1986, 33, 179–201. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia (NICHD/NHLBI/ORD Workshop Summary). Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Benitz, W.E. Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2016, 137, e20153730. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Ensor, J.; I E Snell, K.; E Harrell, F.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.Y.; Donner, A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat. Methods Med. Res. 2013, 22, 661–670. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
- Hayes, R.; Hartnett, J.; Semova, G.; Murray, C.; Murphy, K.; Carroll, L.; Plapp, H.; Hession, L.; O’tOole, J.; McCollum, D.; et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr. Res. 2023, 93, 1141–1148. [Google Scholar] [CrossRef]
- Wang, Y.; Florez, I.D.; Morgan, R.L.; Foroutan, F.; Chang, Y.; Crandon, H.N.; Zeraatkar, D.; Bala, M.M.; Mao, R.Q.; Tao, B.; et al. Probiotics, Prebiotics, Lactoferrin, and Combination Products for Prevention of Mortality and Morbidity in Preterm Infants: A Systematic Review and Network Meta-Analysis. JAMA Pediatr. 2023, 177, 1158–1167. [Google Scholar] [CrossRef]
- Razak Asif, A.H. Lactoferrin Supplementation to Prevent Late-Onset Sepsis in Preterm Infants: A Meta-Analysis. Am. J. Perinatol. 2021, 38, 283–290. [Google Scholar] [CrossRef]
- Piersigilli, F.; Van Grambezen, B.; Hocq, C.; Danhaive, O. Nutrients and microbiota in lung diseases of prematurity: The placenta-gut-lung triangle. Nutrients 2020, 12, 469. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, L.; Lu, C.; Wang, Q.; Pan, B.; Wang, Q.; Tian, J.; Ge, L. Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta Analysis With Trial Sequential Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1186. [Google Scholar] [CrossRef]
- Teng, M.; Wu, T.-J.; Jing, X.; Day, B.W.; Pritchard, K.A.; Naylor, S.; Teng, R.-J. Temporal Dynamics of Oxidative Stress and Inflammation in Bronchopulmonary Dysplasia. Int. J. Mol. Sci. 2024, 25, 10145. [Google Scholar] [CrossRef]
- Thompson, A.; Bhandari, V. Pulmonary Biomarkers of Bronchopulmonary Dysplasia. Biomark Insights. 2008, 3, 361–373. [Google Scholar] [CrossRef]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory effects of lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef]
- Gordon, S.M.; Srinivasan, L.; Catherine Harris, M.; Kilpatrick, L.E. CHAPTER 121-Cytokines and Inflammatory Response in the Fetus and Neonate. In Fetal and Neonatal Physiology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Melville, J.M.; Moss, T.J.M. The immune consequences of preterm birth. Front. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef]
- Lee, A.H.; Shannon, C.P.; Amenyogbe, N.; Bennike, T.B.; Diray-Arce, J.; Idoko, O.T.; Gill, E.E.; Ben-Othman, R.; Pomat, W.S.; van Haren, S.D.; et al. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nat. Commun. 2019, 10, 1092. [Google Scholar] [CrossRef]
- de la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin acts as an alarmin to promote the recruitment and activation of antigen-presenting cells and antigen-specific immune responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A modulator of immune and inflammatory responses. Cell Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-A.; Kruzel, M.L.; Actor, J.K. Recombinant human lactoferrin modulates human PBMC derived macrophage responses to BCG and LPS. Tuberculosis 2016, 101, S53–S62. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D.; Elass, E.; Pierce, A.; Mazurier, J. Lactoferrin and host defence: An overview of its immuno-modulating and anti-inflammatory properties. BioMetals 2004, 17, 225–229. [Google Scholar] [CrossRef]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative stress in preterm newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Lorenzetti, S.; Plösch, T.; Teller, I.C. Antioxidative molecules in human milk and environmental contaminants. Antioxidants 2021, 10, 550. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, S.; Deng, X.; Luo, Z.; Chen, A.; Yu, R. Effects of Antioxidants in Human Milk on Bronchopulmonary Dysplasia Prevention and Treatment: A Review. Front. Nutr. 2022, 9, 924036. [Google Scholar] [CrossRef]
- Bolesławska, I.; Bolesławska-Król, N.; Jakubowski, K.; Przysławski, J.; Drzymała-Czyż, S. Lactoferrin—A Regulator of Iron Homeostasis and Its Implications in Cancer. Molecules 2025, 30, 1507. [Google Scholar] [CrossRef]
- June, D.; Konstantin, A.T.; Lumbanradja, L.A.; Aryani, A.; Hengky, A. Lactoferrin as treatment for iron-deficiency anemia in children: A systematic review. Turk. J. Pediatr. 2023, 65, 543–554. [Google Scholar] [CrossRef]
| Condition | Diagnostic Criteria | References * |
|---|---|---|
| NEC | Diagnosed according to the modified Bell staging criteria. | [32] |
| ROP | Classified with the International Classification of Retinopathy of Prematurity. | [33] |
| BPD | Dependency on supplemental oxygen for ≥28 days, with severity graded at 36 weeks post-menstrual age as mild (room air), moderate (<30% O2), or severe (≥30% O2 and/or positive-pressure support), per National Institute of Child Health and Human Development criteria. | [34] |
| hsPDA | Echocardiographic evidence of left-to-right shunting (ductal diameter > 1.5 mm, left-atrium–to-aortic-root > 1.5, and/or diastolic flow reversal in the descending aorta) plus clinical signs of pulmonary over-circulation and systemic hypoperfusion requiring medical or surgical intervention. | [35] |
| Brain injury | Pathologic findings on standardized cranial imaging (ultrasonography and/or magnetic resonance imaging) obtained at discharge. | - |
| Overall mortality | All causes of mortality during hospitalization. | - |
| Mortality due to LOS | Any death occurring after a LOS episode in the absence of other evident causes. | - |
| bLf (n = 50) | Placebo (n = 53) | p Value | |
|---|---|---|---|
| Maternal age, years | 33.82 ± 5.14 | 33.81 ± 4.01 | 0.992 |
| Preeclampsia | 11 (22) | 10 (18.9) | 0.693 |
| Maternal diabetes | 2 (4) | 4 (7.5) | 0.679 |
| Maternal chorioamnionitis | 5 (10) | 9 (17) | 0.301 |
| Complete course of antenatal corticosteroid | 36 (72) | 40 (75.5) | 0.689 |
| Maternal antibiotic administration | 25 (50) | 24 (45.3) | 0.632 |
| Vertical sepsis risk factors | 21 (42) | 23 (43.4) | 0.886 |
| bLf (n = 50) | Placebo (n = 53) | p Value | |
|---|---|---|---|
| General characteristics | |||
| GA, weeks | 30.04 ± 2.12 | 30.15 ± 2.23 | 0.797 |
| GA ≤ 29 weeks | 17 (34) | 18 (34) | 0.997 |
| Sex, male | 29 (58) | 25 (47.2) | 0.271 |
| Birth weight, g | 1357.02 ± 312.53 | 1298.75 ± 289.51 | 0.328 |
| Birth weight, z-score | −0.28 ± 0.90 | −0.52 ± 0.95 | 0.178 |
| Birth length, cm | 39.23± 3.19 | 39.08 ± 3.46 | 0.820 |
| Birth length, z-score | −0.10 ± 1.00 | −0.22 ± 1.15 | 0.574 |
| Head circumference at birth, cm | 27.61 ± 2.47 | 27.47 ± 2.00 | 0.754 |
| Head circumference at birth, z-score | −0.05 ± 1.12 | −0.17 ± 1.15 | 0.598 |
| Therapeutic interventions | |||
| Surfactant | 30 (60) | 30 (56.6) | 0.842 |
| Intubation | 26 (52) | 21 (39.60) | 0.208 |
| Intubation ≥ 48 h | 14 (28) | 12 (22.6) | 0.532 |
| Duration of respiratory support, days | 18.94 ± 25.83 | 19.17 ± 22.39 | 0.961 |
| Blood product transfusion | 22 (44) | 19 (35.8) | 0.398 |
| Postnatal antibiotic use | 31 (62) | 33 (62.3) | 0.978 |
| PPIs use | 22 (44) | 23 (43.4) | 0.951 |
| Nutritional characteristics | |||
| Duration of parenteral nutrition, days | 15.14 ± 13.76 | 15.04 ± 14.90 | 0.971 |
| Time to first feed, days | 1.84 ± 1.14 | 1.57 ± 0.89 | 0.180 |
| Time to achieve full enteral feeding, days | 16.75 ± 12.32 | 16.68 ± 11.27 | 0.976 |
| Complete enteral feeding with exclusively own mother’s milk or mixed feeding * | 37 (77.1) | 38 (71.7) | 0.503 |
| Characteristics at discharge † | |||
| Total hospital stay, days | 49.40 ± 22.86 | 50.43 ± 20.91 | 0.811 |
| Weight at discharge, g | 2612.65 ± 400.96 | 2581.28 ± 320.93 | 0.664 |
| Weight at discharge, z-score | −1.00 ± 0.86 | −1.01 ± 1.06 | 0.970 |
| Length at discharge, cm | 47.10 ± 2.30 | 47.24 ± 2.31 | 0.762 |
| Length at discharge, z-score | −0.68 ± 0.93 | −0.58 ± 1.29 | 0.667 |
| Head circumference at discharge, cm | 33.13 ± 1.02 | 33.32 ± 1.12 | 0.371 |
| Head circumference at discharge, z-score | −0.33 ± 0.75 | −0.17 ± 0.90 | 0.362 |
| bLf | Placebo | Crude RR with bLf (95% CI) | aRR (95% CI) a | p Value b | |
|---|---|---|---|---|---|
| LOS | 11/50 (22) | 21/53 (39.6) | 0.55 (0.299–1.031) | 0.536 (0.308–0.934) | 0.028 |
| BPD | 9/48 (18.8) | 17/53 (32.1) | 0.585 (0.288–1.186) | 0.695 (0.398–1.214) | 0.201 |
| BPD moderate/severe | 4/48 (8.3) | 4/53 (7.5) | 1.104 (0.292–4.174) | - | 1 † |
| NEC | 3/50 (6) | 2/53 (3.8) | 1.590 (0.277–9.122) | - | 0.672 † |
| hsPDA | 8/50 (16) | 9/53 (17) | 0.942 (0.395–2.250) | - | 0.893 |
| ROP | 1/48 (2.1) | 2/53 (3.8) | 0.552 (0.052–5.897) | - | 1 † |
| Brain injury at discharge | 2/50 (4) | 4/53 (7.5) | 0.530 (0.102–2.767) | - | 0.679 † |
| Mortality | 2/50 (4) | 0/53 (0) | - | - | 0.233 † |
| Mortality due to LOS | 1/50 (2) | 0/53 (0) | - | - | 0.485 † |
| bLf | Placebo | p Value | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Baseline | Final Intervention | N | Baseline | Final Intervention | Time | T × I | |
| Cytokines | ||||||||
| IFNγ, pg/mL | 50 | 3.58 [0.61–10.87] | 16.03 [8.21–30.04] b | 53 | 0.61 [0.61–12.51] | 13.74 [5.63–35.5] b | <0.001 | 0.502 |
| IL10, pg/mL | 50 | 34.71 [18.61–122.62] | 15.04 [9.29–26.26] b | 53 | 40.27 [21.06–132.54] | 14.61 [9.65–23.24] b | <0.001 | 0.497 |
| IL1β, pg/mL | 50 | 0.58 [0.01–1.60] | 1.59 [0.29–3.01] | 53 | 0.68 [0.01–3.59] | 1.55 [0.67–2.64] | 0.137 | 0.564 |
| IL6, pg/mL | 50 | 11.48 [4.81–29.57] | 0.66 [0.03–3.19] b | 53 | 14.14 [4.51–81.65] | 1.34 [0.03–5.07] b | <0.001 | 0.601 |
| IL8, pg/mL | 50 | 59.38 [29.11–106.80] | 26.28 [13.79–76.79] b | 53 | 101.4 [45.73–291.81] a | 22.81 [12.36–67.46] b | <0.001 | 0.073 |
| TNFα, pg/mL | 50 | 31.52 [22.77- 48.42] | 29.13 [20.64–42.63] | 53 | 36.82 [24.88–44.44] | 26.33 [18.81–41.83] b | 0.004 | 0.276 |
| MCP-1, pg/mL | 50 | 466.90 [348.31–758.67] | 457.95 [304.43–581.16] | 53 | 544.91 [333.16–1251.92] | 362.5 [257.73–524.18] a,b | <0.001 | 0.022 |
| Antioxidant Enzyme Activities and TAC | ||||||||
| GR, U enzyme/g Hb | 22 | 1.27 [1.01–1.67] | 1.28 [1.07–1.58] | 24 | 1.11 [0.77–1.64] | 1.31 [0.96–1.65] | 0.290 | 0.715 |
| GPx, U enzyme/g Hb | 23 | 27.38 [19.35–35.57] | 29.76 [18.90–35.27] | 24 | 25.89 [19.98–28.31] | 24.18 [17.26–30.36] | 0.871 | 0.131 |
| SOD, U enzyme/mg Hb | 23 | 0.11 [0.07–0.17] | 0.12 [0.09–0.14] | 24 | 0.10 [0.08–0.13] | 0.11 [0.08–0.14] | 0.400 | 0.213 |
| CAT, U enzyme/mg Hb | 23 | 1.05 [0.98–1.08] | 1.01 [0.94–1.06] b | 24 | 1.06 [1.01–1.10] | 1.05 [0.98–1.09] | 0.007 | 0.528 |
| TAC, mmol of Trolox/L of cell lysate | 49 | 0.88 [0.57–1.00] | 0.85 [0.69–0.96] | 48 | 0.91 [0.78–1.05] | 0.78 [0.62–0.96] b | 0.006 | 0.108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Astasio, V.; Pastor-Villaescusa, B.; Rico-Prados, M.C.; Mesa-García, M.D.; Párraga-Quiles, M.J.; Ruiz-González, M.D.; Jaraba-Caballero, P.; Tofé-Valera, I.; de la Torre-Aguilar, M.J.; Ordóñez-Díaz, M.D. Preventing Sepsis in Preterm Infants with Bovine Lactoferrin: A Randomized Trial Exploring Immune and Antioxidant Effects. Nutrients 2025, 17, 3154. https://doi.org/10.3390/nu17193154
Plaza-Astasio V, Pastor-Villaescusa B, Rico-Prados MC, Mesa-García MD, Párraga-Quiles MJ, Ruiz-González MD, Jaraba-Caballero P, Tofé-Valera I, de la Torre-Aguilar MJ, Ordóñez-Díaz MD. Preventing Sepsis in Preterm Infants with Bovine Lactoferrin: A Randomized Trial Exploring Immune and Antioxidant Effects. Nutrients. 2025; 17(19):3154. https://doi.org/10.3390/nu17193154
Chicago/Turabian StylePlaza-Astasio, Virginia, Belén Pastor-Villaescusa, Mª Cruz Rico-Prados, María Dolores Mesa-García, María José Párraga-Quiles, María Dolores Ruiz-González, Pilar Jaraba-Caballero, Inés Tofé-Valera, María José de la Torre-Aguilar, and María Dolores Ordóñez-Díaz. 2025. "Preventing Sepsis in Preterm Infants with Bovine Lactoferrin: A Randomized Trial Exploring Immune and Antioxidant Effects" Nutrients 17, no. 19: 3154. https://doi.org/10.3390/nu17193154
APA StylePlaza-Astasio, V., Pastor-Villaescusa, B., Rico-Prados, M. C., Mesa-García, M. D., Párraga-Quiles, M. J., Ruiz-González, M. D., Jaraba-Caballero, P., Tofé-Valera, I., de la Torre-Aguilar, M. J., & Ordóñez-Díaz, M. D. (2025). Preventing Sepsis in Preterm Infants with Bovine Lactoferrin: A Randomized Trial Exploring Immune and Antioxidant Effects. Nutrients, 17(19), 3154. https://doi.org/10.3390/nu17193154








