The Association Between Short-Chain Fatty Acids and the Incidence of Food Allergies—Systematic Review
Abstract
1. Introduction
1.1. Overview of Characteristics of Short-Chain Fatty Acids (SCFAs)
1.2. Brief Characteristics of Branched-Chain Fatty Acids (BCFAs)
1.3. Mechanism of Action of Short-Chain Fatty Acids
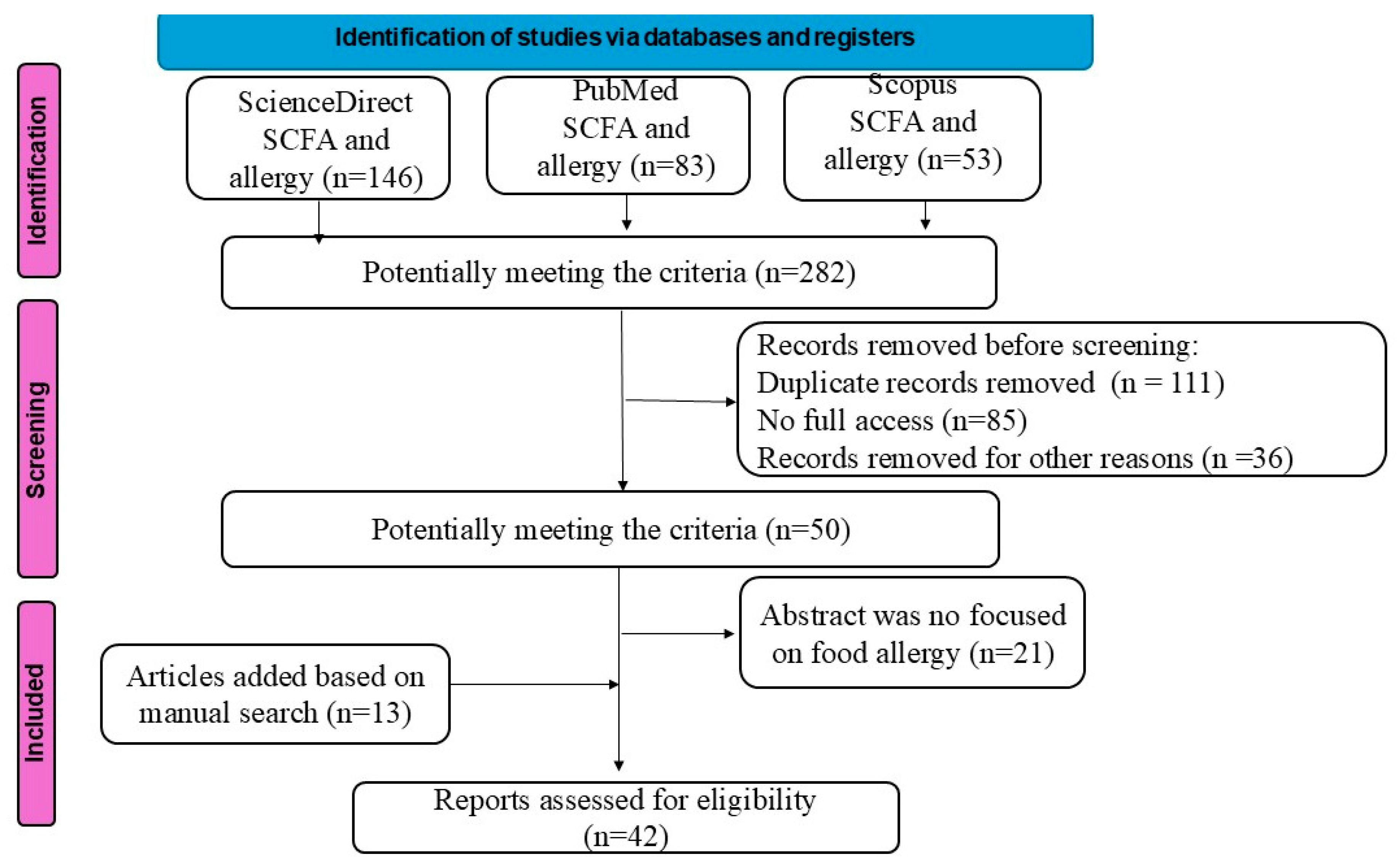
2. Material and Methods
3. Results
3.1. Factors Influencing SCFA Levels
3.2. Effect of SCFAs on Allergy-Related Symptoms
3.3. Mutual Proportions of Short-Chain Fatty Acids
3.4. SCFA Levels and Food Allergies
3.5. Results from Animal Model Studies
3.6. Results from Human Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FOS | fructooligosaccharides |
| GCPRs | G-protein-coupled receptors |
| GOS | galactooligosaccharides |
| SCFAs | short-chain fatty acids |
References
- Gio-Batta, M.; Spetz, K.; Barman, M.; Bråbäck, L.; Norin, E.; Björkstén, B.; Wold, A.E.; Sandin, A. Low Concentration of Fecal Valeric Acid at 1 Year of Age Is Linked with Eczema and Food Allergy at 13 Years of Age: Findings from a Swedish Birth Cohort. Int. Arch. Allergy Immunol. 2022, 183, 398–408. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. NutrSoc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Barman, M.; Gio-Batta, M.; Andrieux, L.; Stråvik, M.; Saalman, R.; Fristedt, R.; Rabe, H.; Sandin, A.; Wold, A.E.; Sandberg, A.-S. Short-chain fatty acids (SCFA) in infants’ plasma and corresponding mother’s milk and plasma in relation to subsequent sensitisation and atopic disease. EBio Med. 2024, 101, 104999. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Blachier, F.; Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Audebert, M.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. High-protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clin. Nutr. 2019, 38, 1012–1022. [Google Scholar] [CrossRef]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-chain fatty acids—An underexplored class of dairy-derived fatty acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef]
- Gozdzik, P.; Magkos, F.; Sledzinski, T.; Mika, A. Monomethyl branched-chain fatty acids: Health effects and biological mechanisms. Prog. Lipid Res. 2023, 90, 101226. [Google Scholar] [CrossRef] [PubMed]
- Murayama, M.; Hosonuma, M.; Kuramasu, A.; Kobayashi, S.; Sasaki, A.; Baba, Y.; Narikawa, Y.; Toyoda, H.; Isobe, J.; Funayama, E.; et al. Isobutyric acid enhances the anti-tumour effect of anti-PD-1 antibody. Sci. Rep. 2024, 14, 11325. [Google Scholar] [CrossRef] [PubMed]
- Gio-Batta, M.; Sjöberg, F.; Jonsson, K.; Barman, M.; Lundell, A.-C.; Adlerberth, I.; Hesselmar, B.; Sandberg, A.-S.; Wold, A.E. Fecal short chain fatty acids in children living on farms and a link between valeric acid and protection from eczema. Sci. Rep. 2020, 10, 22449. [Google Scholar] [CrossRef]
- Zhang, W.; Mackay, C.R.; Gershwin, M.E. Immunomodulatory Effects of MicrobiotaDerived Short-Chain Fatty Acids in Autoimmune Liver Diseases. J. Immunol. 2023, 210, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Taitz, J.; Nanan, R.; Grau, G.; Macia, L. Dysbiotic Gut Microbiota-Derived Metabolites and Their Role in Non-Communicable Diseases. Int. J. Mol. Sci. 2023, 24, 15256. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, M.M.; Shetty, R.; Bang-Berthelsen, C.H.; Mudnakudu-Nagaraju, K.K. Role of mesenchymal stem cells and short chain fatty acids in allergy: A prophylactic therapy for future. Immunol. Lett. 2023, 260, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.; Sikora, M.; Maciejewska, M.; Paralusz-Stec, K.; Michalska, M.; Sikorska, E.; Rudnicka, L. Bacterial Metabolites: A Link between Gut Microbiota and Dermatological Diseases. Int. J. Mol. Sci. 2023, 24, 3494. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular Mechanisms Underlying the Protective Effects of Microbial SCFAs on Intestinal Tolerance and Food Allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; Berg, M.P.M.v.D.; de Bruijn, M.J.W.; van Ijcken, W.F.J.; Junt, T.; Tam, S.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978. [Google Scholar] [CrossRef]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Andreassen, M.; Rudi, K.; Angell, I.L.; Dirven, H.; Nygaard, U.C. Allergen Immunization Induces Major Changes in Microbiota Composition and Short-Chain Fatty Acid Production in Different Gut Segments in a Mouse Model of Lupine Food Allergy. Int. Arch. Allergy Immunol. 2018, 177, 311–323. [Google Scholar] [CrossRef]
- van Esch, B.C.; Abbring, S.; Diks, M.A.; Dingjan, G.M.; Harthoorn, L.F.; Vos, A.P.; Garssen, J. Post-sensitization administration of non-digestible oligosaccharides and Bifidobacterium breve M-16V reduces allergic symptoms in mice. Immun. Inflamm. Dis. 2016, 4, 155–165. [Google Scholar] [CrossRef]
- Ho, H.E.; Chun, Y.; Jeong, S.; Jumreornvong, O.; Sicherer, S.H.; Bunyavanich, S. Multidimensional study of the oral microbiome, metabolite, and immunologic environment in peanut allergy. J. Allergy Clin. Immunol. 2021, 148, 627–632.e3. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Mor, H.; Neriya, D.M.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial signature in IgE-mediated food allergies. Genome Med. 2020, 12, 92. [Google Scholar] [CrossRef]
- Yu, Z.D.; Yue, L.L.; Wang, Z.H.; Wang, R.Z.; Li, L.F.; Zhang, W.C.; Li, X.Q. Specific changes in gut microbiota and short-chain fatty acid levels in infants with cow’s milk protein allergy. Zhongguo Dang Dai Er Ke Za Zhi 2024, 26, 236–243. (In Chinese) [Google Scholar] [PubMed]
- Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Nielsen, D.S.G.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of Diet-Modulated Butyrate Production on Intestinal Barrier Function and Inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’aLessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef]
- Blanco-Pérez, F.; Steigerwald, H.; Schülke, S.; Vieths, S.; Toda, M.; Scheurer, S. The Dietary Fiber Pectin: Health Benefits and Potential for the Treatment of Allergies by Modulation of Gut Microbiota. Curr. Allergy Asthma Rep. 2021, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Rupani, P.F.; Azman, S.; Dewil, R.; Appels, L. A redox-based strategy to enhance propionic and butyric acid production during anaerobic fermentation. Bioresour. Technol. 2022, 361, 127672. [Google Scholar] [CrossRef]
- Jovandaric, M.Z.; Jovanović, K.; Raus, M.; Babic, S.; Igic, T.; Kotlica, B.; Milicevic, S. The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review. Medicina 2024, 60, 1969. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Eviston, D.; Hsu, P.; Mariño, E.; Chidgey, A.; Santner-Nanan, B.; Wong, K.; Richards, J.L.; Yap, Y.-A.; Collier, F.; et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat. Commun. 2019, 10, 3031. [Google Scholar] [CrossRef]
- Jones, J.M.; Reinke, S.N.; Mousavi-Derazmahalleh, M.; Garssen, J.; Jenmalm, M.C.; Srinivasjois, R.; Silva, D.; Keelan, J.; Prescott, S.L.; Palmer, D.J.; et al. Maternal prebiotic supplementation during pregnancy and lactation modifies the microbiome and short chain fatty acid profile of both mother and infant. Clin. Nutr. 2024, 43, 969–980. [Google Scholar] [CrossRef]
- Bozorgmehr, T.; Boutin, R.C.; Woodward, S.E.; Donald, K.; Chow, J.M.; Buck, R.H.; Finlay, B.B. Early Life Exposure to Human Milk Oligosaccharides Reduces Allergic Response in a Murine Asthma Model. J. Immunol. Res. 2023, 2023, 9603576. [Google Scholar] [CrossRef]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbø, M.; Johnson, C.C.; Wegienka, G.; du Toit, E.; Shimojo, N.; Munblit, D.; Campbell, D.E.; et al. Human Milk from Atopic Mothers Has Lower Levels of Short Chain Fatty Acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Zakharova, I.; Dmitrieva, Y. Oligosaccharides in infant formula: More evidence to validate the role of prebiotics. Br. J. Nutr. 2015, 113, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Shi, J.; Dong, P.; Liu, C.; Xu, Y.; Zheng, M.; Cheng, L.; Wang, J.; Raghavan, V. Lactobacillus rhamnosus Probio-M9 alleviates OVA-sensitized food allergy through modulating gut microbiota and its metabolism. Food Funct. 2023, 14, 10784–10795. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Mu, K.; Xue, W. Lactobacillus paracasei AH2 isolated from Chinese sourdough alleviated gluten-induced food allergy through modulating gut microbiota and promoting short-chain fatty acid accumulation in a BALB/c mouse model. J. Sci. Food Agric. 2024, 104, 664–674. [Google Scholar] [CrossRef]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Szczuko, M.; Kikut, J.; Maciejewska, D.; Kulpa, D.; Celewicz, Z.; Ziętek, M. The Associations of SCFA with Anthropometric Parameters and Carbohydrate Metabolism in Pregnant Women. Int. J. Mol. Sci. 2020, 21, 9212. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.S.; Xing, Y.; Fernández-Rivas, M.; Wong, G.W. The Relationship Between Dietary Patterns and the Epidemiology of Food Allergy. Allergy 2024, 80, 690–702. [Google Scholar] [CrossRef]
- Szczuko, M.; Duliban, G.; Drozd, A.; Sochaczewska, D.; Pokorska-Niewiada, K.; Ziętek, M. The Association of Short-Chain FattyAcids with the Occurrence of Gastrointestinal Symptoms in Infants. Int. J. Mol. Sci. 2024, 25, 12487. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Animals | Study Design | Tested Material | Observed Effect |
|---|---|---|---|---|
| M Andreassen et al., 2018 [18] | 24 mice (13 were immunised) | The SCFA content in the gastrointestinal tract of lupine-sensitised mice in various sections of the intestine | Blood, stool, and tissue samples from the distal part of the small intestine, the cecum, and the colon | Mice that were immunised had higher concentrations of acetic, iso-butyric, and iso-valeric acids in the cecal segment of the intestine. |
| Van Esch BC. et al., 2016 [19] | 40 mice | The effect of diet on egg white allergy: control diet: allergic and non-allergic group scFOSlcFOS diet, B.breveiscFOSlc-FOS diet | Blood serum samples, spleen cells | The use of the scFOSIcFOS + B.breve diet resulted in an increase in total SCFAs, a reduction in allergic symptoms to minor allergic shocks, activation of Th1 cells in the spleen, and a decrease in mast cell degranulation with a tendency to decrease OVA-IgE. |
| Author, Year | Number | Tested Material | Observed Effect |
|---|---|---|---|
| Ho He et al., 2021 [20] | 105 people: 56 with peanut allergies and 49 healthy | Saliva | Lower levels of SCFA in the saliva of people with allergies, particularly butyric and propionic acids. |
| Goldberg et al., 2020 [21] | 66 people with a milk allergy, 38 people with a sesame allergy, 71 people with a peanut allergy, 58 people with a tree nut allergy; 58 in the control group- healthy | Faeces | Individuals with food allergies had lower concentrations of acetic, butyric, and propionic acids compared to the control group. Significant differences in acetate concentrations were found between the group without allergies and groups with allergies to peanuts, sesame, and tree nuts. The level of butyric acid was lower in the milk and peanut allergy groups. However, the group with a peanut allergy had the lowest concentration of propionate. |
| Yu Z. et al., 2024 [22] | 50 infants: 25 with allergies (cow milk protein allergy) 25 in the control group | Faeces | Lower SCFAs, particularly acetic, butyric, and isovaleric acid, in the group with cow milk protein allergy. |
| Gio-Batta et al., 2022 [1] | 110 people -1 year of age with allergy at 13 years of age | Faeces | At the age of 1, the concentration of valeric acid was inversely correlated with the occurrence of food allergies to peanuts, tree nuts, fruits, milk, and eggs at the age of 13. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szukalska, I.; Ziętek, M.; Brodowski, J.; Szczuko, M. The Association Between Short-Chain Fatty Acids and the Incidence of Food Allergies—Systematic Review. Nutrients 2025, 17, 3117. https://doi.org/10.3390/nu17193117
Szukalska I, Ziętek M, Brodowski J, Szczuko M. The Association Between Short-Chain Fatty Acids and the Incidence of Food Allergies—Systematic Review. Nutrients. 2025; 17(19):3117. https://doi.org/10.3390/nu17193117
Chicago/Turabian StyleSzukalska, Iga, Maciej Ziętek, Jacek Brodowski, and Małgorzata Szczuko. 2025. "The Association Between Short-Chain Fatty Acids and the Incidence of Food Allergies—Systematic Review" Nutrients 17, no. 19: 3117. https://doi.org/10.3390/nu17193117
APA StyleSzukalska, I., Ziętek, M., Brodowski, J., & Szczuko, M. (2025). The Association Between Short-Chain Fatty Acids and the Incidence of Food Allergies—Systematic Review. Nutrients, 17(19), 3117. https://doi.org/10.3390/nu17193117






