Enteral Nutrition in Pediatric Crohn’s Disease: New Perspectives
Abstract
1. Introduction
1.1. How Does EEN Work in CD?
1.1.1. Mucosal Barrier
1.1.2. Immunological Barrier
1.1.3. Microbiological Barrier
1.2. Is It Possible to Enhance the Efficacy of EEN by Modulating Its Nutritional Composition?
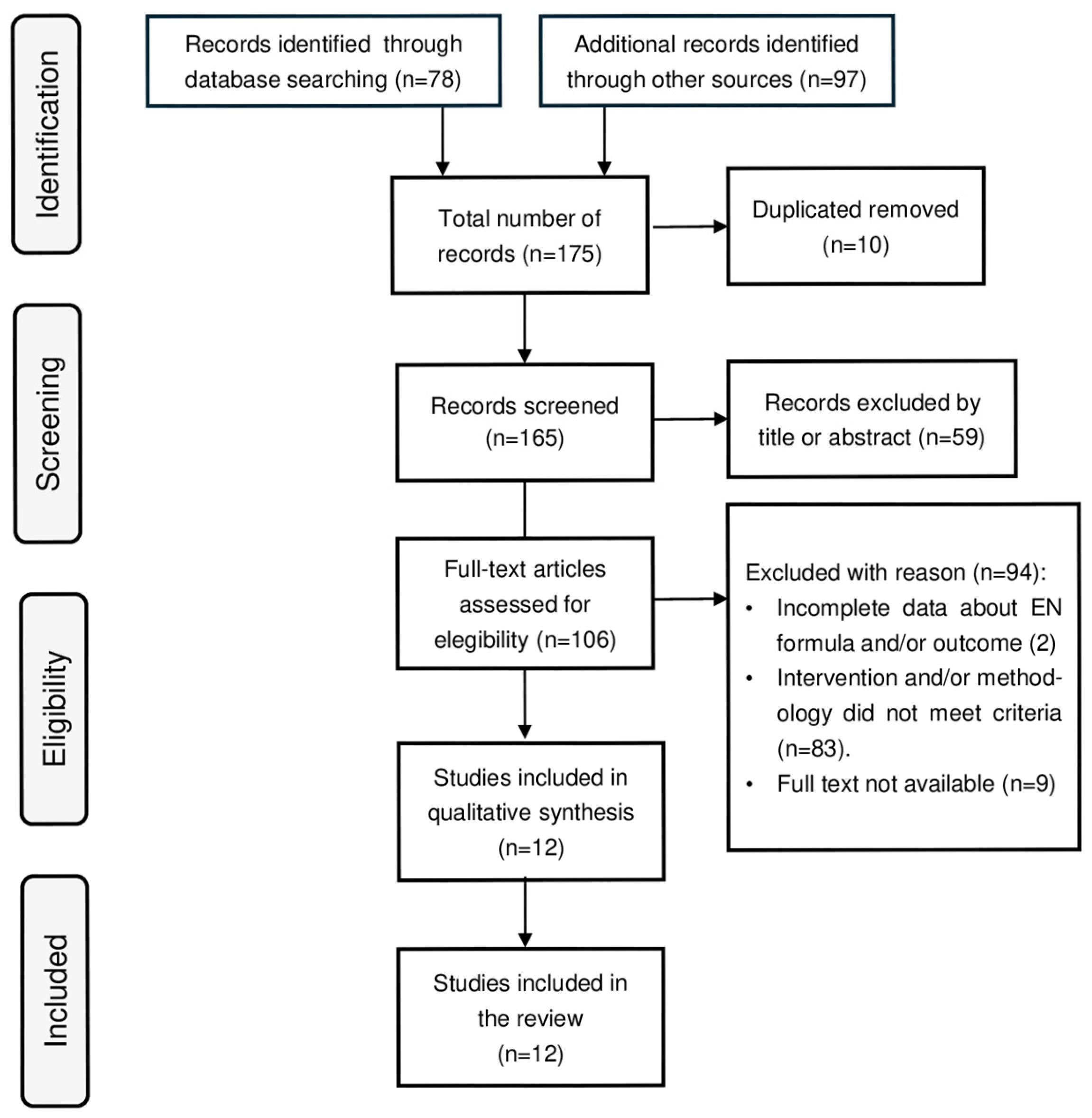
2. Materials and Methods
3. Literature Review
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIEC | Adherent-Invasive Escherichia coli |
| CD | Crohn’s Disease |
| CDAI | Crohn’s Disease Activity Index |
| CDED | Crohn’s Disease Exclusion Diet |
| CDEIS | Crohn’s Disease Endoscopic Index of Severity |
| CEN | Continuous Enteral Nutrition |
| CHO | Carbohydrate |
| CRP | C-Reactive Protein |
| EEN | Exclusive Enteral Nutrition |
| EN | Enteral Nutrition |
| ESR | Erythrocyte Sedimentation Rate |
| GALT | Gut-Associated Lymphoid Tissue |
| HBI | Harvey–Bradshaw Index |
| IBD | Inflammatory Bowel Disease |
| IFN-γ | Interferon γ |
| IL | Interleukin |
| LBP | LPS-Binding Protein |
| LPS | Lipopolysaccharide |
| MCT | Medium-Chain Triglyceride |
| MD-2 | Myeloid Differentiation Factor 2 |
| MHC | Major Histocompatibility Complex |
| MUFA | Monounsaturated Fatty Acid |
| PCDAI | Pediatric Crohn’s Disease Activity Index |
| PN | Parenteral Nutrition |
| PP | Per Protocol |
| PUFA | Polyunsaturated Fatty Acid |
| RCT | Randomized Controlled Trial |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acid |
| SFA | Saturated Fatty Acid |
| TGF-β | Transforming Growth Factor- β |
| Th | T-Helper Lymphocyte |
| TLR4 | Toll-Like Receptor 4 |
| Treg | T-Regulatory Lymphocyte |
| VHAI | Van Hees Activity Index |
| ω3 | Omega-3 Fatty Acid |
| ω6 | Omega-6 Fatty Acid |
References
- Gordon, H.; Minozzi, S.; Kopylov, U.; Verstockt, B.; Chaparro, M.; Buskens, C.; Warusavitarne, J.; Agrawal, M.; Allocca, M.; Atreya, R.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2024, 18, 1531–1555. [Google Scholar] [CrossRef] [PubMed]
- Voitk, A.J.; Echave, V.; Feller, J.H.; Brown, R.A.; Gurd, F.N. Experience with elemental diet in the treatment of inflammatory bowel disease. Is this primary therapy? Arch. Surg. 1973, 107, 329–333. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E.; Assa, A.; Boneh, R.S.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8. [Google Scholar] [CrossRef]
- Day, A.S.; Lopez, R.N. Exclusive enteral nutrition in children with Crohn’s disease. World J. Gastroenterol. 2015, 21, 6809–6816. [Google Scholar] [CrossRef]
- Covello, C.; Becherucci, G.; Di Vincenzo, F.; Del Gaudio, A.; Pizzoferrato, M.; Cammarota, G.; Gasbarrini, A.; Scaldaferri, F.; Mentella, M.C. Parenteral Nutrition, Inflammatory Bowel Disease, and Gut Barrier: An Intricate Plot. Nutrients 2024, 16, 2288. [Google Scholar] [CrossRef]
- Sanderson, I.R.; Croft, N.M. The anti-inflammatory effects of enteral nutrition. JPEN J. Parenter. Enteral Nutr. 2005, 29 (Suppl. S4), S134–S188. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.J.; Gavin, J.; Beattie, R.M. Exclusive enteral nutrition in Crohn’s disease: Evidence and practicalities. Clin. Nutr. 2019, 38, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, I.R.; Boulton, P.; Menzies, I.; Walker-Smith, J.A. Improvement of abnormal lactulose/rhamnose permeability in active Crohn’s disease of the small bowel by an elemental diet. Gut 1987, 28, 1073–1076. [Google Scholar] [CrossRef]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef]
- Levine, A.; Wine, E. Effects of enteral nutrition on Crohn’s disease: Clues to the impact of diet on disease pathogenesis. Inflamm. Bowel Dis. 2013, 19, 1322–1329. [Google Scholar] [CrossRef]
- Di Caro, S.; Fragkos, K.C.; Keetarut, K.; Koo, H.F.; Sebepos-Rogers, G.; Saravanapavan, H.; Barragry, J.; Rogers, J.; Mehta, S.J.; Rahman, F. Enteral Nutrition in Adult Crohn’s Disease: Toward a Paradigm Shift. Nutrients 2019, 11, 2222. [Google Scholar] [CrossRef] [PubMed]
- Verburgt, C.M.; Ghiboub, M.; Benninga, M.A.; de Jonge, W.J.; Van Limbergen, J.E. Nutritional Therapy Strategies in Pediatric Crohn’s Disease. Nutrients 2021, 13, 212. [Google Scholar] [CrossRef]
- MacLellan, A.; Moore-Connors, J.; Grant, S.; Cahill, L.; Langille, M.G.I.; Van Limbergen, J. The Impact of Exclusive Enteral Nutrition (EEN) on the Gut Microbiome in Crohn’s Disease: A Review. Nutrients 2017, 9, 447. [Google Scholar] [CrossRef]
- Quince, C.; Ijaz, U.Z.; Loman, N.; A Eren, M.; Saulnier, D.; Russell, J.; Haig, S.J.; Calus, S.T.; Quick, J.; Barclay, A.; et al. Extensive Modulation of the Fecal Metagenome in Children with Crohn’s Disease During Exclusive Enteral Nutrition. Am. J. Gastroenterol. 2015, 110, 1718–1730. [Google Scholar] [CrossRef]
- Mitrev, N.; Huang, H.; Hannah, B.; Kariyawasam, V.C. Review of exclusive enteral therapy in adult Crohn’s disease. BMJ Open Gastroenterol. 2021, 8, e000745. [Google Scholar] [CrossRef]
- Belzer, C.; de Vos, W.M. Microbes inside--from diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Dunn, K.A.; Moore-Connors, J.; MacIntyre, B.; Stadnyk, A.W.; Thomas, N.A.; Noble, A.; Mahdi, G.; Rashid, M.; Otley, A.R.; Bielawski, J.P.; et al. Early Changes in Microbial Community Structure Are Associated with Sustained Remission After Nutritional Treatment of Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Nagler-Anderson, C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 2001, 1, 59–67. [Google Scholar] [CrossRef]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef]
- Chen, L.; Ruan, G.; Cheng, Y.; Yi, A.; Chen, D.; Wei, Y. The role of Th17 cells in inflammatory bowel disease and the research progress. Front. Immunol. 2023, 13, 1055914. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Pallone, F.; Monteleone, G. Interleukin-12 and Th1 immune response in Crohn’s disease: Pathogenetic relevance and therapeutic implication. World J. Gastroenterol. 2006, 12, 5606–5610. [Google Scholar] [CrossRef]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Di Sabatino, A.; Gordon, J.N. Immunopathogenesis of Crohn’s disease. JPEN J. Parenter. Enteral Nutr. 2005, 29 (Suppl. S4), S118–S188. [Google Scholar] [CrossRef]
- Toms, C.; Powrie, F. Control of intestinal inflammation by regulatory T cells. Microbes Infect. 2001, 3, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, G.; Nancey, S.; Sardi, F.; Roblin, X.; Flourié, B.; Kaiserlian, D. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 160–170. [Google Scholar] [CrossRef]
- Schwerd, T.; Frivolt, K.; Clavel, T.; Lagkouvardos, I.; Katona, G.; Mayr, D.; Uhlig, H.H.; Haller, D.; Koletzko, S.; Bufler, P. Exclusive enteral nutrition in active pediatric Crohn disease: Effects on intestinal microbiota and immune regulation. J. Allergy Clin. Immunol. 2016, 138, 592–596. [Google Scholar] [CrossRef]
- Sanderson, I.R. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J. Nutr. 2004, 134, 2450S–2454S. [Google Scholar] [CrossRef] [PubMed]
- Geesala, R.; Gongloor, P.; Recharla, N.; Shi, X.Z. Mechanisms of Action of Exclusive Enteral Nutrition and Other Nutritional Therapies in Crohn’s Disease. Nutrients 2024, 16, 3581. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Ma, X.; Lu, X.; Zhang, W.; Yang, L.; Wang, D.; Xu, J.; Jia, Y.; Wang, X.; Xie, H.; Li, S.; et al. Gut microbiota in the early stage of Crohn’s disease has unique characteristics. Gut Pathog. 2022, 14, 46. [Google Scholar] [CrossRef]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD-what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Glasser, A.L.; Boudeau, J.; Barnich, N.; Perruchot, M.H.; Colombel, J.F.; Darfeuille-Michaud, A. Adherent invasive Escherichia coli strains from patients with Crohn’s disease survive and replicate within macrophages without inducing host cell death. Infect. Immun. 2001, 69, 5529–5537. [Google Scholar] [CrossRef]
- Qiao, J.; Dong, C.; Wang, X.; Liu, Y.; Ma, L. One-step production of bioactive human lipopolysaccharide binding protein from LPS-eliminated E. coli. Protein Expr. Purif. 2019, 157, 17–20. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Galeazzi, T.; Franceschini, E.; Annibali, R.; Albano, V.; Verma, A.K.; De Angelis, M.; Lionetti, M.E.; Catassi, C. Effects of the Exclusive Enteral Nutrition on the Microbiota Profile of Patients with Crohn’s Disease: A Systematic Review. Nutrients 2017, 9, 832. [Google Scholar] [CrossRef]
- Gerasimidis, K.; Bertz, M.; Hanske, L.; Junick, J.; Biskou, O.; Aguilera, M.; Garrick, V.; Russell, R.K.; Blaut, M.; McGrogan, P.; et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn’s disease during enteral nutrition. Inflamm. Bowel Dis. 2014, 20, 861–871. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Bridonneau, C.; Robert, V.; Sokol, H.; Bermudez-Humaran, L.G.; Thomas, M.; Langella, P. Ecology and metabolism of the beneficial intestinal commensal bacterium Faecalibacterium prausnitzii. Gut Microbes 2014, 5, 146–151. [Google Scholar] [CrossRef]
- Serban, D.E. Microbiota in Inflammatory Bowel Disease Pathogenesis and Therapy: Is It All About Diet? Nutr. Clin. Pract. 2015, 30, 760–779. [Google Scholar] [CrossRef] [PubMed]
- Lochs, H.; Steinhardt, H.J.; Klaus-Wentz, B.; Zeitz, M.; Vogelsang, H.; Sommer, H.; Fleig, W.E.; Bauer, P.; Schirrmeister, J.; Malchow, H. Comparison of enteral nutrition and drug treatment in active Crohn’s disease. Results of the European Cooperative Crohn’s Disease Study. IV. Gastroenterology 1991, 101, 881–888. [Google Scholar] [CrossRef]
- Gonzalez-Huix, F.; de Leon, R.; Fernandez-Banares, F.; Esteve, M.; Cabre, E.; Acero, D.; Abad-Lacruz, A.; Figa, M.; Guilera, M.; Planas, R. Polymeric enteral diets as primary treatment of active Crohn’s disease: A prospective steroid controlled trial. Gut 1993, 34, 778–782. [Google Scholar] [CrossRef]
- Beattie, R.M.; Schiffrin, E.J.; Donnet-Hughes, A.; Huggett, A.C.; Domizio, P.; Macdonald, T.T.; Walker-Smith, J.A. Polymeric nutrition as the primary therapy in children with small bowel Crohn’s disease. Aliment. Pharmacol. Ther. 1994, 8, 609–615. [Google Scholar] [CrossRef]
- Terrin, G.; Berni Canani, R.; Ambrosini, A.; Viola, F.; Bueno de Mesquita, M.; Di Nardo, G.; Dito, L.; Cucchiara, S. A semielemental diet (Pregomin) as primary therapy for inducing remission in children with active Crohn’s disease. Ital. J. Pediatr. 2002, 28, 401–405. [Google Scholar]
- Sakurai, T.; Matsui, T.; Yao, T.; Takagi, Y.; Hirai, F.; Aoyagi, K.; Okada, M. Short-term efficacy of enteral nutrition in the treatment of active Crohn’s disease: A randomized, controlled trial comparing nutrient formulas. JPEN J. Parenter. Enteral. Nutr. 2002, 26, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Gassull, M.A.; Fernandez-Banares, F.; Cabre, E.; Papo, M.; Giaffer, M.H.; Sanchez-Lombrana, J.L.; Richart, C.; Malchow, H.; Gonzalez-Huix, F.; Esteve, M. Fat composition may be a clue to explain the primary therapeutic effect of enteral nutrition in Crohn’s disease: Results of a double blind randomised multicentre European trial. Gut 2002, 51, 164–168. [Google Scholar] [CrossRef]
- Borrelli, O.; Cordischi, L.; Cirulli, M.; Paganelli, M.; Labalestra, V.; Uccini, S.; Russo, P.M.; Cucchiara, S. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: A randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 2006, 4, 744–753. [Google Scholar] [CrossRef]
- Rubio, A.; Pigneur, B.; Garnier-Lengliné, H.; Talbotec, C.; Schmitz, J.; Canioni, D.; Goulet, O.; Ruemmele, F.M. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment. Pharmacol. Ther. 2011, 33, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Pigneur, B.; Lepage, P.; Mondot, S.; Schmitz, J.; Goulet, O.; Doré, J.; Ruemmele, F.M. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition vs Steroid-based Induction Therapy-A Randomised Prospective Clinical Trial in Children with Crohn’s Disease. J. Crohns Colitis 2019, 13, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Boneh, R.S.; Van Limbergen, J.; Wine, E.; Assa, A.; Shaoul, R.; Milman, P.; Cohen, S.; Kori, M.; Peleg, S.; On, A.; et al. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients with Active Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2021, 19, 752–759. [Google Scholar] [CrossRef]
- Verburgt, C.M.; A Dunn, K.; Ghiboub, M.; Lewis, J.D.; Wine, E.; Boneh, R.S.; Gerasimidis, K.; Shamir, R.; Penny, S.; Pinto, D.M.; et al. Successful Dietary Therapy in Paediatric Crohn’s Disease is Associated with Shifts in Bacterial Dysbiosis and Inflammatory Metabotype Towards Healthy Controls. J. Crohns Colitis 2023, 17, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.; Wands, D.I.F.; Logan, M.; Bremner, G.; Efklides, S.; Benn, L.; Henderson, P.; Grant, H.; Meredith, J.; Armstrong, K.; et al. Comparing Effectiveness of a Generic Oral Nutritional Supplement with Specialized Formula in the Treatment of Active Pediatric Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 1859–1864. [Google Scholar] [CrossRef]
- Narula, N.; Dhillon, A.; Zhang, D.; Sherlock, M.E.; Tondeur, M.; Zachos, M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2018, 4, CD000542. [Google Scholar] [CrossRef]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2021, 15, jjaa161. [Google Scholar] [CrossRef]
- Fell, J.M. Control of systemic and local inflammation with transforming growth factor beta containing formulas. JPEN J. Parenter. Enteral Nutr. 2005, 29 (Suppl. S4), S126–S188. [Google Scholar] [CrossRef]
- Schiffrin, E.J.; El Yousfi, M.; Faure, M.; Combaret, L.; Donnet, A.; Blum, S.; Obled, C.; Breuillé, D. Milk casein-based diet containing TGF-beta controls the inflammatory reaction in the HLA-B27 transgenic rat model. JPEN J. Parenter. Enteral Nutr. 2005, 29 (Suppl. S4), S141–S188. [Google Scholar] [CrossRef]
- Marek, A.; Brodzicki, J.; Liberek, A.; Korzon, M. TGF-beta (transforming growth factor-beta) in chronic inflammatory conditions—A new diagnostic and prognostic marker? Med. Sci. Monit. 2002, 8, RA145–RA151. [Google Scholar] [PubMed]
- Zorzi, F.; Calabrese, E.; Di Fusco, D.; De Cristofaro, E.; Biancone, L.; Casella, S.; Palmieri, G.; Monteleone, G. High Smad7 in the early post-operative recurrence of Crohn’s disease. J. Transl. Med. 2020, 18, 395. [Google Scholar] [CrossRef] [PubMed]
- Italian Society of Human Nutrition (SINU). LARN—Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana. In Documento di Sintesi per il XXXVCongresso Nazionale, S.I.N.U.; SICS: Milan, Italy, 2012. [Google Scholar]
- Tina, K.; Jiatong, N.; Deborah, H.; Tobias, S.; Doriane, A.; Dirk, H. Therapeutic mechanisms of exclusive enteral nutrition in Crohn’s disease. Semin. Immunopathol. 2025, 47, 28. [Google Scholar] [CrossRef] [PubMed]
- Lunken, G.R.; Tsai, K.; Schick, A.; Lisko, D.J.; Cook, L.; Vallance, B.A.; Jacobson, K. Prebiotic Enriched Exclusive Enteral Nutrition Suppresses Colitis via Gut Microbiome Modulation and Expansion of Anti-inflammatory T Cells in a Mouse Model of Colitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1251–1266. [Google Scholar] [CrossRef]
- Moreau, N.M.; Martin, L.J.; Toquet, C.S.; Laboisse, C.L.; Nguyen, P.G.; Siliart, B.S.; Dumon, H.J.; Champ, M.M.J. Restoration of the integrity of rat caeco-colonic mucosa by resistant starch, but not by fructo-oligosaccharides, in dextran sulfate sodium-induced experimental colitis. Br. J. Nutr. 2003, 90, 75–85. [Google Scholar] [CrossRef]
- Montroy, J.; Berjawi, R.; Lalu, M.M.; Podolsky, E.; Peixoto, C.; Sahin, L.; Stintzi, A.; Mack, D.; Fergusson, D.A. The effects of resistant starches on inflammatory bowel disease in preclinical and clinical settings: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 372. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
| CD Pathophysiological Features | Specific EEN Actions | |
|---|---|---|
| Mucosal barrier [5,9,10,11,12,13,14,15,16,17,18,19] | ↓ Thickness of mucus layer | ↑ Thickness of mucus layer by:
|
| ↑ Epithelial layer permeability | ↓ Epithelial layer permeability by:
| |
| ↑ Tight junction permeability | ↓ Tight junction permeability by:
| |
| Immunological barrier [5,9,20,21,22,23,24,25,26,27,28,29,30,31] | ↑ Antigen presentation by dendritic cells, together with II MHC compatibility | ↓ Capacity for antigen presentation via changes in gene expression of class II MHC |
| ↓ Treg | ↑ Treg | |
| ↑ Switch to Th1 response | ↓ Switch to Th1 response by inhibition of IL-1β, IL-6, IL-8, IFN-γ, and TNF-α | |
| ↓ Cell apoptosis | ↑ Cells apoptosis by inhibition of IL-2, IL-15, IL-6, IL-12, and IL-18 | |
| Microbiological barrier [19,22,26,32,33,34,35,36,37,38,39,40,41,42,43,44,45] | ↓ Firmicutes | ↓ Firmicutes (F. prausnitzii) ↓ Proteobacteria (Enterobacteriaceae) |
| ↓ Akkermansia muciniphila | ↑ Akkermansia muciniphila | |
| ↑ AIEC strains | ↓ AIEC strains |
| References | Interventions | Patients | Age | Female (n, %) | CD Activity Score | Albumin | CRP and ESR |
|---|---|---|---|---|---|---|---|
| Lochs et al. [46] | EN (EN group) vs. corticosteroids and sulfasalazine (drug group) | Total: 107 patients EN group: 55 patients Drug group: 52 patients | Adult | EN group: 33, 60% Drug group: 37, 71% | CDAI < 150 within 6 weeks of interventions in: -43.6% (EN group) -67.3% (drug group) | Increased in both groups | N/A |
| González-Huix et al. [47] | EN (EN group) vs. corticosteroids (steroid group) | Total: 32 patients EN group:15 patients Steroid group: 17 patients | Adult | EN group: 8, 53.3% Steroid group: 7, 41.2% | Reduction in VHAI (%): -32.28% (EN group) -34.8% (steroid group) | Increased in: -26.7% (EN group) -29.4% (steroid group) | CRP decreased in: -20% (EN group) -23.5% (steroid group) ESR decreased in: -46.7% (EN group) -41.2% (steroid group) |
| Beattie et al. [48] | EN | Total: 7 patients | Pediatric | 3, 42.9% | Significant improvement in LSI (p < 0.001) | Significantly increased (p < 0.001) | Both CRP and ESR decreased significantly (p < 0.001) |
| Terrin et al. [49] | EN (EN group) vs. corticosteroids (steroid group) | Total: 20 patients EN group: 10 patients Steroid group: 10 patients | Pediatric | N/A | Both treatments were effective in significantly reducing PCDAI, but only EN showed significantly lower post-intervention scores (p < 0.01 vs. p = NS) | Significantly increased only in the EN group (p < 0.01 vs. p = NS) | CRP decreased significantly in both groups, especially in the EN group (p < 0.01 vs. p < 0.05). ESR decreased significantly in both groups (p < 0.01 in both) |
| Sakurai et al. [50] | EN with elemental formula (ED group) vs. EN with semi-elemental formula (TL group) | Total: 36 patients ED group: 18 patients TL group: 18 patients | Young adults: ED group: 26.3 ± 8.0 years TL group: 25.3 ± 7.4 years | ED group: 4, 22.2% TL group: 2, 11.1% | Decreased in both groups. Over 6 weeks, CDAI decreased from 213 ± 8 to 102 in the ED group and from 195 ± 4.5 to 82 in the TL group | Increased in both groups, without significant differences | Both CRP and ESR decreased in both groups, without significant differences |
| Gassul et al. [51] | Polymeric enteral formula 1 (PEN1) vs. polymeric enteral formula 2 (PEN2) vs. corticosteroids (steroid group) | Total: 62 randomized patients (n = 44 PP) PEN1 group: 20 patients PEN2 group: 23 patients Steroid group: 19 patients | Adult | PEN1 group: 11, 55% PEN2 group: 13, 56.5% Steroid group: 10, 52.6% | Decreased in all groups, without significant differences between groups | Increased in all groups, without significant differences between groups | Both CRP and ESR decreased in all groups, without significant differences between groups |
| Borrelli et al. [52] | EN (EN group) vs. corticosteroids (steroid group) | Total: 37 randomized patients (n = 32 PP) EN group: 17 patients Steroid group: 15 patients | Pediatric | EN group: 12, 63.2% Steroid group: 10, 55.6% | PCDAI significantly decreased in each group (p < 0.001), with no differences between groups (p = NS) | Significantly increased in each group (p < 0.001), with no differences between groups (p = NS) | Both CRP and ESR decreased significantly in each group (p < 0.001), with no differences between groups (p = NS) |
| Rubio et al. [53] | Continuous EN (EN group) vs. oral fractionated EN (oral group) | Total = 106 patients EN group: 61 patients Oral group: 45 patients | Pediatric | EN group: 22, 36% Oral group: 14, 31% | PCDAI significantly decreased in both groups (p < 0.001), with no differences between groups (p = NS) | Increased in each group (p < 0.01), with no differences between groups (p = NS) | Both CRP and ESR decreased in each group (p < 0.01), with no differences between groups (p = NS) |
| Pigneur et al. [54] | EN (EN group) vs. corticosteroids (steroid group) | Total: 19 patients EN group: 13 patients Steroid group: 6 patients | Pediatric | 4, 21% | HBI significantly improved, especially in the EN group (p < 0.05 compared to the steroid group) | Increased in each group, with no differences between groups (p = NS) | Both CRP and ESR decreased significantly in each group, with no differences between groups (p = NS) |
| Sigall Boneh et al. [55] | EN (EN group) vs. CDED (CDED group) | Total: 73 patients EN group: 34 patients CDED group: 39 patients | Pediatric | 27, 37% | PCDAI significantly decreased in each group (p < 0.001) | N/A | CRP decreased significantly in each group (p < 0.001) ESR data N/A |
| Dawson et al. [57] | EN with two different polymeric formulas (Fortisip group vs. Modulen group) | Total: 171 patients Fortisip group: 106 patients Modulen group: 65 patients | Pediatric | 70, 41% | N/A | N/A | No difference between the two groups in patients with normalization of CRP and ESR (p = NS) Fortisip patients had higher median CRP values compared to the Modulen group (p < 0.001) |
| Reference | Rem. Rate (%) | P. | S. | E. | Protein | Type of Protein | Fat | SFAs | MCTs | MUFAs | ω6 | ω3 | ω6:ω3 | CHO | Glucose Polymers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terrin et al. [49] | 90 | X | 1.8 | Whey | 3.4 | 2.1 | 0 | 0.7 | 0.44 | 0.054 | 8.1:1 | 7.2 | 6.1 | ||
| Rubio et al. [53] | 85 | X | 3.5 | Casein | 4.6 | 2.6 | 1.2 | 0.78 | 0.42 | 0.004 | 10:1 | 10.8 | 4.6 | ||
| Sigal Bonneh et al. [55] | 85 | X | 3.5 | Casein | 4.6 | 2.6 | 1.2 | 0.78 | 0.42 | 0.004 | 10:1 | 10.8 | 4.6 | ||
| González-Huix et al. [47] | 80 | X | 5.5 | NR | 3.6 | 1 | 0.5 | 1.47 | NR | NR | NR | 11.4 | / | ||
| Borrelli et al. [52] | 74 | X | 3.5 | Casein | 4.6 | 2.6 | 1.2 | 0.78 | 0.42 | 0.004 | 10:1 | 10.8 | 4.6 | ||
| Sakurai et al. [50] | 72 | X | 3.38 | / | 0.3 | / | / | / | 0.09 | 0.015 | 6:1 | 163 | NR | ||
| Sakurai et al. [50] | 67 | X | 3.2 | NR | 5 | / | 2 | / | 1.3 | / | / | 11.8 | / | ||
| Dawson et al. [57] | 64 | X | 3.5 | Casein | 4.6 | 2.6 | 1.2 | 0.78 | 0.42 | 0.004 | 10:1 | 10.8 | 4.6 | ||
| Gassul et al. [51] | 63 | X | 5.4 | Casein | 3.3 | 0.34 | 0.19 | 2.6 | 0.21 | 0.05 | 4.2:1 | 11.6 | 11.6 | ||
| Dawson et al. [57] | 63 | X | 5.9 | Casein | 5.8 | 0.6 | / | 3.5 | 0.7 | 0.14 | 5:1 | 18.4 | 11.7 | ||
| Pigneur et al. [54] | 62 | X | 3.5 | Casein | 4.6 | 2.6 | 1.2 | 0.78 | 0.42 | 0.004 | 10:1 | 10.8 | 4.6 | ||
| Lochs et al. [46] | 53 | X | 2.8 | Whey | 3.9 | 2.2 | 1.8 | 0.5 | 1.02 | 0.093 | 10.9:1 | 13.7 | 12 | ||
| Beattie et al. [48] | 29 | X | 2.8 | Casein | 6.25 | 3.8 | NR | NR | 0.7 | NR | NR | 10.8 | 10.8 | ||
| Gassul et al. [51] | 27 | X | 5.4 | Casein | 3.3 | 0.55 | 0.26 | 0.93 | 1.5 | 0.05 | 30:1 | 11.6 | 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brindicci, V.F.; Grieco, R.; Ruiz, R.G.; Cardile, S.; Capriati, T.; Trovato, C.M.; Bolasco, G.; Knafelz, D.; Bracci, F.; Alterio, A.; et al. Enteral Nutrition in Pediatric Crohn’s Disease: New Perspectives. Nutrients 2025, 17, 3124. https://doi.org/10.3390/nu17193124
Brindicci VF, Grieco R, Ruiz RG, Cardile S, Capriati T, Trovato CM, Bolasco G, Knafelz D, Bracci F, Alterio A, et al. Enteral Nutrition in Pediatric Crohn’s Disease: New Perspectives. Nutrients. 2025; 17(19):3124. https://doi.org/10.3390/nu17193124
Chicago/Turabian StyleBrindicci, Viviana Fara, Rosangela Grieco, Roberta Giusy Ruiz, Sabrina Cardile, Teresa Capriati, Chiara Maria Trovato, Giulia Bolasco, Daniela Knafelz, Fiammetta Bracci, Arianna Alterio, and et al. 2025. "Enteral Nutrition in Pediatric Crohn’s Disease: New Perspectives" Nutrients 17, no. 19: 3124. https://doi.org/10.3390/nu17193124
APA StyleBrindicci, V. F., Grieco, R., Ruiz, R. G., Cardile, S., Capriati, T., Trovato, C. M., Bolasco, G., Knafelz, D., Bracci, F., Alterio, A., Ferretti, F., Elia, D., Spinetti, E., Francavilla, R., & Diamanti, A. (2025). Enteral Nutrition in Pediatric Crohn’s Disease: New Perspectives. Nutrients, 17(19), 3124. https://doi.org/10.3390/nu17193124








