Dynamic Variations in Endogenous Peptides in Chinese Human Milk Across Lactation and Geographical Regions
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals and Samples
2.2. Dietary Frequency
2.3. Sample Preparation
2.4. Mass Spectrometry Analysis
2.5. Bioactive Peptide Prediction
2.6. Data Analysis
3. Results and Discussion
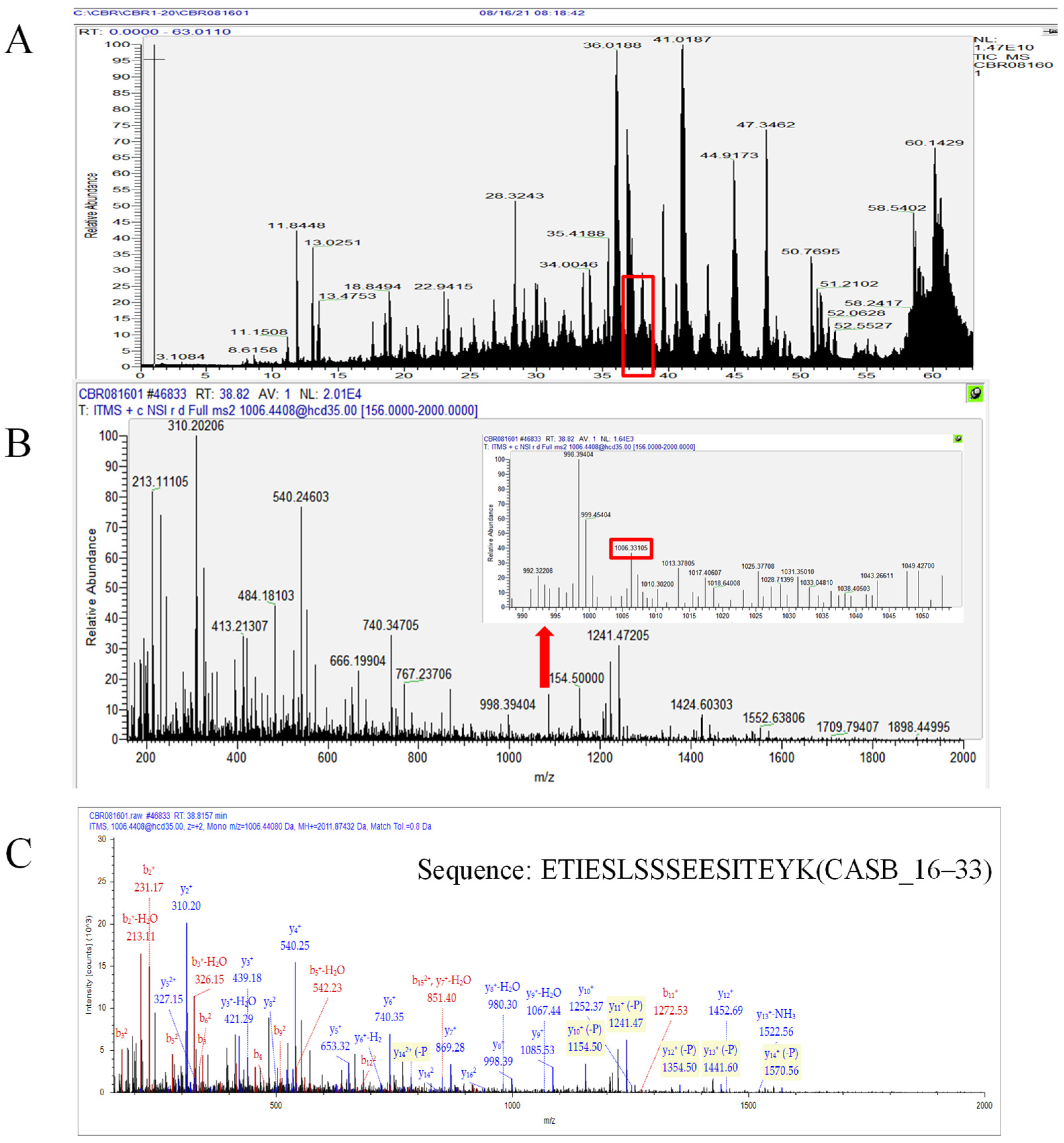
3.1. Comprehensive Profiling of Endogenous Peptides in Human Milk
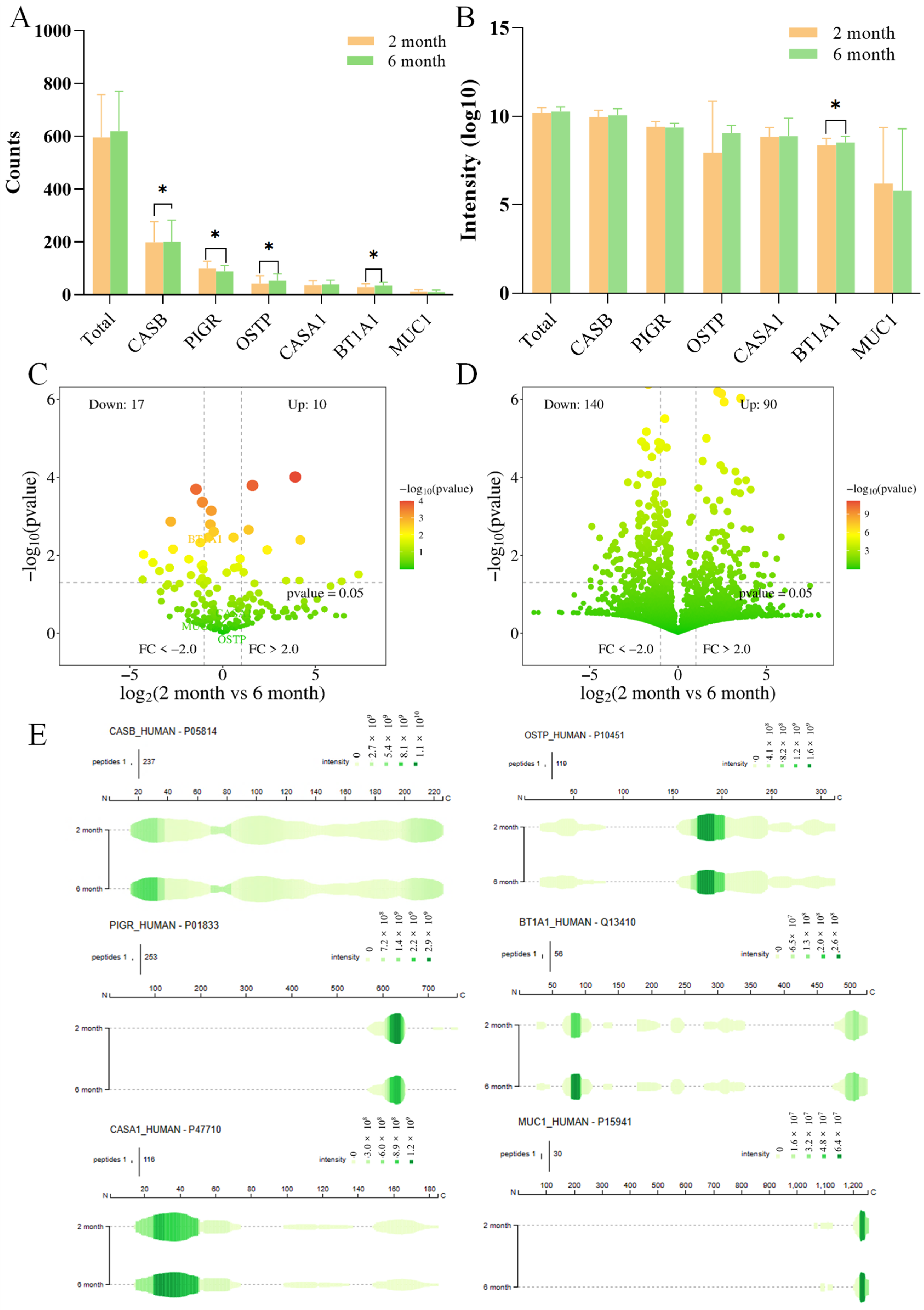
3.2. Dynamics of Milk Peptide Profiles Across Lactation Stages
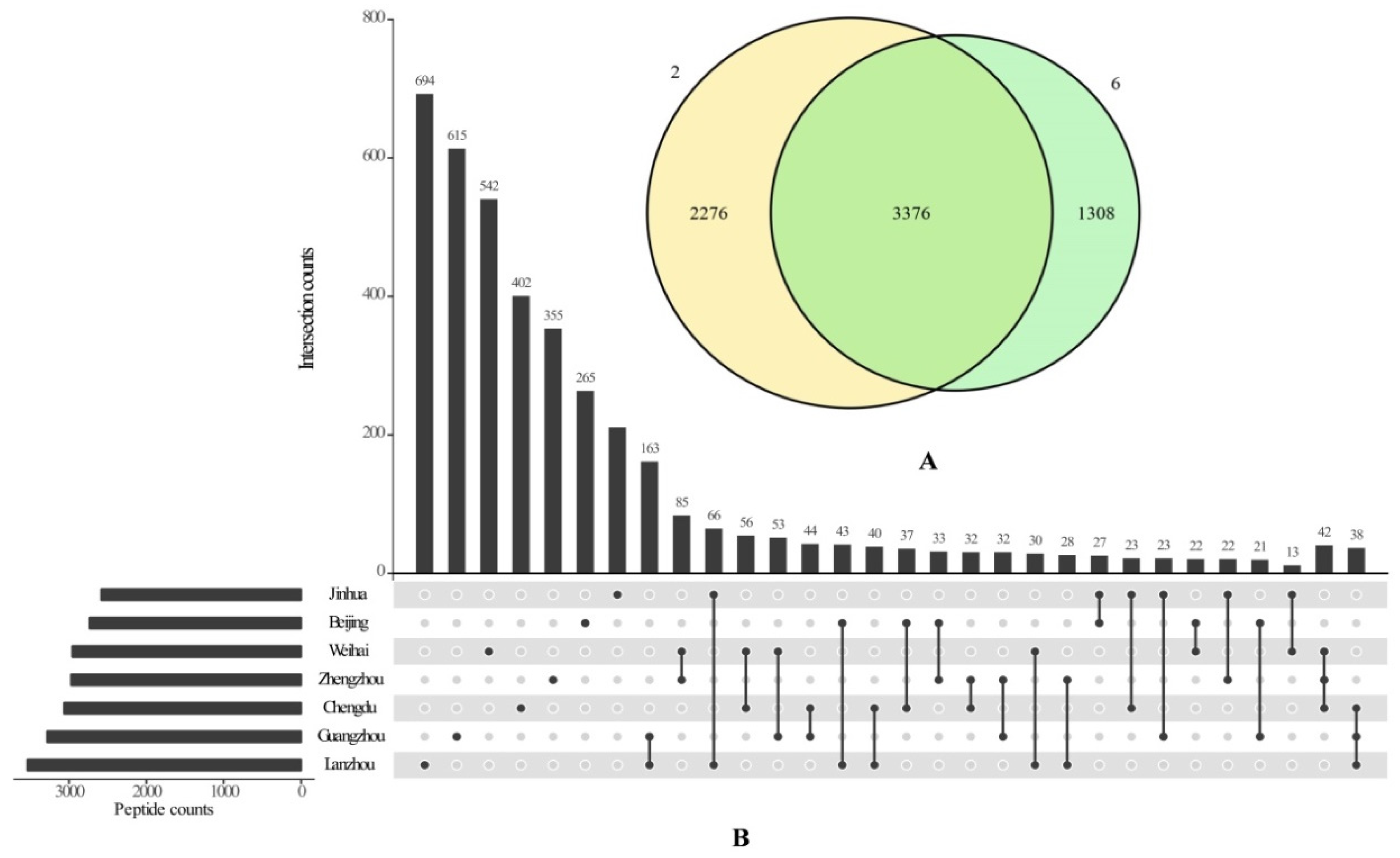
3.3. Geographic Variation in Human Milk Peptide Composition
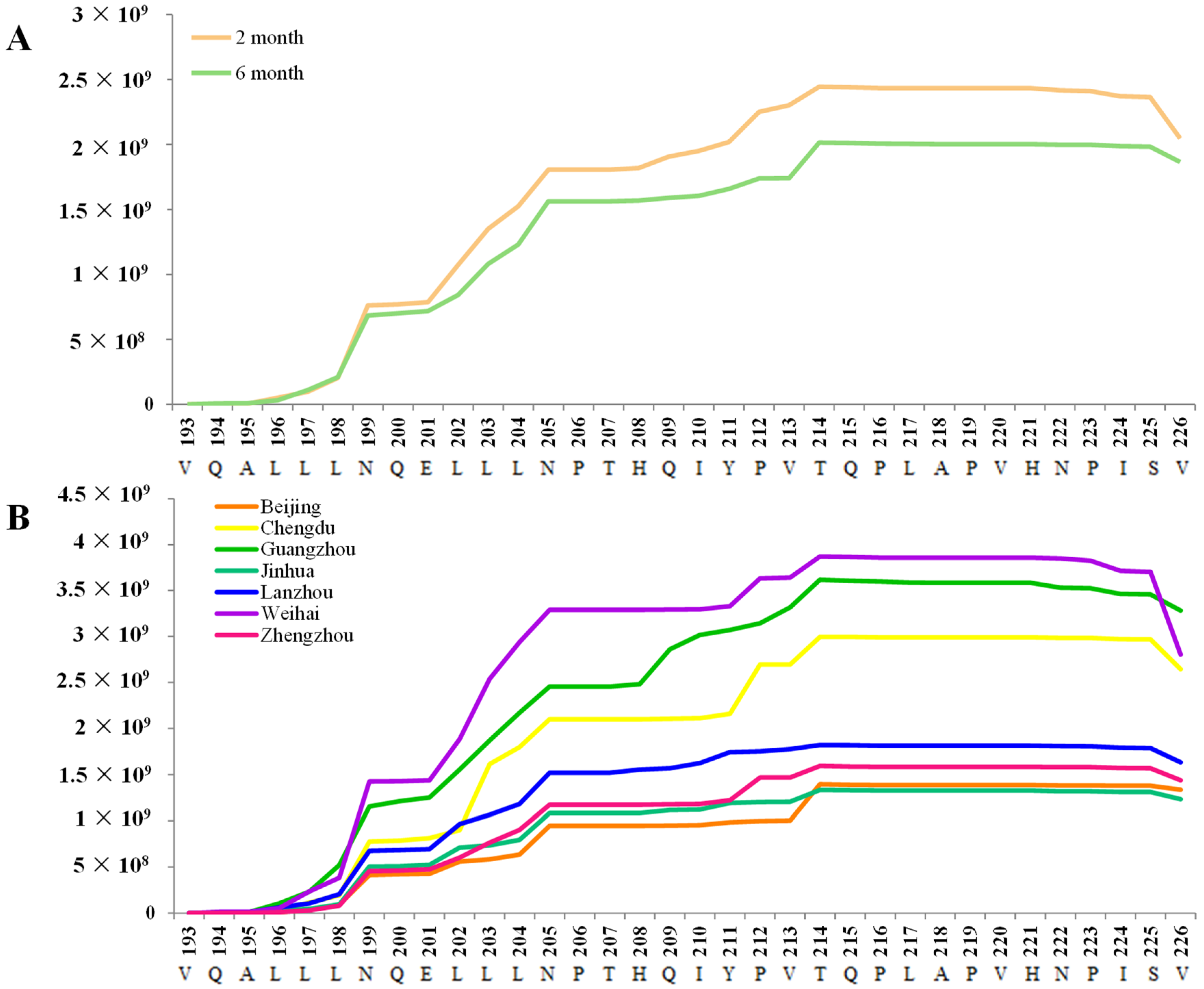
3.4. Bioactive Peptides in Human Milk and Functional Potential
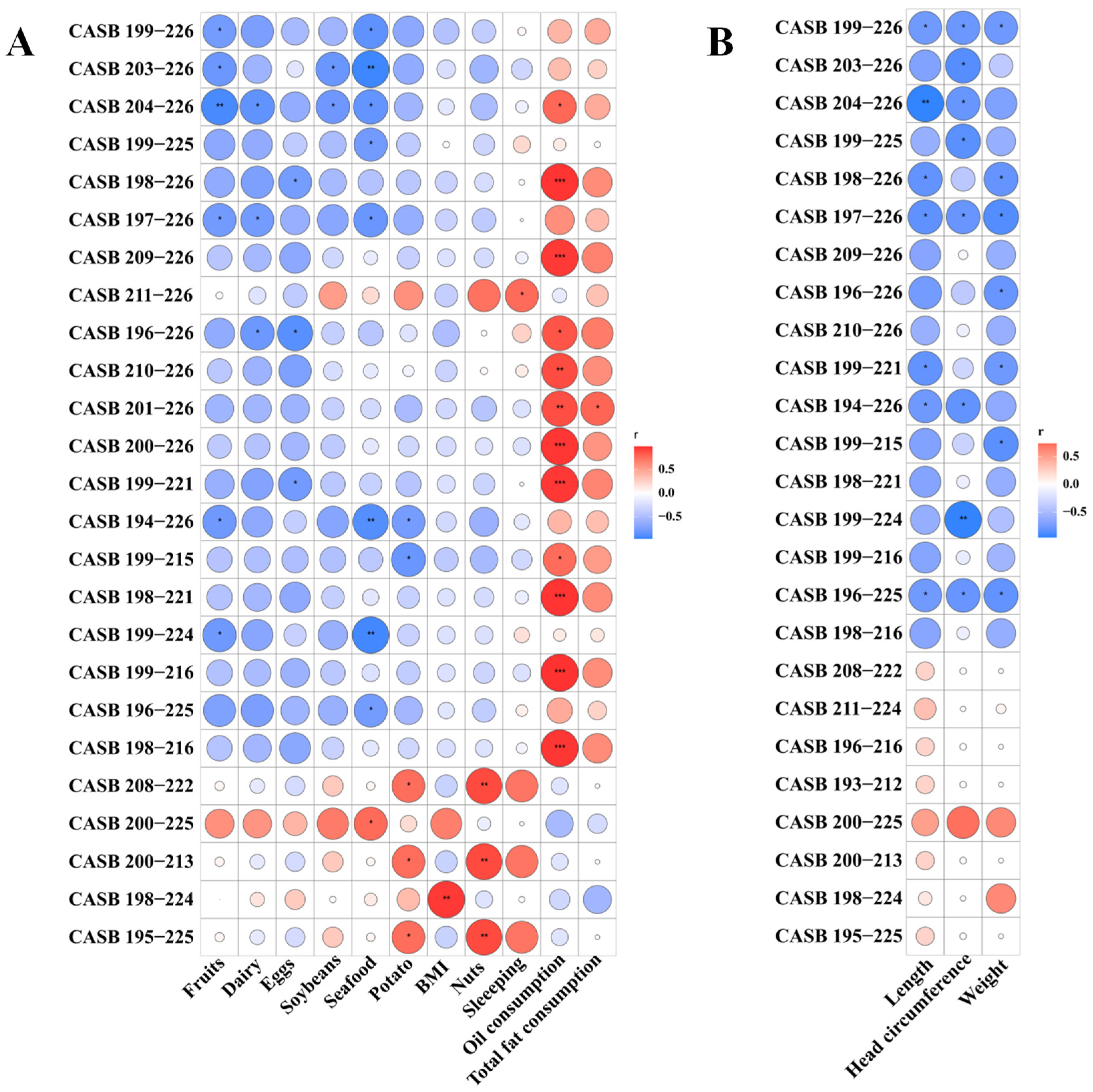
3.5. Associations Between Maternal Diet and Antimicrobial Peptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of infant feeding: Key features of breast milk and infant formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of human milk bioactives on infants’ gut and immune health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef]
- Gan, J.; Robinson, R.C.; Wang, J.; Krishnakumar, N.; Manning, C.J.; Lor, Y.; Breck, M.; Barile, D.; German, J.B. Peptidomic profiling of human milk with LC–MS/MS reveals pH-specific proteolysis of milk proteins. Food Chem. 2019, 274, 766–774. [Google Scholar] [CrossRef]
- Dingess, K.A.; De Waard, M.; Boeren, S.; Vervoort, J.; Lambers, T.T.; Van Goudoever, J.B.; Hettinga, K. Human milk peptides differentiate between the preterm and term infant and across varying lactational stages. Food Funct. 2017, 8, 3769–3782. [Google Scholar] [CrossRef]
- Guerrero, A.; Dallas, D.C.; Contreras, S.; Chee, S.; Parker, E.A.; Sun, X.; Dimapasoc, L.; Barile, D.; German, J.B.; Lebrilla, C.B. Mechanistic peptidomics: Factors that dictate specificity in the formation of endogenous peptides in human milk. Mol. Cell Proteom. 2014, 13, 3343–3351. [Google Scholar] [CrossRef]
- Ning, J.; Li, M.; Chen, W.; Zhao, H.; Chen, J.; Yang, M.; Cao, X.; Yue, X. Peptidomics as a tool to analyze endogenous peptides in milk and milk-related peptides. Food Biosci. 2022, 50, 102199. [Google Scholar] [CrossRef]
- Baum, F.; Fedorova, M.; Ebner, J.; Hoffmann, R.; Pischetsrieder, M. Analysis of the endogenous peptide profile of milk: Identification of 248 mainly casein-derived peptides. J. Proteome Res. 2013, 12, 5447–5462. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Uniacke-lowe, T.; Ryan, A.C.; Kelly, A.L. The composition and physico-chemical properties of human milk: A review. Trends Food Sci. Technol. 2021, 112, 608–621. [Google Scholar] [CrossRef]
- Enjapoori, A.K.; Kukuljan, S.; Dwyer, K.M.; Sharp, J.A. In vivo endogenous proteolysisyielding beta-caseinderived bioactive beta-casomorphin peptides in human breast milk for infant nutrition. Nutrition 2019, 57, 259–267. [Google Scholar] [CrossRef]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk derived antimicrobial bioactive peptides: A review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Howland, V.; Klaedtke, M.; Ruhnau, J.; Dhople, V.M.; Grabe, H.J.; Völker, U.; Heckmann, M.; Hammer, E. Impact of storage conditions on the breast milk peptidome. Nutrients 2020, 12, 2733. [Google Scholar] [CrossRef] [PubMed]
- Montone, C.M.; Capriotti, A.L.; Cerrato, A.; Antonelli, M.; La Barbera, G.; Piovesana, S.; Laganà, A.; Cavaliere, C. Identification of bioactive short peptides in cow milk by high-performance liquid chromatography on C18 and porous graphitic carbon coupled to high-resolution mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 3395–3404. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Dallas, D.C. Peptides released from foremilk and hindmilk proteins by breast milk proteases are highly similar. Front. Nutr. 2017, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Smink, C.J.; Robinson, R.C.; Tian, T.; Guerrero, A.; Parker, E.A.; Smilowitz, J.T.; Hettinga, K.A.; Underwood, M.A.; Lebrilla, C.B.; et al. Endogenous human milk peptide release is greater after preterm birth than term birth. J. Nutr. 2015, 145, 425–433. [Google Scholar] [CrossRef]
- Bravi, F.; Di Maso, M.; Eussen, S.R.B.M.; Agostoni, C.; Salvatori, G.; Profeti, C.; Tonetto, P.; Quitadamo, P.A.; Kazmierska, I.; Vacca, E.; et al. Dietary patterns of breastfeeding mothers and human milk composition: Data from the italian MEDIDIET study. Nutrients 2021, 13, 1722. [Google Scholar] [CrossRef]
- Leghi, G.E.; Netting, M.; Muhlhausler, B.S. The short-term impact of dietary fat and sugar intake on breast milk composition: A clinical trial protocol. Nutr. Health 2020, 26, 65–72. [Google Scholar] [CrossRef]
- Aumeistere, L.; Ciproviča, I.; Zavadska, D.; Andersons, J.; Volkovs, V.; Ceļmalniece, K. Impact of maternal diet on human milk composition among lactating women in Latvia. Med. Lith. 2019, 55, 173. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, Y.M. Dietary patterns and their association with breast milk macronutrient composition among lactating women. Int. Breastfeed. J. 2020, 15, 52. [Google Scholar] [CrossRef]
- Keikha, M.; Shayan-Moghadam, R.; Bahreynian, M.; Kelishadi, R. Nutritional supplements and mother’s milk composition: A systematic review of interventional studies. Int. Breastfeed. J. 2021, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lerma, J.; Selma-Royo, M.; Hervas, D.; Yang, B.; Intonen, L.; González, S.; Martínez-Costa, C.; Linderborg, K.M.; Collado, M.C. Breast milk lipidome is associated with maternal diet and infants’ growth. Front. Nutr. 2022, 9, 854786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Vervoort, J.; Pan, J.; Gao, P.; Zhu, H.; Wang, X.; Zhang, Y.; Chen, B.; Liu, Y.; Li, Y.; et al. Comparison of twelve human milk oligosaccharides in mature milk from different areas in China in the Chinese human milk project (CHMP) study. Food Chem. 2022, 395, 133554. [Google Scholar] [CrossRef]
- Zhang, W.; Li, K.; Zheng, C.; Sun, H.; Pan, J.; Li, Y.; Liu, Y.; Wang, W.; Ju, M.; Xu, Y.; et al. Human milk metabolomics are related to maternal adiposity, infant growth rate and allergies: The Chinese human milk project. Nutrients 2022, 14, 2097. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Guerrero, A.; Khaldi, N.; Castillo, P.A.; Martin, W.F.; Smilowitz, J.T.; Bevins, C.L.; Barile, D.; German, J.B.; Lebrilla, C.B. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J. Proteome Res. 2013, 12, 2295–2304. [Google Scholar] [CrossRef]
- Ferranti, P.; Traisci, M.V.; Picariello, G.; Nasi, A.; Boschi, V.; Siervo, M.; Falconi, C.; Chianese, L.; Addeo, F. Casein proteolysis in human milk: Tracing the pattern of casein breakdown and the formation of potential bioactive peptides. J. Dairy. Res. 2004, 71, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Khaldi, N.; Borghese, R.; Bhandari, A.; Underwood, M.A.; Lebrilla, C.B.; German, J.B.; Barile, D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J. Nutr. 2014, 144, 815–820. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Azuma, N.; Nagaune, S.I.; Ishino, Y.; Mori, H.; Kaminogawa, S.; Yamauchi, K. DNA-Synthesis stimulating peptides from human β-casein. J. Agric. Food Chem. 1989, 53, 2631–2634. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, A.; Lai, S.; Yuan, Q.; Jia, X.; Wang, P.; Zhang, Y. Longitudinal changes in the concentration of major human milk proteins in the first six months of lactation and their effects on infant growth. Nutrients 2021, 13, 1476. [Google Scholar] [CrossRef]
- Dekker, P.M.; Boeren, S.; Van Goudoever, J.B.; Vervoort, J.J.M.; Hettinga, K.A. Exploring human milk dynamics: Interindividual variation in milk proteome, peptidome, and metabolome. J. Proteome Res. 2022, 21, 1002–1016. [Google Scholar] [CrossRef]
- Caldeo, V.; Downey, E.; Shea, C.O.; Affolter, M.; Volger, S.; Courtet-compondu, M.; Antonio, C.; Castros, D.; Mahony, J.A.O.; Ryan, C.A.; et al. Protein levels and protease activity in milk from mothers of pre-term infants: A prospective longitudinal study of human milk macronutrient composition. Clin. Nutr. 2020, 40, 3567–3577. [Google Scholar] [CrossRef]
- Zhu, J.; Dingess, K.A.; Mank, M.; Stahl, B.; Heck, A.J.R. Personalized profiling reveals donor- and lactation-specific trends in the human milk proteome and peptidome. J. Nutr. 2021, 151, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Newburg, D.S.; Walker, W.A. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr. Res. 2007, 61, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; Fontes, E.A.F.; López, C.J.R.; Sousa, W.V.; Gomes, I.S.; Barros, E.; Coimbra, J.S.D.R.; Franceschini, S.D.C.C.; Silva, D.A.; Baracat-Pereira, M.C. Proteoforms of β-casein, α-s1-casein, α-lactalbumin, serum albumin, and lactotransferrin identified in different lactation phases of human milk by proteomics approach. Mol. Nutr. Food Res. 2023, 67, e2200308. [Google Scholar] [CrossRef]
- Al-Waith, H.K.; Mahmood, F.A.; Al-Gburi, O.S. Association between BTN1A1 gene polymorphism and some reproductive efficiency indicator and heat toleranceinholsteincows. Plant Archives. 2019, 19, 1959–1964. [Google Scholar]
- Han, L.; Zhang, M.; Xing, Z.; Coleman, D.N.; Liang, Y.; Loor, J.J. Knockout of butyrophilin subfamily 1 member A1 (BTN1A1) alters lipid droplet formation and phospholipid composition in bovine mammary epithelial cells. J. Anim. Sci. Biotechnol. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liang, A.; Wang, X.; Zhang, W.; Zhang, Y.; He, X.; Liu, Y.; Jiang, S.; Lu, J.; Lv, J. Comparative analysis of triglycerides from different regions and mature lactation periods in Chinese human milk project (CHMP) study. Front. Nutr. 2021, 8, 798821. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, Q.; Liu, P.; Zhang, T.; Zetterstr, R. Fatty acid composition of diet, cord blood and human milk in Chinese mothers with different dietary habits. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 325–330. [Google Scholar] [CrossRef]
- Halleux, V.; De Rigo, J. Variability in human milk composition: Benefit of individualized fortification in very-low-birth-weight infants. Am. J. Clin. Nutr. 2013, 98, 529S–535S. [Google Scholar] [CrossRef]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef]
- Affolter, M.; Garcia-rodenas, C.L.; Vinyes-pares, G.; Jenni, R.; Roggero, I.; Avanti-nigro, O.; Castro, C.A.; De Zhao, A.; Zhang, Y.; Wang, P.; et al. Temporal changes of protein composition in breast milk of Chinese urban mothers and impact of caesarean section delivery. Nutrients 2016, 8, 504. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Zhou, Q.; Giuffrida, F.; Munblit, D.; Verhasselt, V.; Thakkar, S.K. Nutritional and non-nutritional composition of human milk is modulated by maternal, infant, and methodological factors. Front. Nutr. 2020, 7, 576133. [Google Scholar] [CrossRef] [PubMed]
- Umnyakova, E.S.; Zharkova, M.S.; Berlov, M.N.; Shamova, O.V.; Kokryakov, V.N. Human antimicrobial peptides in autoimmunity. Autoimmunity 2020, 53, 137–147. [Google Scholar] [CrossRef]
- Fu, Y.; Ji, C.; Chen, X.; Cui, X.; Wang, X.; Feng, J.; Li, Y.; Qin, R.; Guo, X. Investigation into the antimicrobial action and mechanism of a novel endogenous peptide β-casein 197 from human milk. AMB Express 2017, 7, 119. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Y.; Wang, F.; You, L.; Cao, Y.; Tang, R.; Wen, J.; Cui, X. A novel endogenous antimicrobial peptide CAMP211-225 derived from casein in human milk. Food Funct. 2020, 11, 2291–2298. [Google Scholar] [CrossRef]
- Baricelli, J.; Rocafull, M.A.; Vázquez, D.; Bastidas, B.; Báez-Ramirez, E.; Thomas, L.E. β-defensin-2 in breast milk displays a broad antimicrobial activity against pathogenic bacteria. J. Pediatr. 2015, 91, 36–43. [Google Scholar] [CrossRef]
- Ma, G.; Shi, Y.; Meng, L.; Fan, H.; Tang, X.; Luo, H.; Wang, D.; Zhou, J.; Xiao, X. Factors affecting the early establishment of neonatal intestinal flora and its intervention measures. Front. Cell Infect. Microbiol. 2023, 13, 1295111. [Google Scholar] [CrossRef]
- Salzman, N.H.; Hung, K.; Haribhai, D.; Chu, H.; Karlsson-Sjöberg, J.; Amir, E.; Teggatz, P.; Barman, M.; Hayward, M.; Eastwood, D.; et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 2010, 11, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Trend, S.; Strunk, T.; Hibbert, J.; Kok, C.H.; Zhang, G.; Doherty, D.A.; Richmond, P.; Burgner, D.; Simmer, K.; Davidson, D.J.; et al. Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PLoS ONE 2015, 10, e0117038. [Google Scholar]
- Freitas, R.F.; De Souza Macedo, M.; Do Carmo Lessa, A.; Pinto, N.A.V.D.; Teixeira, R.A. Relationship between the diet quality index in nursing mothers and the fatty acid profile of mature breast milk. Rev. Paul. Pediatr. 2021, 39, e2019089. [Google Scholar]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-e16. [Google Scholar] [CrossRef] [PubMed]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The relationship between breast milk components and the infant gut microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, P.; Xing, M.; He, Z.; Dong, C.; Cao, Y.; Liu, Q. Fatty acid conjugation enhances the activities of antimicrobial peptides. Recent Pat. Food Nutr. Agric. 2013, 5, 52–56. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin d and the host-gut microbiome: A brief overview. Acta Histochem. Cytochem. 2020, 5, 33–42. [Google Scholar] [CrossRef]
- Ma, L.; Huang, S.; Xie, H.; Ma, P.; Jia, B.; Yao, Y.; Gao, Y.; Li, W.; Song, J.; Zhang, W. Influence of chain length on the anticancer activity of the antimicrobial peptide CAMEL with fatty acid modification. Eur. J. Med. Chem. 2022, 239, 114557. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shang, L.; Zeng, X.; Li, N.; Liu, H.; Cai, S. Risks related to high-dosage recombinant antimicrobial peptide microcin J25 in mice model: Intestinal microbiota, intestinal barrier function and immune rregulation. J. Agric. Food Chem. 2018, 66, 11301–11310. [Google Scholar] [CrossRef] [PubMed]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef]
- Regional, E. Calcium in dairy products. J. Dairy Sci. 1987, 70, 2429–2438. [Google Scholar] [CrossRef]
- Zheng, H.; Lorenzen, J.K.; Astrup, A.; Larsen, L.H.; Yde, C.C.; Clausen, M.R.; Bertram, H.C. Metabolic effects of a 24-week energy-restricted intervention combined with low or high dairy intake in overweight women: An NMR-based metabolomics investigation. Nutrients 2016, 8, 108. [Google Scholar] [CrossRef]
- Liu, Y.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Matthijs, B.; Voorspoels, S. Egg-derived bioactive peptides with ACE-inhibitory properties: A literature update. Food Funct. 2017, 8, 3847–3855. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.J.; Gold, M.S.; Makrides, M. Effect of cooked and raw egg consumption on ovalbumin content of human milk: A randomized, double-blind, cross-over trial. Clin. Exp. Allergy 2005, 35, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.J.; Khara, J.; Wright, V.J.; Levy, O.; Kampmann, B. Antimicrobial proteins and peptides in early life: Ontogeny and translational opportunities. Front. Immunol. 2016, 7, 309. [Google Scholar] [CrossRef]
- Venter, C.; Brown, K.R.; Maslin, K.; Palmer, D.J. Maternal dietary intake in pregnancy and lactation and allergic disease outcomes in offspring. Pediatr. Allergy Immunol. 2017, 28, 135–143. [Google Scholar] [CrossRef]

| Characteristics | Total | 2nd Month | 6th Month | Beijing | Chengdu | Weihai | Lanzhou | Jinhua | Zheng Zhou | Guang Zhou | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample information | Number | 200 | 106 | 94 | 28 | 29 | 20 | 37 | 30 | 32 | 24 |
| Peptide concentrations mg/mL | 0.41 | 0.44 | 0.37 | 0.35 | 0.37 | 0.59 | 0.48 | 0.34 | 0.39 | 0.38 | |
| Infant characteristic | Girls (Boys) | 86 (114) | 46 (60) | 40 (54) | 14 (14) | 10 (19) | 12 (8) | 14 (23) | 12 (18) | 14 (18) | 10 (14) |
| Length (cm) | 60.83 | 55.79 | 66.50 | 64.57 | 60.01 | 58.06 | 61.97 | 61.49 | 61.34 | 56.48 | |
| Weight (kg) | 7.01 | 5.50 | 8.73 | 7.38 | 7.39 | 5.85 | 6.89 | 7.06 | 8.08 | 5.79 | |
| Head circumference (cm) | 40.50 | 38.34 | 42.96 | 41.70 | 39.57 | 38.37 | 40.25 | 42.20 | 40.28 | 40.02 | |
| Delivery mode | Virginal | 123 | 67 | 56 | 18 | 14 | 16 | 29 | 19 | 17 | 10 |
| Cesarean | 61 | 34 | 27 | 6 | 13 | 3 | 7 | 10 | 13 | 9 | |
| Assisted | 15 | 5 | 10 | 4 | 2 | 1 | 1 | 0 | 2 | 5 | |
| other | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Maternal BMI | Normal (BMI 18–24.9) | 156 | 86 | 70 | 22 | 27 | 15 | 32 | 20 | 20 | 20 |
| Overweight (BMI 25–29.9) | 42 | 20 | 24 | 6 | 2 | 5 | 5 | 9 | 11 | 4 | |
| Obese (BMI ≥ 30) | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Li, K.; Wang, X.; Zhang, W.; Han, S.; Zhang, Y.; Wang, Y.; Pang, X.; Xie, Q.; Lu, J.; et al. Dynamic Variations in Endogenous Peptides in Chinese Human Milk Across Lactation and Geographical Regions. Nutrients 2025, 17, 3131. https://doi.org/10.3390/nu17193131
Chen B, Li K, Wang X, Zhang W, Han S, Zhang Y, Wang Y, Pang X, Xie Q, Lu J, et al. Dynamic Variations in Endogenous Peptides in Chinese Human Milk Across Lactation and Geographical Regions. Nutrients. 2025; 17(19):3131. https://doi.org/10.3390/nu17193131
Chicago/Turabian StyleChen, Baorong, Kaifeng Li, Xiaodan Wang, Wenyuan Zhang, Sun Han, Yumeng Zhang, Yunna Wang, Xiaoyang Pang, Qinggang Xie, Jing Lu, and et al. 2025. "Dynamic Variations in Endogenous Peptides in Chinese Human Milk Across Lactation and Geographical Regions" Nutrients 17, no. 19: 3131. https://doi.org/10.3390/nu17193131
APA StyleChen, B., Li, K., Wang, X., Zhang, W., Han, S., Zhang, Y., Wang, Y., Pang, X., Xie, Q., Lu, J., Jiang, S., Zhang, S., & Lv, J. (2025). Dynamic Variations in Endogenous Peptides in Chinese Human Milk Across Lactation and Geographical Regions. Nutrients, 17(19), 3131. https://doi.org/10.3390/nu17193131







