Impact of Nutritional Status on Clinical Outcomes of Patients Undergoing PRGF Treatment for Knee Osteoarthritis—A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
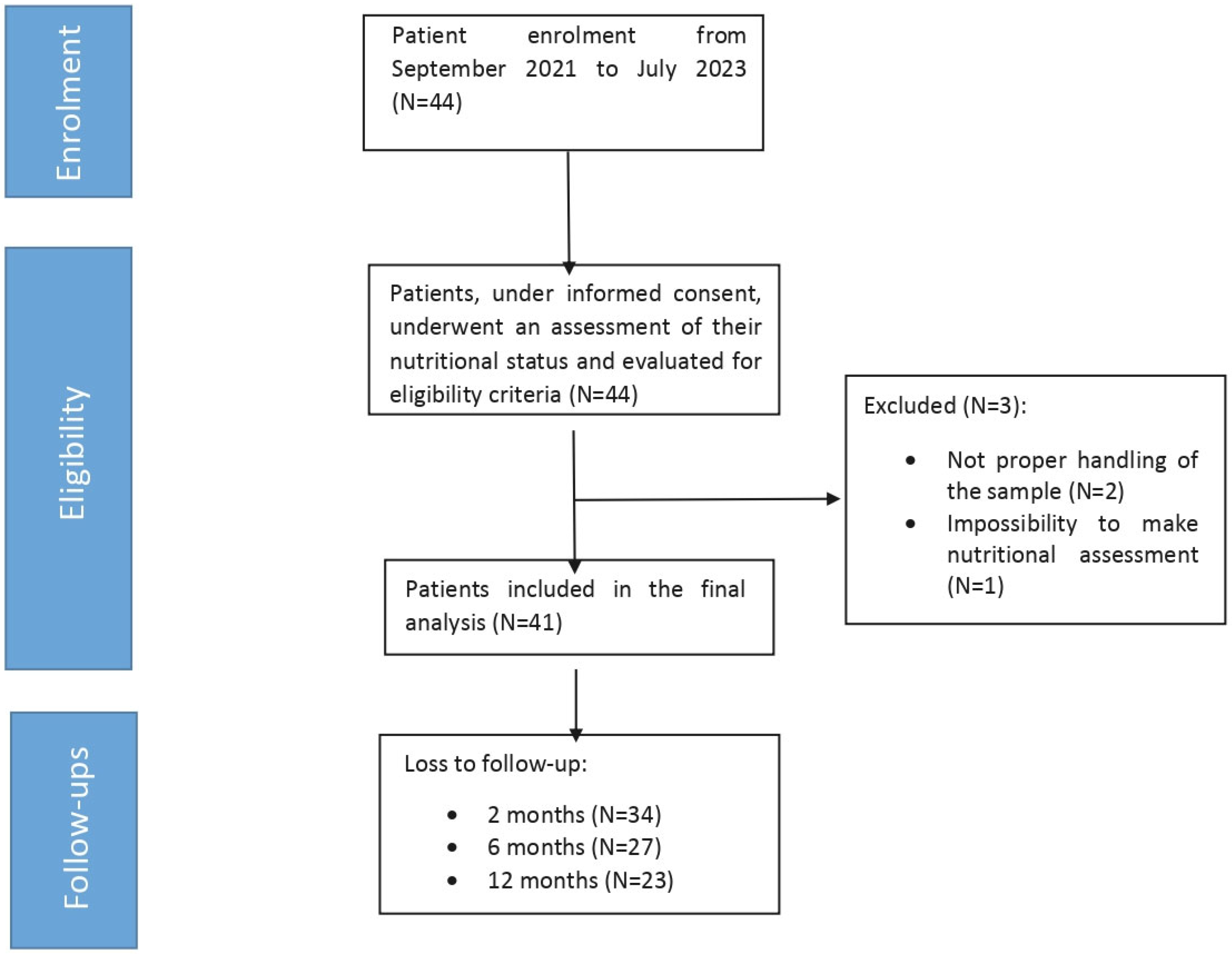
2.1. Study Design, Patient Enrolment, and Data Collection
2.2. Anthropometric, Metabolic, and Nutritional Evaluations
2.3. PRGF Collection
2.4. Isolation of EVs from PRGF Samples
2.5. Nanoparticles Analysis with the Multiplex Bead-Based Platform
2.6. Flow Cytometry Analysis
2.7. Analysis of the Selected Proteins Released by Luminex® Assays
2.8. Statistical Analysis
3. Results
3.1. Collected Data Evaluation and Anthropometric/Nutritional Assessments of Enrolled Patients
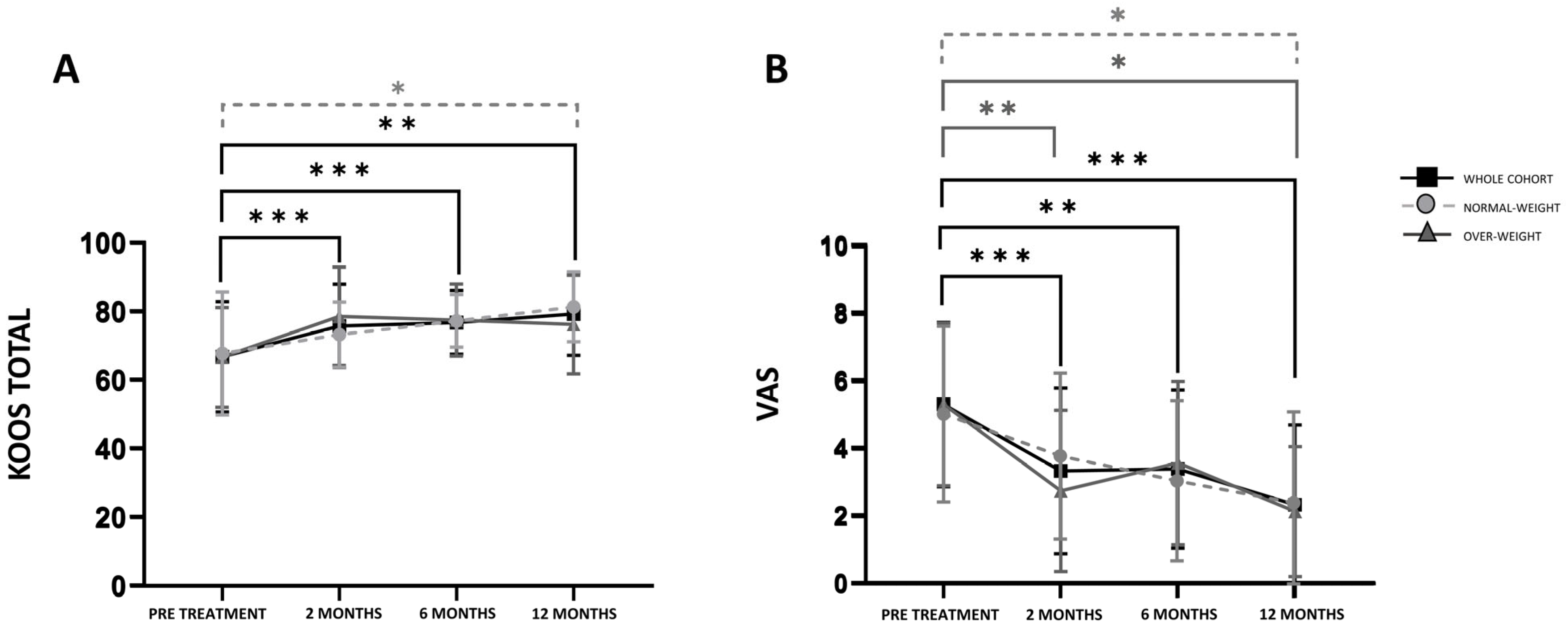
3.2. Evaluation of the Clinical Outcomes
3.3. PRGF Characteristics in Different Patient Subgroups
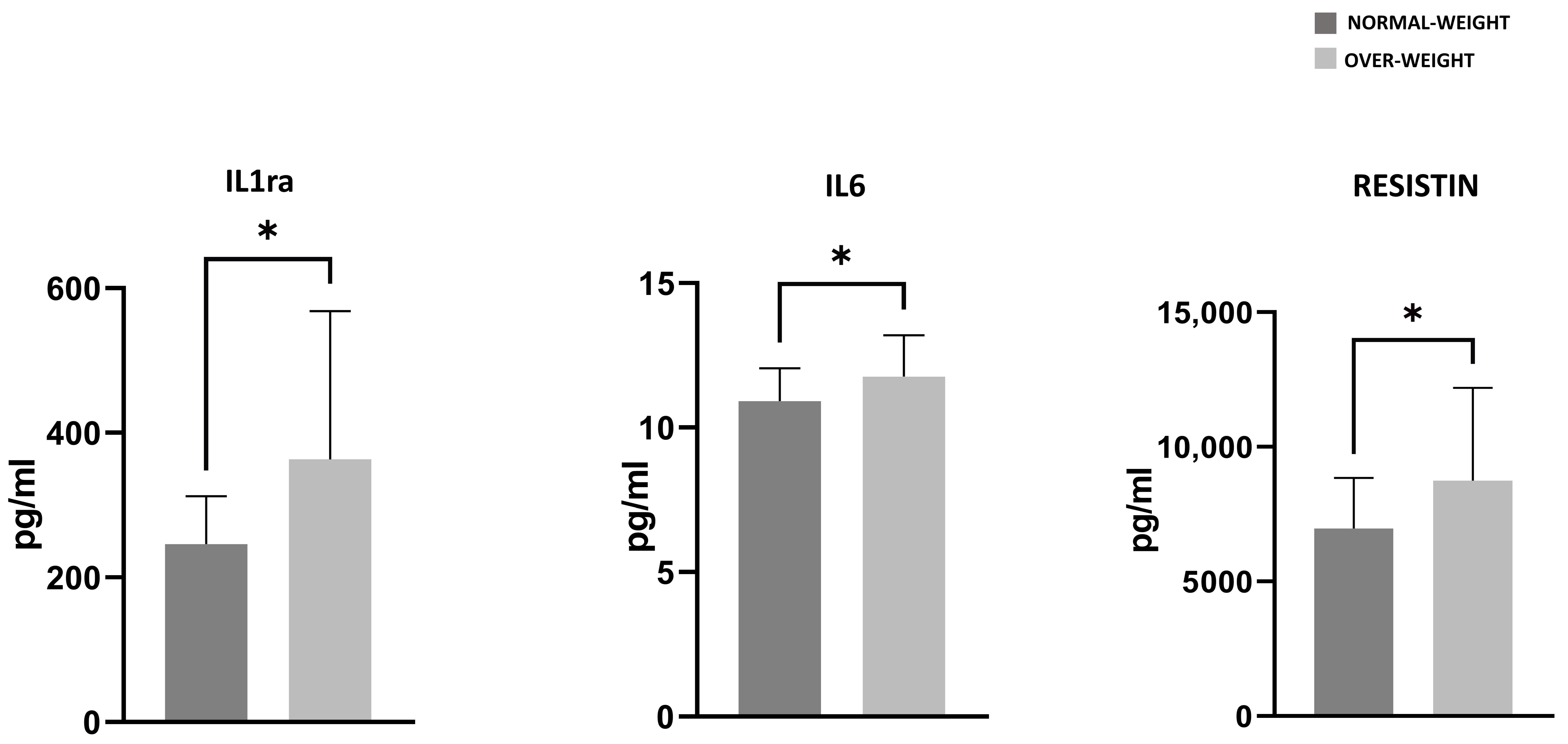
3.4. PRGF Characteristics of Normal Weight and Overweight Patients
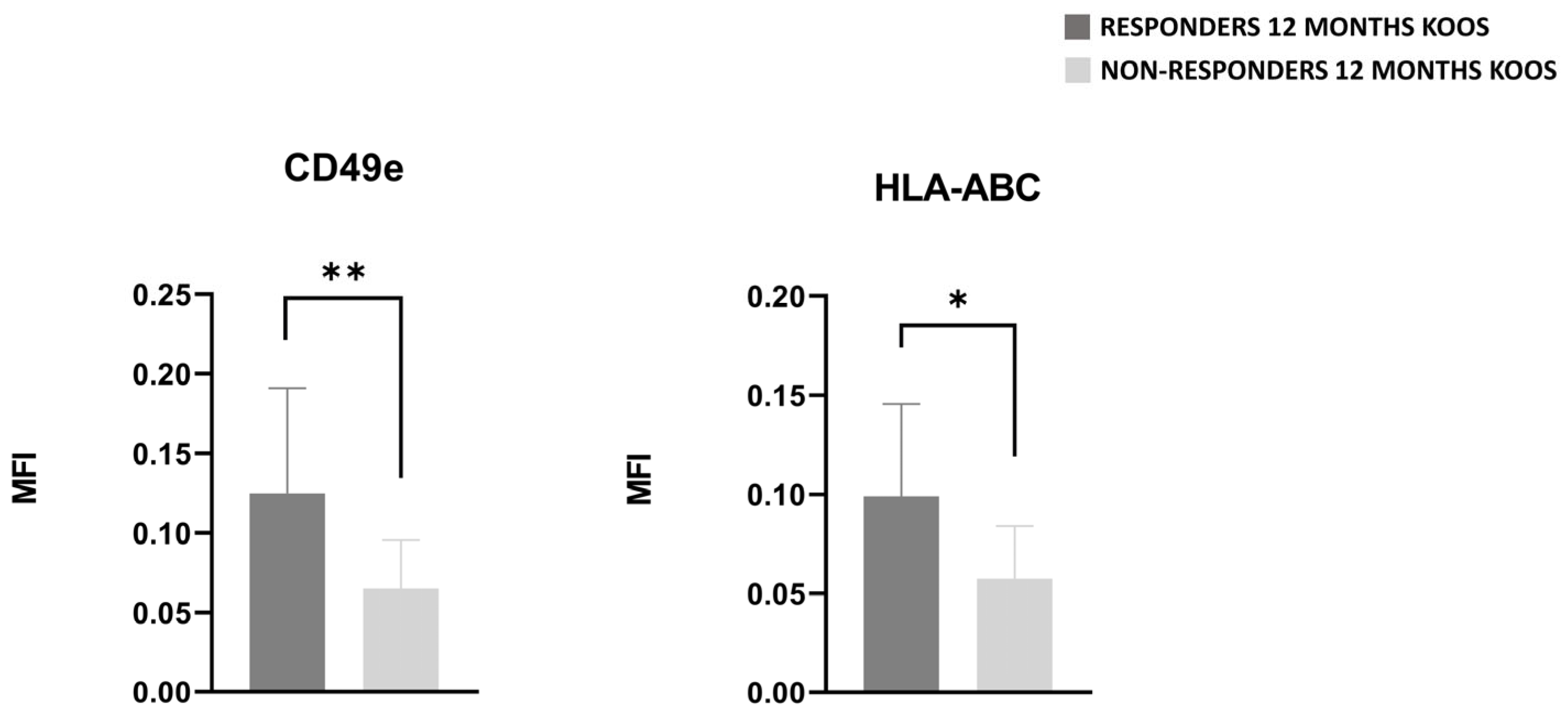
3.5. PRGF Characteristics of Responsive and Nonresponsive Patients
3.6. PRGF Characteristics of Normocholesterolemic and Hypercholesterolemic Patients
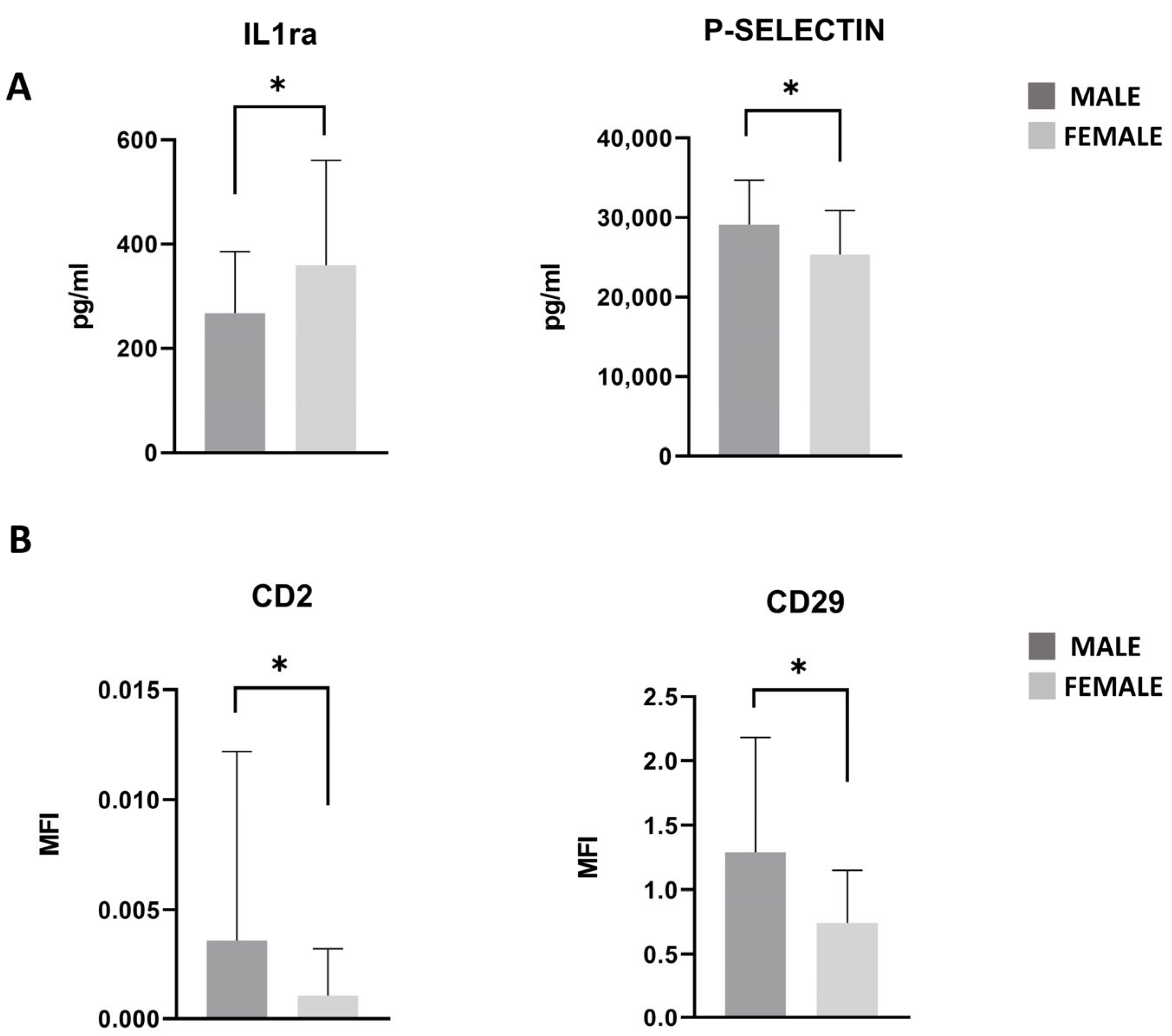
3.7. PRGF Characteristics in Male and Female Patients
3.8. Statistical Modeling and Predictive Factors
3.8.1. Multivariate Logistic Regression Model
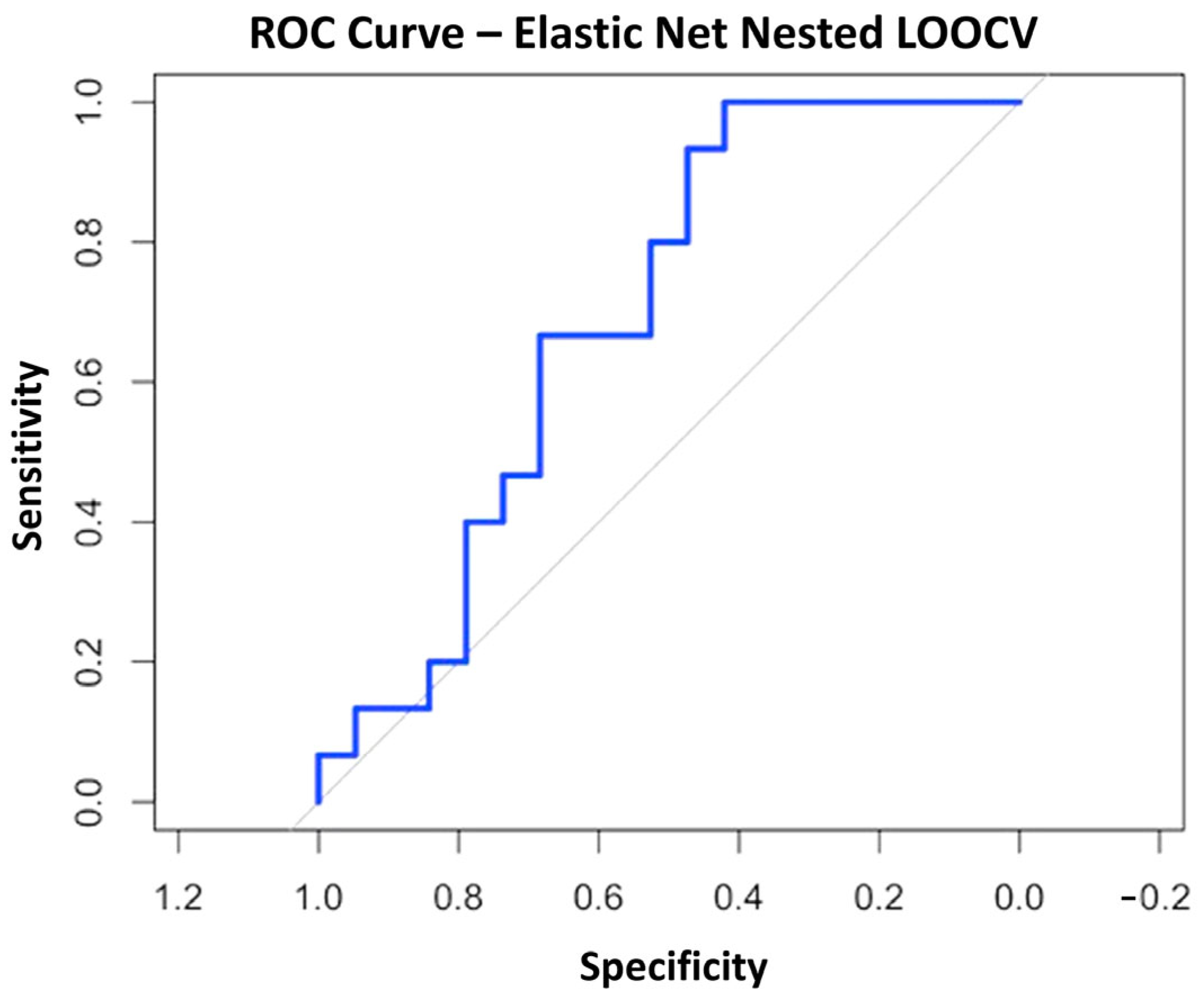
3.8.2. Elastic Net Regularization Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| APC | Allophycocyanin |
| AUC | Area under the curve |
| BMAC | Bone marrow aspirate concentrate |
| BMI | Body mass index |
| EV | Extracellular vesicles |
| FFM | Free fatty mass |
| FM | Fatty mass |
| G-CSF | Granulocyte colony-stimulating factor |
| KOOS | Knee Injury and Osteoarthritis Outcome Score |
| LOOCV | Leave-one-out cross-validation |
| MFAT | Micro-fragmented adipose tissue |
| MPB | MACSPlex Buffer |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OA | Osteoarthritis |
| PBS | Phosphate-buffer solution |
| PRGF | Platelet-rich growth factors |
| PROMs | Patient-reported outcome measures |
| PRP | Platelet-rich plasma |
| SYSADOA | Symptomatic slow-acting drugs for OA |
| SVF | Stromal vascular fraction |
| VAS | Visual Analog Scale |
| VIF | Variance Inflation Factor |
References
- Loef, M.; Schoones, J.W.; Kloppenburg, M.; Ioan-Facsinay, A. Fatty Acids and Osteoarthritis: Different Types, Different Effects. Jt. Bone Spine 2019, 86, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an Inflammatory Disease (Osteoarthritis Is Not Osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L. Mechanoflammation in Osteoarthritis Pathogenesis. Semin. Arthritis Rheum. 2019, 49, S36–S38. [Google Scholar] [CrossRef]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. eClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Honvo, G.; Reginster, J.-Y.; Rabenda, V.; Geerinck, A.; Mkinsi, O.; Charles, A.; Rizzoli, R.; Cooper, C.; Avouac, B.; Bruyère, O. Safety of Symptomatic Slow-Acting Drugs for Osteoarthritis: Outcomes of a Systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 65–99. [Google Scholar] [CrossRef]
- Van Spil, W.E.; Kubassova, O.; Boesen, M.; Bay-Jensen, A.-C.; Mobasheri, A. Osteoarthritis Phenotypes and Novel Therapeutic Targets. Biochem. Pharmacol. 2019, 165, 41–48. [Google Scholar] [CrossRef]
- Balusani, P.; Shrivastava, S.; Pundkar, A.; Kale, P. Navigating the Therapeutic Landscape: A Comprehensive Review of Platelet-Rich Plasma and Bone Marrow Aspirate Concentrate in Knee Osteoarthritis. Cureus 2024, 16, e54747. [Google Scholar] [CrossRef]
- Du, D.; Liang, Y. A Meta-Analysis and Systematic Review of the Clinical Efficacy and Safety of Platelet-Rich Plasma Combined with Hyaluronic Acid (PRP + HA) versus PRP Monotherapy for Knee Osteoarthritis (KOA). J. Orthop. Surg. Res. 2025, 20, 57. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. IJMS 2021, 22, 5492. [Google Scholar] [CrossRef]
- Shahbaz, A.; Alzarooni, A.; Veeranagari, V.R.; Patel, K.; Mohammed, C.; Kuruba, V.; Rajkumar, N.; Mirza, B.A.; Rauf, M.; Maldonado Ramirez, J.G.; et al. Efficacy of Platelet-Rich Plasma Intra-Articular Injections in Hip and Knee Osteoarthritis. Cureus 2024, 16, e69656. [Google Scholar] [CrossRef] [PubMed]
- Bensa, A.; Bianco Prevot, L.; Moraca, G.; Sangiorgio, A.; Boffa, A.; Filardo, G. Corticosteroids, Hyaluronic Acid, Platelet-Rich Plasma, and Cell-Based Therapies for Knee Osteoarthritis-Literature Trends Are Shifting in the Injectable Treatments’ Evidence: A Systematic Review and Expert Opinion. Expert. Opin. Biol. Ther. 2025, 25, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Boffa, A.; De Marziani, L.; Andriolo, L.; Di Martino, A.; Romandini, I.; Zaffagnini, S.; Filardo, G. Influence of Platelet Concentration on the Clinical Outcome of Platelet-Rich Plasma Injections in Knee Osteoarthritis. Am. J. Sports Med. 2024, 52, 3223–3231. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, T.; Franco, D.; Di Gioia, G.; De Biase, C.; Morisco, C.; Trimarco, B.; Barbato, E. Impact of Genetic Polymorphisms on Platelet Function and Response to Anti Platelet Drugs. Cardiovasc. Diagn. Ther. 2018, 8, 610–620. [Google Scholar] [CrossRef]
- Monticone, M.; Ferrante, S.; Salvaderi, S.; Rocca, B.; Totti, V.; Foti, C.; Roi, G.S. Development of the Italian Version of the Knee Injury and Osteoarthritis Outcome Score for Patients with Knee Injuries: Cross-Cultural Adaptation, Dimensionality, Reliability, and Validity. Osteoarthr. Cartil. 2012, 20, 330–335. [Google Scholar] [CrossRef]
- Durnin, J.V.; Womersley, J. Body Fat Assessed from Total Body Density and Its Estimation from Skinfold Thickness: Measurements on 481 Men and Women Aged from 16 to 72 Years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a Literature-Based Adherence Score to Mediterranean Diet: The MEDI-LITE Score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef]
- Kikuchi, N.; Yoshioka, T.; Taniguchi, Y.; Sugaya, H.; Arai, N.; Kanamori, A.; Yamazaki, M. Optimization of Leukocyte-Poor Platelet-Rich Plasma Preparation: A Validation Study of Leukocyte-Poor Platelet-Rich Plasma Obtained Using Different Preparer, Storage, and Activation Methods. J. Exp. Orthop. 2019, 6, 24. [Google Scholar] [CrossRef]
- Alessio-Mazzola, M.; Lovisolo, S.; Sonzogni, B.; Capello, A.G.; Repetto, I.; Formica, M.; Felli, L. Clinical Outcome and Risk Factor Predictive for Failure of Autologous PRP Injections for Low-to-Moderate Knee Osteoarthritis. J. Orthop. Surg. 2021, 29, 23094990211021922. [Google Scholar] [CrossRef]
- Rossi, L.; Ranalletta, M.; Pasqualini, I.; Zicaro, J.P.; Paz, M.C.; Camino, P.; Piuzzi, N.S. Substantial Variability in Platelet-Rich Plasma Composition Is Based on Patient Age and Baseline Platelet Count. Arthrosc. Sports Med. Rehabil. 2023, 5, e853–e858. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and Misleading Statistical Results. Korean J. Anesth. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hastie, T. Regularization and Variable Selection Via the Elastic Net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Lewis, M.J.; Spiliopoulou, A.; Goldmann, K.; Pitzalis, C.; McKeigue, P.; Barnes, M.R. Nestedcv: An R Package for Fast Implementation of Nested Cross-Validation with Embedded Feature Selection Designed for Transcriptomics and High-Dimensional Data. Bioinform. Adv. 2023, 3, vbad048. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, R.; Jin, C.; Liang, J.; Wang, X.; Fan, W.; Wu, X.; Zou, M. Association Between Blood Urea Nitrogen/Albumin and the Incidence as Well as Progression of Type 2 Diabetes. Nutrients 2024, 17, 113. [Google Scholar] [CrossRef]
- Roos, E.M.; Lohmander, L.S. The Knee Injury and Osteoarthritis Outcome Score (KOOS): From Joint Injury to Osteoarthritis. Health Qual. Life Outcomes 2003, 1, 64. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Blanco, J.F.; Sánchez, M.; Moreno, V.; Granero-Moltó, F.; Sánchez-Guijo, F.; Crespo-Cullel, Í.; Mora, G.; San Vicente, D.D.; Pompei-Fernández, O.; et al. Phase II Multicenter Randomized Controlled Clinical Trial on the Efficacy of Intra-Articular Injection of Autologous Bone Marrow Mesenchymal Stem Cells with Platelet Rich Plasma for the Treatment of Knee Osteoarthritis. J. Transl. Med. 2020, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wen, X.; Zhou, L.; Fang, X. The Value of Platelet-Rich Plasma-Derived Extracellular Vesicles in Modern Medicine. Ann. Med. 2023, 55, 2287705. [Google Scholar] [CrossRef] [PubMed]
- Beitia, M.; Guadilla, J.; Mercader Ruiz, J.; Marijuan Pinel, D.; Sánchez, P.; Iriondo, A.; Andrade, R.; Espregueira-Mendes, J.; Delgado, D.; Sánchez, M. The Effect of Long-Term Cryopreservation on the Properties and Functionality of Platelet-Rich Plasma. Int. J. Mol. Sci. 2025, 26, 721. [Google Scholar] [CrossRef]
- Berrigan, W.A.; Bailowitz, Z.; Park, A.; Reddy, A.; Liu, R.; Lansdown, D. A Greater Platelet Dose May Yield Better Clinical Outcomes for Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2025, 41, 809–817.e2. [Google Scholar] [CrossRef]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef]
- Sampath, S.J.P.; Venkatesan, V.; Ghosh, S.; Kotikalapudi, N. Obesity, Metabolic Syndrome, and Osteoarthritis—An Updated Review. Curr. Obes. Rep. 2023, 12, 308–331. [Google Scholar] [CrossRef]
- Marmotti, A.; Bonasia, D.E.; Bruzzone, M.; Rossi, R.; Castoldi, F.; Collo, G.; Realmuto, C.; Tarella, C.; Peretti, G.M. Human Cartilage Fragments in a Composite Scaffold for Single-Stage Cartilage Repair: An in Vitro Study of the Chondrocyte Migration and the Influence of TGF-Β1 and G-CSF. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1819–1833. [Google Scholar] [CrossRef] [PubMed]
- Jayasuriya, C.T.; Chen, Q. Potential Benefits and Limitations of Utilizing Chondroprogenitors in Cell-Based Cartilage Therapy. Connect. Tissue Res. 2015, 56, 265–271. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Larsen, M.; Artym, V.V.; Green, J.A.; Yamada, K.M. The Matrix Reorganized: Extracellular Matrix Remodeling and Integrin Signaling. Curr. Opin. Cell Biol. 2006, 18, 463–471. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Vallet, S.D. Fragments Generated upon Extracellular Matrix Remodeling: Biological Regulators and Potential Drugs. Matrix Biol. 2019, 75–76, 170–189. [Google Scholar] [CrossRef]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin Trafficking in Cells and Tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, N.; Ozols, M.; Bell, M.; Bradley, E.; Gilmore, A.; Debelle, L.; Sherratt, M.J. Matrikines as Mediators of Tissue Remodelling. Adv. Drug Deliv. Rev. 2022, 185, 114240. [Google Scholar] [CrossRef]
- Miao, M.Z.; Su, Q.P.; Cui, Y.; Bahnson, E.M.; Li, G.; Wang, M.; Yang, Y.; Collins, J.A.; Wu, D.; Gu, Q.; et al. Redox-Active Endosomes Mediate A5β1 Integrin Signaling and Promote Chondrocyte Matrix Metalloproteinase Production in Osteoarthritis. Sci. Signal 2023, 16, eadf8299. [Google Scholar] [CrossRef]
- Štok, U.; Blokar, E.; Lenassi, M.; Holcar, M.; Frank-Bertoncelj, M.; Erman, A.; Resnik, N.; Sodin-Šemrl, S.; Čučnik, S.; Perdan Pirkmajer, K.; et al. Characterization of Plasma-Derived Small Extracellular Vesicles Indicates Ongoing Endothelial and Platelet Activation in Patients with Thrombotic Antiphospholipid Syndrome. Cells 2020, 9, 1211. [Google Scholar] [CrossRef]
- Cosgrove, L.J.; Vaughan, H.A.; Tjandra, J.J.; Thurlow, P.J.; McKenzie, I.F. HLA (Class I) Antigens on Platelets Are Involved in Platelet Function. Immunol. Cell Biol. 1988, 66 Pt 1, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Zhang, A.; Wang, M.; Fang, Z.; Zhang, J. Exosomes: An Emerging Factor in Atherosclerosis. Biomed. Pharmacother. 2019, 115, 108951. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, B. Exploring Liquid-Liquid Phase Separation-Related Diagnostic Biomarkers in Osteoarthritis Based on Machine Learning Algorithms and Experiment. Immunobiology 2024, 229, 152825. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, G. CD31 as a Therapeutic Target in Atherosclerosis. Circ. Res. 2020, 126, 1178–1189. [Google Scholar] [CrossRef]
- Mabey, T.; Honsawek, S.; Saetan, N.; Poovorawan, Y.; Tanavalee, A.; Yuktanandana, P. Angiogenic Cytokine Expression Profiles in Plasma and Synovial Fluid of Primary Knee Osteoarthritis. Int. Orthop. 2014, 38, 1885–1892. [Google Scholar] [CrossRef]
- Melinte, R.; Jung, I.; Georgescu, L.; Gurzu, S. VEGF and CD31 Expression in Arthritic Synovium and Cartilage of Human Knee Joints. Rom. J. Morphol. Embryol. 2012, 53, 911–915. [Google Scholar]
- Enomoto, H.; Inoki, I.; Komiya, K.; Shiomi, T.; Ikeda, E.; Obata, K.; Matsumoto, H.; Toyama, Y.; Okada, Y. Vascular Endothelial Growth Factor Isoforms and Their Receptors Are Expressed in Human Osteoarthritic Cartilage. Am. J. Pathol. 2003, 162, 171–181. [Google Scholar] [CrossRef]
- Mehta, S.; Akhtar, S.; Porter, R.M.; Önnerfjord, P.; Bajpayee, A.G. Interleukin-1 Receptor Antagonist (IL-1Ra) Is More Effective in Suppressing Cytokine-Induced Catabolism in Cartilage-Synovium Co-Culture than in Cartilage Monoculture. Arthritis Res. Ther. 2019, 21, 238. [Google Scholar] [CrossRef]
- George, R.; Bhatt, A.; Narayani, J.; Thulaseedharan, J.V.; Sivadasanpillai, H.; Tharakan, J.A. Enhanced P-Selectin Expression on Platelet-a Marker of Platelet Activation, in Young Patients with Angiographically Proven Coronary Artery Disease. Mol. Cell Biochem. 2016, 419, 125–133. [Google Scholar] [CrossRef]
- Théorêt, J.-F.; Yacoub, D.; Hachem, A.; Gillis, M.-A.; Merhi, Y. P-Selectin Ligation Induces Platelet Activation and Enhances Microaggregate and Thrombus Formation. Thromb. Res. 2011, 128, 243–250. [Google Scholar] [CrossRef]
- Koelling, S.; Kruegel, J.; Irmer, M.; Path, J.R.; Sadowski, B.; Miro, X.; Miosge, N. Migratory Chondrogenic Progenitor Cells from Repair Tissue during the Later Stages of Human Osteoarthritis. Cell Stem Cell 2009, 4, 324–335. [Google Scholar] [CrossRef] [PubMed]


| ID | Age (Years) | Sex | Weight (kg) | Height (m) | BMI (kg/m2) | Waist Circumference (cm) | Arm Circumference (cm) | Biceps Fold (mm) | Triceps Fold (mm) | Subcrestal Fold (mm) | Subscapular Fold (mm) | FFM % | FM% | MD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID 1 | 47 | M | 68.5 | 1.69 | 24.1 | 87.5 | 29 | 4 | 4.8 | 9.8 | 15 | 80.9 | 19.1 | 12 |
| ID 2 | 32 | M | 77 | 1.76 | 25.0 | 99.5 | 30.5 | 7.2 | 9.2 | 22.4 | 18.6 | 77 | 23 | 10 |
| ID 3 | 53 | F | 51.5 | 1.66 | 18.7 | 67 | 25.5 | 3 | 7 | 5 | 7.5 | 77.1 | 22.9 | 11 |
| ID 4 | 54 | F | 51 | 1.63 | 19.2 | 72 | 24.5 | 4 | 13.8 | 11.2 | 11.4 | 69.6 | 30.4 | 11 |
| ID 5 | 47 | M | 107 | 1.81 | 32.7 | 108 | 36 | 8.2 | 17.8 | 36 | 29.2 | 67 | 33 | 11 |
| ID 6 | 68 | F | 85 | 1.62 | 32.4 | 106 | 33 | 8.2 | 13 | 20 | 22.4 | 63.6 | 36.4 | 8 |
| ID 7 | 50 | M | 76 | 1.77 | 24.3 | 91 | 34.5 | 8 | 12 | 20.4 | 17.8 | 71.3 | 28.7 | 11 |
| ID 8 | 48 | M | 79 | 1.78 | 24.9 | 96 | 32.5 | 6.6 | 12.8 | 25.8 | 18.8 | 72 | 28 | 10 |
| ID 9 | 44 | F | 68 | 1.60 | 26.7 | 86 | 32 | 5.2 | 25 | 27.2 | 23 | 63.1 | 26.9 | 9 |
| ID 10 | 29 | M | 87 | 1.77 | 27.8 | 96.5 | 33.5 | 7 | 16 | 27 | 15 | 77.9 | 22.1 | 10 |
| ID 11 | 41 | M | 78.5 | 1.78 | 24.8 | 92 | 32 | 5.4 | 14.8 | 14 | 12.4 | 76.4 | 23.6 | 11 |
| ID 12 | 24 | M | 72.5 | 1.72 | 24.5 | 92 | 31 | 4.2 | 20 | 21.4 | 18.6 | 78.1 | 21.9 | 12 |
| ID 13 | 57 | F | 60.5 | 1.68 | 21.6 | 77 | 26 | 6.6 | 16 | 20.4 | 14.6 | 64.9 | 35.1 | 11 |
| ID 14 | 65 | M | 85 | 1.75 | 27.9 | 102 | 33 | 4 | 15.6 | 23.6 | 27.8 | 68.1 | 31.9 | 4 |
| ID 15 | 60 | M | 72.5 | 1.68 | 25.6 | 96.5 | 31 | 4.2 | 5.2 | 15.4 | 14.6 | 77.3 | 22.7 | 9 |
| ID 16 | 51 | M | 71 | 1.93 | 19.2 | 84 | 30 | 3.2 | 8.8 | 10.4 | 7.2 | 81.6 | 18.4 | 10 |
| ID 17 | 35 | M | 80 | 1.88 | 22.6 | 82 | 30 | 3.4 | 6.8 | 33 | 10.6 | 84.6 | 15.4 | 13 |
| ID 18 | 34 | M | 81 | 1.73 | 27.1 | 85.5 | 38.5 | 5 | 6.4 | 20.6 | 11.4 | 80 | 20 | 5 |
| ID 19 | 65 | M | 94 | 1.78 | 29.8 | 103 | 33 | 6.4 | 14.8 | 21.8 | 15.6 | 71.2 | 28.8 | 12 |
| ID 20 | 48 | F | 60 | 1.64 | 22.4 | 85.5 | 27.5 | 5.8 | 13.8 | 16.8 | 13.6 | 69.1 | 30.9 | 12 |
| ID 21 | 51 | F | 50 | 1.54 | 19.4 | 71 | 25.5 | 6.2 | 14.2 | 20.2 | 11.6 | 66.2 | 33.8 | 12 |
| ID 22 | 51 | M | 86.5 | 1.77 | 27.8 | 94.5 | 35.5 | 5.8 | 14.4 | 25.8 | 13 | 71.1 | 28.9 | 11 |
| ID 23 | 64 | M | 72 | 1.68 | 25.5 | 91.5 | 31.5 | 3.8 | 16.8 | 19.4 | 16.4 | 71.8 | 28.2 | 11 |
| ID 24 | 65 | F | 76 | 1.67 | 27.2 | 96 | 28.5 | 6.4 | 24 | 21 | 20.8 | 61.8 | 38.2 | 11 |
| ID 25 | 57 | M | 81 | 1.80 | 25.1 | 93 | 34 | 3.2 | 9 | 18.4 | 12.8 | 75.8 | 24.2 | 11 |
| ID 26 | 54 | F | 68 | 1.62 | 25.9 | 97 | 31 | 4.4 | 18.6 | 22.2 | 12.8 | 64.8 | 35.2 | 8 |
| ID 27 | 44 | M | 76 | 1.80 | 23.5 | 84 | 32.5 | 3.8 | 5.8 | 10 | 10.4 | 82.4 | 17.6 | 7 |
| ID 28 | 59 | F | 62 | 1.60 | 24.1 | 82.5 | 32.5 | 14 | 31 | 20 | 19.6 | 59.7 | 40.3 | 14 |
| ID 29 | 45 | M | 87 | 1.79 | 27 | 96.5 | 36 | 7 | 20.3 | 18.6 | 15 | 72.7 | 27.3 | 14 |
| ID 30 | 41 | M | 80 | 1.85 | 24 | 92 | 33.5 | 5 | 14 | 27 | 12.8 | 73.2 | 26.8 | 12 |
| ID 31 | 61 | F | 71.5 | 1.57 | 28.8 | 106.5 | 32.5 | 16 | 24.8 | 23 | 18 | 60.2 | 39.8 | 11 |
| ID 32 | 48 | F | 57 | 1.53 | 24.3 | 80.5 | 29.5 | 14.4 | 26.2 | 25 | 13.2 | 66.4 | 36.6 | / |
| ID 33 | 52 | M | 70 | 1.82 | 21.13 | 79 | 28.5 | 4.2 | 11.4 | 8 | 10.6 | 79.5 | 20.5 | 12 |
| ID 34 | 44 | M | 65 | 1.68 | 23.17 | 88.5 | 32 | 5.4 | 13.6 | 23 | 11.4 | 72.6 | 27.4 | 14 |
| ID 35 | 48 | M | 93 | 1.78 | 29.4 | 102.5 | 39.5 | 2 | 23.4 | 19.4 | 14.4 | 73.1 | 26.9 | 11 |
| ID 36 | 52 | F | 72 | 1.60 | 29.1 | 89 | 31.5 | 21.4 | 26.2 | 21.4 | 24.2 | 58.4 | 41.6 | 10 |
| ID 37 | 45 | M | 71.6 | 1.18 | 23 | 91.5 | 32 | 8.8 | 18.6 | 18 | 8.4 | 74.4 | 25.6 | 12 |
| ID 38 | 56 | F | 80 | 1.63 | 30.3 | 103 | 33 | 14.8 | 34 | 31 | 25 | 56.8 | 43.2 | 13 |
| ID 39 | 59 | M | 97 | 1.75 | 31.5 | 106 | 40 | 9.2 | 15 | 19.2 | 16 | 71 | 29 | 4 |
| ID 40 | 52 | F | 60.45 | 1.63 | 22.7 | 77 | 28.5 | 6 | 16 | 9 | 12 | 68.7 | 31.3 | 9 |
| ID 41 | 62 | F | 50 | 1.65 | 18.4 | 72 | 24 | 3.6 | 16 | 11 | 9.2 | 69.7 | 30.3 | 13 |
| Coefficients | Estimate | STD Error | z Value | p Value |
|---|---|---|---|---|
| Intercept | −3.8435 | 6.4810 | −0.5930 | 0.5532 |
| BMI | 0.2308 | 0.1703 | 1.3560 | 0.1753 |
| Biceps fold (mm) | −0.5069 | 0.2677 | −1.8940 | 0.0583 # |
| FM% | 0.2086 | 0.1181 | 1.7660 | 0.0774 # |
| Red blood cells count | −2.5749 | 1.3391 | −1.9230 | 0.0545 # |
| Platelets count | 0.0149 | 0.0094 | 1.5820 | 0.1136 |
| Neutrophils cells count | 1.0055 | 0.5216 | 1.9280 | 0.0539 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Grieco, G.; Landoni, S.; Caradonna, E.; Pascale, V.; Ragni, E.; de Girolamo, L. Impact of Nutritional Status on Clinical Outcomes of Patients Undergoing PRGF Treatment for Knee Osteoarthritis—A Prospective Observational Study. Nutrients 2025, 17, 3134. https://doi.org/10.3390/nu17193134
De Luca P, Grieco G, Landoni S, Caradonna E, Pascale V, Ragni E, de Girolamo L. Impact of Nutritional Status on Clinical Outcomes of Patients Undergoing PRGF Treatment for Knee Osteoarthritis—A Prospective Observational Study. Nutrients. 2025; 17(19):3134. https://doi.org/10.3390/nu17193134
Chicago/Turabian StyleDe Luca, Paola, Giulio Grieco, Simona Landoni, Eugenio Caradonna, Valerio Pascale, Enrico Ragni, and Laura de Girolamo. 2025. "Impact of Nutritional Status on Clinical Outcomes of Patients Undergoing PRGF Treatment for Knee Osteoarthritis—A Prospective Observational Study" Nutrients 17, no. 19: 3134. https://doi.org/10.3390/nu17193134
APA StyleDe Luca, P., Grieco, G., Landoni, S., Caradonna, E., Pascale, V., Ragni, E., & de Girolamo, L. (2025). Impact of Nutritional Status on Clinical Outcomes of Patients Undergoing PRGF Treatment for Knee Osteoarthritis—A Prospective Observational Study. Nutrients, 17(19), 3134. https://doi.org/10.3390/nu17193134







