Bioavailability and Metabolic Fate of (Poly)phenols from Hull-Less Purple Whole-Grain Barley in Humans
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Whole-Grain Barley Biscuits
2.3. Subjects and Study Design
2.4. Biological Samples Pretreatment
2.4.1. Plasma Analysis
2.4.2. Urine Analysis
2.5. Analysis of (Poly)phenolic Metabolites by UPLC-MS/MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of (Poly)phenolic Compounds in Barley Biscuits
3.2. Plasma Appearance of Phenolic Metabolites
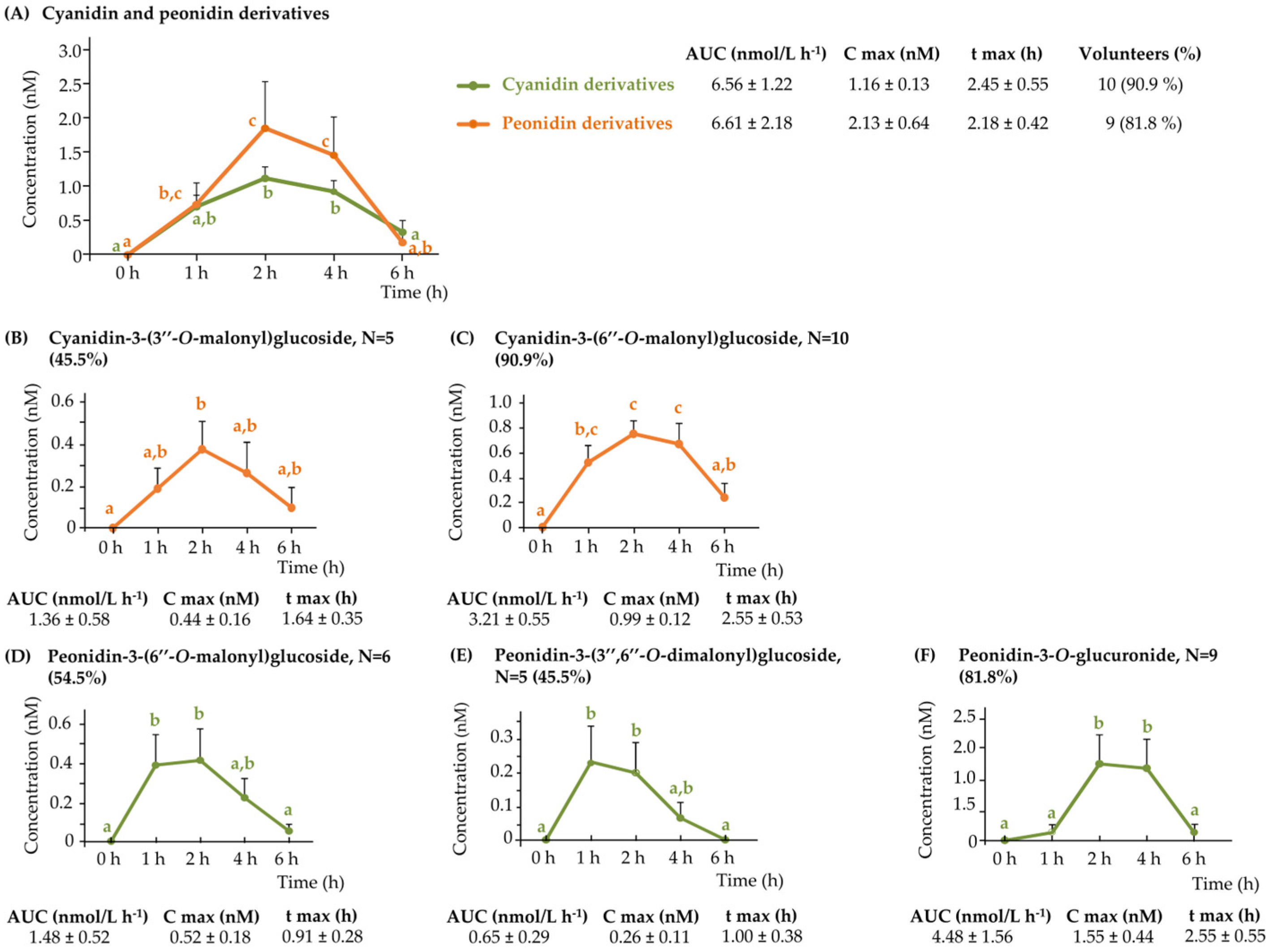
3.2.1. Plasma Anthocyanins
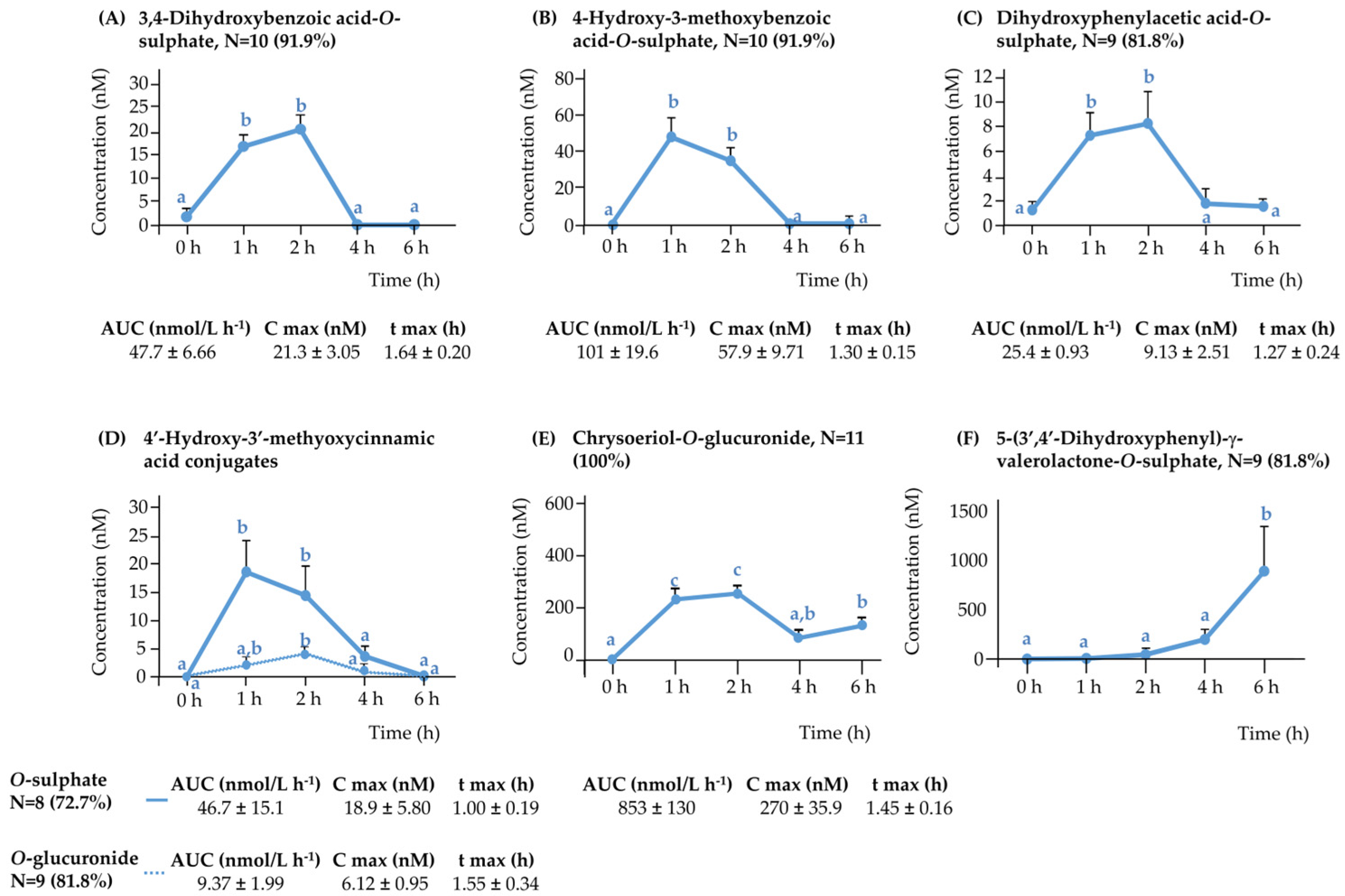
3.2.2. Rest of the (Poly)phenolic Compounds
3.3. Urinary Excretion of Phenolic Metabolites
3.3.1. Urinary Anthocyanins
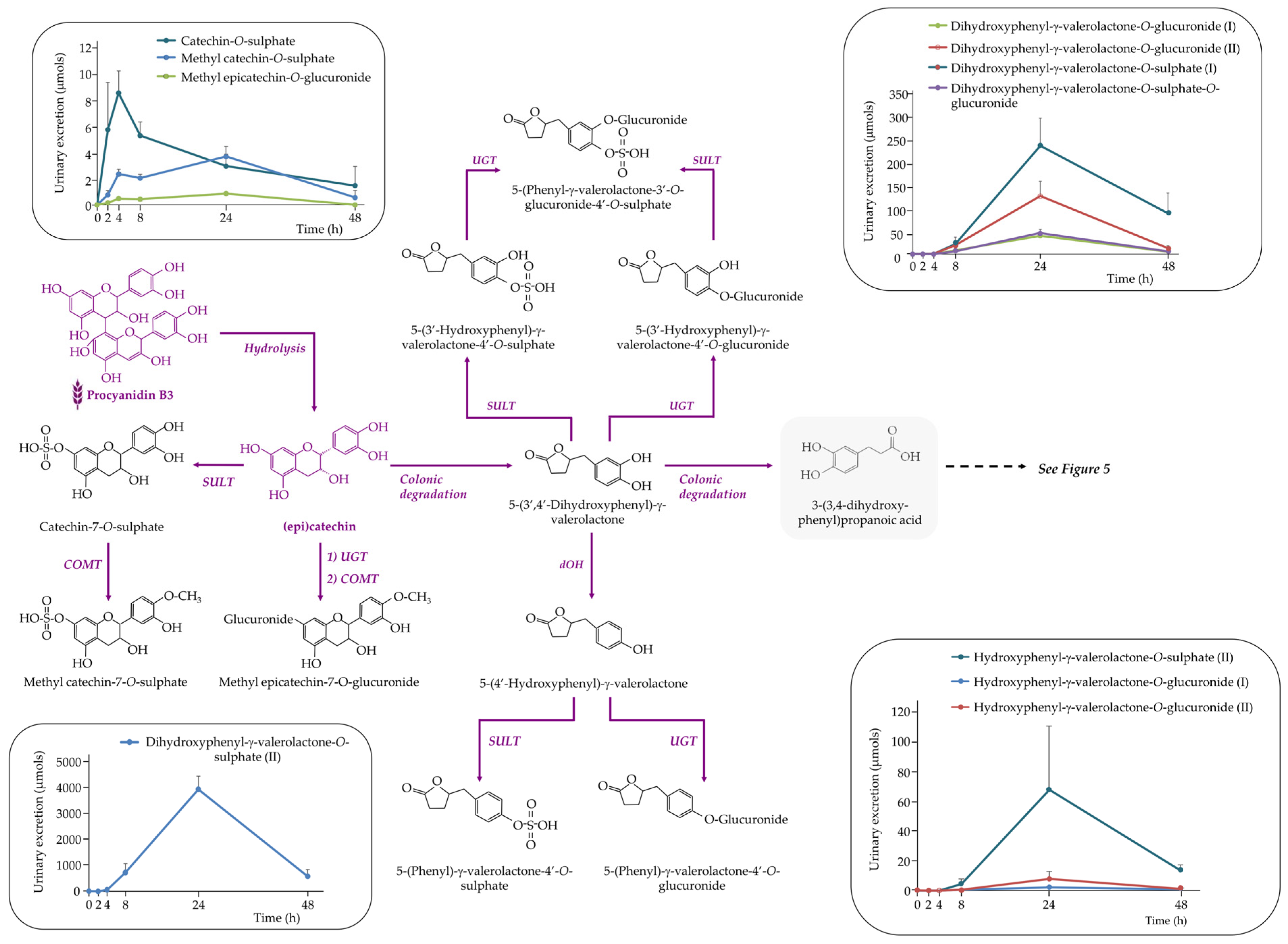
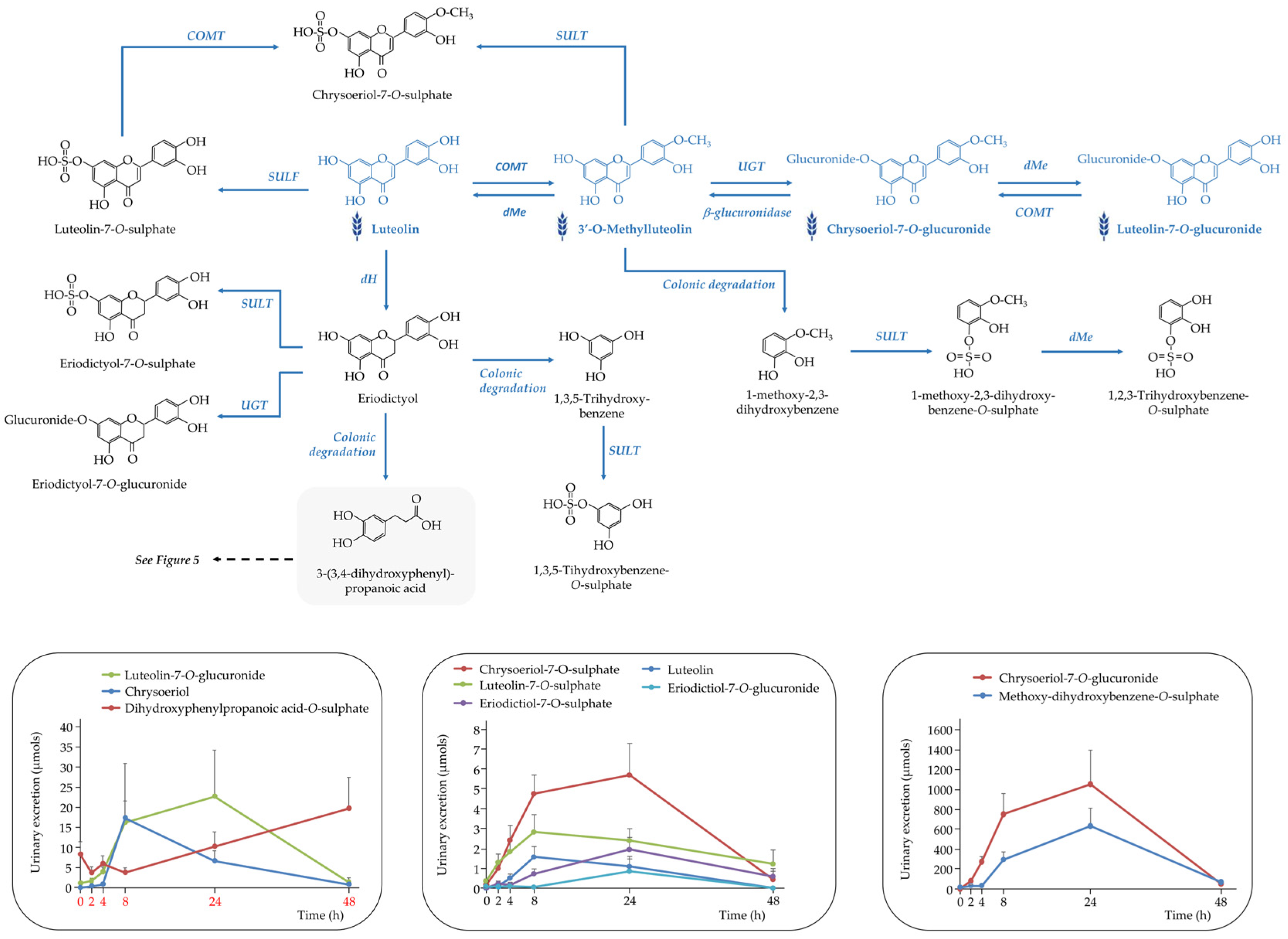
3.3.2. The Rest of (Poly)phenolic Compounds
Flavan-3-ols
Flavones
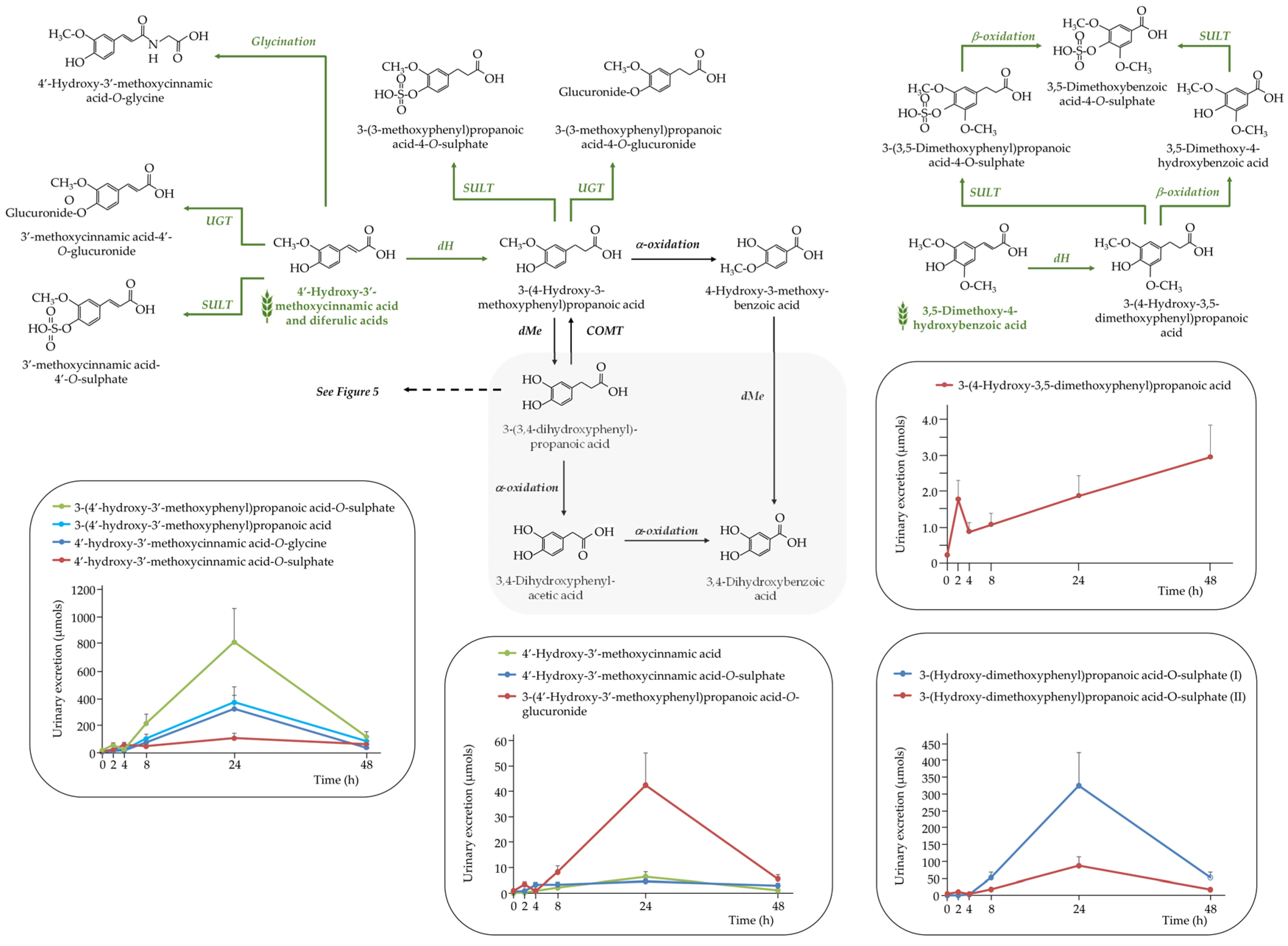
Phenolic Acids
Non-Specific Colonic Metabolites of (Poly)phenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sui, J.; Xu, Y.; Pan, L.; Xia, H.; Sun, G. Effect of whole grain and fiber consumption on chronic liver diseases: A systematic review and meta-analysis. Food Funct. 2024, 15, 9707–9717. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Deng, J.; Yu, M.; Yang, Y.; Liu, T.; Xiang, Z.; Chen, J.; Yang, K.; Zhan, R.; Zhu, B.; Zhu, Y.; et al. Studies of phytochemical constituents by UPLC-QTOF-MS/MS of black hulless barley bran and its antioxidation and α-glucosidase inhibition effect. Arab. J. Chem. 2024, 17, 105644. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Francis, N.; Blanchard, C.; Santhakumar, A. The bioavailability of polyphenols following acute consumption of pigmented barley and wheat. Food Funct. 2024, 15, 9330–9342. [Google Scholar] [CrossRef]
- Deng, J.; Xiang, Z.; Lin, C.; Zhu, Y.; Yang, K.; Liu, T.; Xia, C.; Chen, J.; Zhang, W.; Zhang, Y.; et al. Identification and quantification of free, esterified, and insoluble-bound phenolics in grains of hulless barley varieties and their antioxidant activities. LWT 2021, 151, 112001. [Google Scholar] [CrossRef]
- Gamel, T.H.; Wright, A.J.; Tucker, A.J.; Pickard, M.; Rabalski, I.; Podgorski, M.; Di Ilio, N.; O’Brien, C.; Abdel-Aal, E.-S.M. Absorption and metabolites of anthocyanins and phenolic acids after consumption of purple wheat crackers and bars by healthy adults. J. Cereal Sci. 2019, 86, 60–68. [Google Scholar] [CrossRef]
- Kay, C.D.; Clifford, M.N.; Mena, P.; McDougall, G.J.; Andres-Lacueva, C.; Cassidy, A.; Del Rio, D.; Kuhnert, N.; Manach, C.; Pereira-Caro, G.; et al. Recommendations for standardizing nomenclature for dietary (poly)phenol catabolites. Am. J. Clin. Nutr. 2020, 112, 1051–1068. [Google Scholar] [CrossRef]
- Curti, C.; Clifford, M.N.; Kay, C.D.; Mena, P.; Rodriguez-Mateos, A.; Del Rio, D.; McDougall, G.J.; Williamson, G.; Andres-Lacueva, C.; Bresciani, L.; et al. Extended recommendations on the nomenclature for microbial catabolites of dietary (poly)phenols, with a focus on isomers. Food Funct. 2025, 16, 3963–4000. [Google Scholar] [CrossRef]
- Cortijo-Alfonso, M.-E.; Yuste, S.; Piñol-Felis, C.; Romero, M.-P.; Macià, A.; Rubió-Piqué, L. Finger-prick blood sampling using volumetric absorptive microsampling (VAMS) method for monitoring the main (poly)phenolic metabolites in human blood after barley biscuit intake. J. Chromatogr. B 2025, 1256, 124527. [Google Scholar] [CrossRef]
- Cortijo-Alfonso, M.-E.; Yuste, S.; Friero, I.; Martínez-Subirà, M.; Moralejo, M.; Piñol-Felis, C.; Rubió-Piqué, L.; Macià, A. Metabolic profiling of (poly)phenolic compounds in mouse urine following consumption of hull-less and purple-grain barley. Food Funct. 2024, 15, 8300–8309. [Google Scholar] [CrossRef]
- Friero, I.; Martínez-Subirà, M.; Macià, A.; Romero, M.-P.; Moralejo, M. Exploring the nutritional and techno-functional benefits of purple hull-less barley in extruded ready-to-eat cereals. LWT 2024, 212, 117018. [Google Scholar] [CrossRef]
- Assefa, A.D.; Hur, O.-S.; Hahn, B.-S.; Kim, B.; Ro, N.-Y.; Rhee, J.-H. Nutritional Metabolites of Red Pigmented Lettuce (Lactuca sativa) Germplasm and Correlations with Selected Phenotypic Characters. Foods 2021, 10, 2504. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, D.; Nuñez, V.; Sancho, A.I.; Faulds, C.B.; Williamson, G.; Bartolomé, B.; Gómez-Cordovés, C. Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J. Agric. Food Chem. 2001, 49, 4884–4888. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Gui, H.; Sun, L.; Liu, R.; Si, X.; Li, D.; Wang, Y.; Shu, C.; Sun, X.; Jiang, Q.; Qiao, Y.; et al. Current knowledge of anthocyanin metabolism in the digestive tract: Absorption, distribution, degradation, and interconversion. Crit. Rev. Food Sci. Nutr. 2023, 63, 5953–5966. [Google Scholar] [CrossRef]
- Serra, A.; Macià, A.; Romero, M.-P.; Reguant, J.; Ortega, N.; Motilva, M.-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Mosele, J.I.; Viadel, B.; Yuste, S.; Tomás-Cobos, L.; García-Benlloch, S.; Bailón, M.-T.E.; Estévez, I.G.; Fraile, P.M.; de Rivera, F.R.; Casado, S.d.D.; et al. Application of a dynamic colonic gastrointestinal digestion model to red wines: A study of flavanol metabolism by the gut microbiota and the cardioprotective activity of microbial metabolites. Food Funct. 2025, 16, 885–899. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Ferulic Acid Sugar Esters Are Recovered in Rat Plasma and Urine Mainly as the Sulfoglucuronide of Ferulic Acid. J. Nutr. 2003, 133, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol. Nutr. Food Res. 2013, 57, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, Y.; Oppong, M.B.; Qiu, F. Insights into the intestinal bacterial metabolism of flavonoids and the bioactivities of their microbe-derived ring cleavage metabolites. Drug Metab. Rev. 2018, 50, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Hur, H.-G.; Beger, R.D.; Heinze, T.M.; Lay, J.O.; Freeman, J.P.; Dore, J.; Rafii, F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch. Microbiol. 2002, 178, 75. [Google Scholar] [CrossRef]
- Winter, J.; Popoff, M.R.; Grimont, P.; Bokkenheuser, V.D. Clostridium orbiscindens sp. nov., a Human Intestinal Bacterium Capable of Cleaving the Flavonoid C-Ring. Int. J. Syst. Bacteriol. 1991, 41, 355–357. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. A critical examination of human data for the biological activity of phenolic acids and their phase-2 conjugates derived from dietary (poly)phenols, phenylalanine, tyrosine and catecholamines. Crit. Rev. Food Sci. Nutr. 2024, 9, 1–60. [Google Scholar] [CrossRef] [PubMed]


| FREE (Poly)phenolics | BOUND (Poly)phenolics | |
|---|---|---|
| Cyanidin-3-O-glucoside | 6.20 ± 0.24 | 0.06 ± 0.01 |
| Cyanidin-3-O-(3″-malonyl)glucoside | 4.90 ± 0.20 | n.d. |
| Cyanidin-3-O-(6″-malonyl)glucoside | 12.5 ± 0.50 | n.d. |
| Cyanidin-3-O-(3″,6″-dimalonyl)glucoside | 16.9 ± 0.64 | n.d. |
| Peonidin-3-O-glucoside | 0.38 ± 0.07 | 0.02 ± 0.00 |
| Peonidin-3-O-(3″-O-malonyl)glucoside | 0.31 ± 0.01 | n.d. |
| Peonidin-3-O-(6″-O-malonyl)glucoside | 1.12 ± 0.07 | n.d. |
| Peonidin-3-O-(3″,6″-dimalonyl)glucoside | 0.46 ± 0.01 | n.d. |
| Pelargonidin-3-O-glucoside | 0.27 ± 0.02 | 0.02 ± 0.00 |
| Pelargonidin-3-O-malonylglucoside | 0.22 ± 0.02 | n.d. |
| Pelargonidin-3-O-(6″-malonyl)glucoside | 1.24 ± 0.08 | n.d. |
| Delphinidin-3-O-glucoside | 0.20 ± 0.06 | n.d. |
| Total anthocyanins | 44.7 ± 1.80 | 0.10 ± 0.01 |
| 4-Hydroxybenzoic acid | 0.22 ± 0.02 | 0.87 ± 0.03 |
| Hydroxybenzoic acid | n.d. | 0.18 ± 0.01 |
| 3,4-Dihydroxybenzoic acid (PCA) | 0.16 ± 0.06 | 0.03 ± 0.01 |
| 4-Hydroxy-3-methoxybenzoic acid (VA) | 0.16 ± 0.03 | 0.44 ± 0.01 |
| 4-Hydroxy-3,5-dimethoxybenzoic acid (Syr) | n.d. | 0.16 ± 0.02 |
| Cinnamic acid | n.d. | 0.11 ± 0.01 |
| 4′-Hydroxycinnamic acid | 0.10 ± 0.02 | 1.18 ± 0.04 |
| 3,4-Dihydroxycinnamic acid (CA) | n.d. | 0.02 ± 0.00 |
| 4′-Hydroxy-3′-methoxycinnamic acid (FA) | 1.22 ± 0.14 | 40.8 ± 0.80 |
| 3′-Hydroxy-4′-methoxycinnamic acid (IsoFA) | 0.20 ± 0.07 | 8.64 ± 0.17 |
| 4′-Hydroxy-3,5-dimethoxycinnamic acid | n.d. | 3.07 ± 0.16 |
| 4′-Hydroxy-3,5-dimethoxycinnamic acid-O-glucoside | n.d. | 0.95 ± 0.25 |
| Diferulic acid | n.d. | 12.4 ± 0.65 |
| Diferulic acid DC | n.d. | 1.20 ± 0.24 |
| Triferulic acid | n.d. | 2.06 ± 0.39 |
| Total phenolic acids | 2.06 ± 0.16 | 72.1 ± 1.24 |
| Catechin | 2.62 ± 0.56 | n.d. |
| Catechin glucoside | 2.09 ± 0.20 | n.d. |
| Procyanidin B3 | 8.40 ± 1.49 | n.d. |
| Gallocatechin-catechin or prodelphinidin B4 | 3.67 ± 0.39 | n.d. |
| Total flavan-3-ols | 16.8 ± 1.40 | n.d. |
| Apigenin-7-O-glucoside | n.d. | 0.04 ± 0.01 |
| Apigenin-6-C-arabinoside-8-C-glucoside | 0.46 ± 0.09 | 0.05 ± 0.01 |
| Isovitexin-C-glucoside | 0.56 ± 0.05 | 0.09 ± 0.01 |
| Isovitexin-C-rutinoside | 0.25 ± 0.03 | n.d. |
| Isoscoparin-C-glucoside | 0.89 ± 0.12 | 0.09 ± 0.02 |
| Isoscoparin-C-rutinoside | 0.26 ± 0.04 | n.d. |
| Luteolin | 5.92 ± 0.18 | 0.03 ± 0.00 |
| Methyl luteolin (Chrysoeriol) | 16.2 ± 2.63 | 0.07 ± 0.02 |
| Luteolin-O-glucoside | 0.13 ± 0.02 | n.d. |
| Luteolin-7-O-glucuronide | 32.9 ± 5.91 | 0.46 ± 0.03 |
| Methyl Luteolin-O-glucoside | 0.22 ± 0.03 | n.d. |
| Methyl Luteolin-O-glucuronide | 160 ± 24.6 | 4.64 ± 0.49 |
| Total flavones | 217 ± 33.0 | 5.53 ± 0.50 |
| Total (poly)phenols | 281 ± 32.4 | 77.7 ± 0.73 |
| Total (poly)phenols (Free and Bound) | 359 ± 32.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortijo-Alfonso, M.-E.; Yuste, S.; Martínez-Subirà, M.; Moralejo, M.; Piñol-Felis, C.; Macià, A.; Rubió-Piqué, L. Bioavailability and Metabolic Fate of (Poly)phenols from Hull-Less Purple Whole-Grain Barley in Humans. Nutrients 2025, 17, 3086. https://doi.org/10.3390/nu17193086
Cortijo-Alfonso M-E, Yuste S, Martínez-Subirà M, Moralejo M, Piñol-Felis C, Macià A, Rubió-Piqué L. Bioavailability and Metabolic Fate of (Poly)phenols from Hull-Less Purple Whole-Grain Barley in Humans. Nutrients. 2025; 17(19):3086. https://doi.org/10.3390/nu17193086
Chicago/Turabian StyleCortijo-Alfonso, María-Engracia, Silvia Yuste, Mariona Martínez-Subirà, Marian Moralejo, Carme Piñol-Felis, Alba Macià, and Laura Rubió-Piqué. 2025. "Bioavailability and Metabolic Fate of (Poly)phenols from Hull-Less Purple Whole-Grain Barley in Humans" Nutrients 17, no. 19: 3086. https://doi.org/10.3390/nu17193086
APA StyleCortijo-Alfonso, M.-E., Yuste, S., Martínez-Subirà, M., Moralejo, M., Piñol-Felis, C., Macià, A., & Rubió-Piqué, L. (2025). Bioavailability and Metabolic Fate of (Poly)phenols from Hull-Less Purple Whole-Grain Barley in Humans. Nutrients, 17(19), 3086. https://doi.org/10.3390/nu17193086







