Mucin Alterations in Response to High-Fat Diet and the Potential Protective Role of Chickpea Accessions
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
- CTRL: standard diet with 10% fat, 24% protein, and 66% carbohydrates (3.514 kcal/kg);
- HFD: diet with 45% fat, 19% protein, and 38% carbohydrates (4.496 kcal/kg);
- HFD plus MG_13-chickpeas (HFD + MG_13): diet same as HFD (metabolized energy and composition), except that 10% of MG_13 raw crushed chickpea flour replaced crude fibers and ashes of HFD; details of diet are given in Costantini et al. (2021) [16];
- HFD plus PI358934-chickpeas (HFD + PI): diet same as HFD (metabolized energy and composition), except that 10% of PI358934 raw crushed chickpea flour replaced crude fibers and ashes of HFD; details of diet are given in Costantini et al. (2021) [16]. In respect to MG_13, this diet has a similar content of protein, lipids, carbohydrates, and ash, and a lower phenolic content.
2.2. Section Preparation
2.3. Classic Histochemical Staining
2.4. Lectins
2.5. Enzymatic Treatments
2.6. Immunohistochemistry
2.7. Quantitative Analysis
2.8. Statistical Analysis
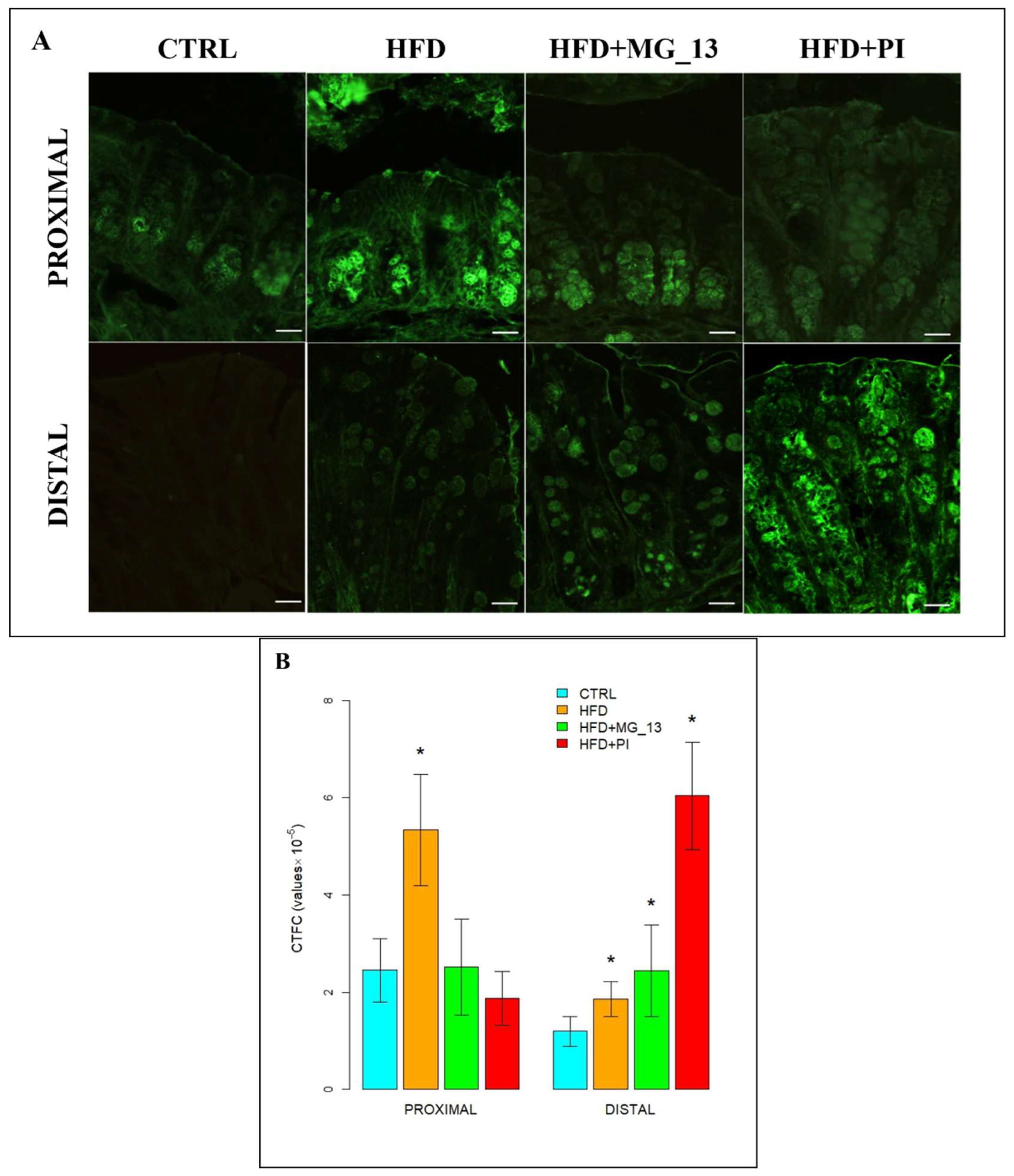
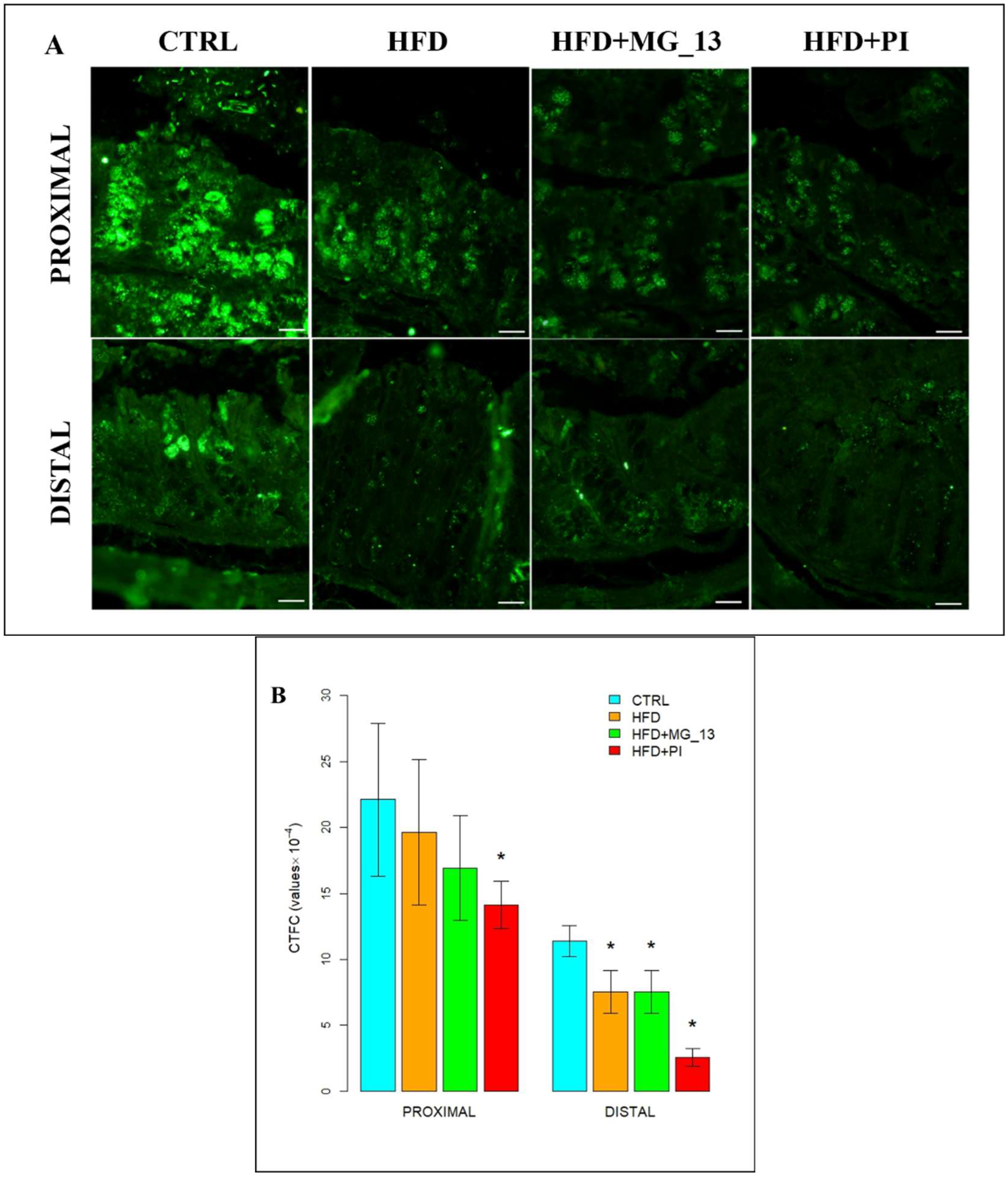
3. Results
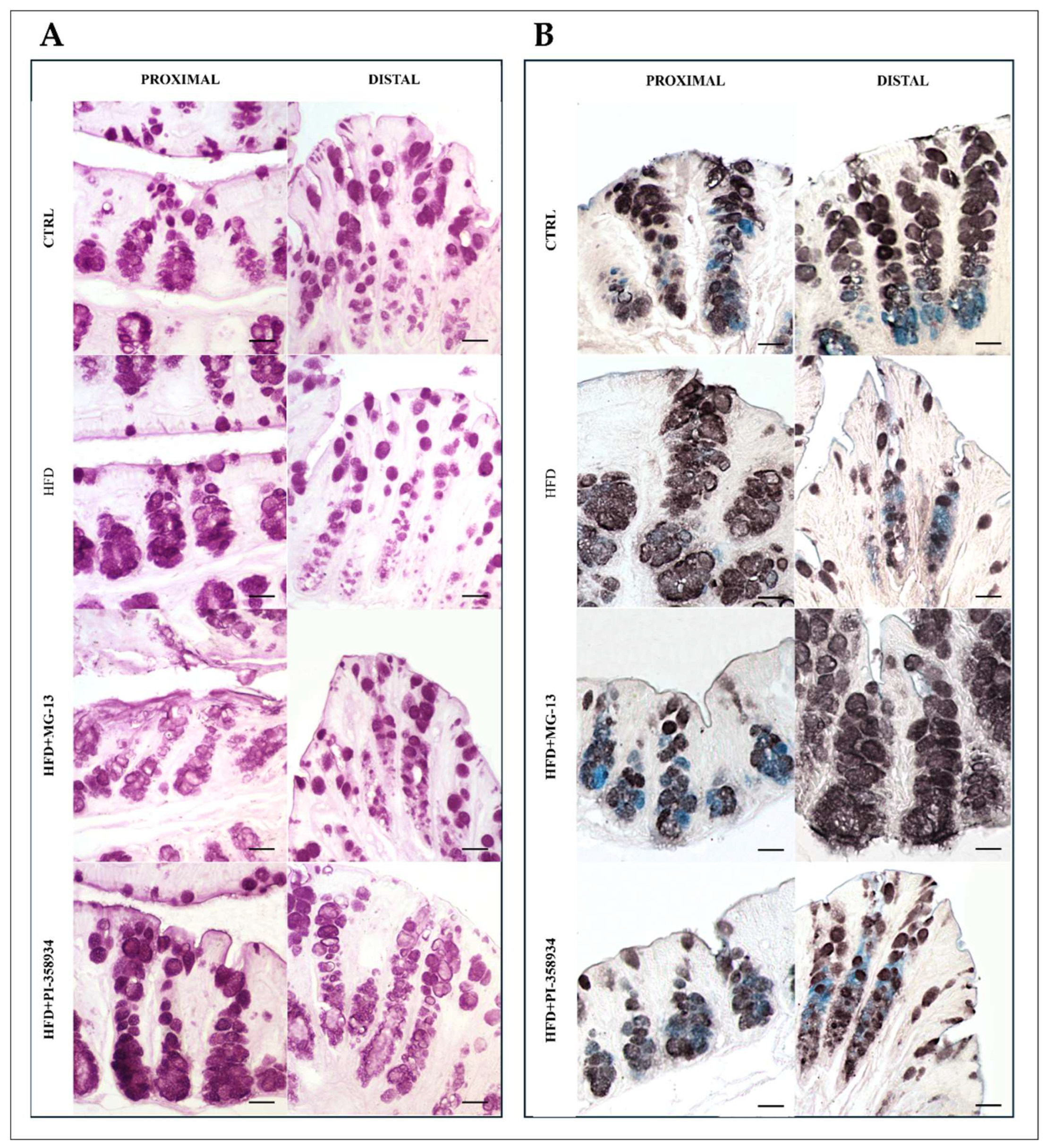
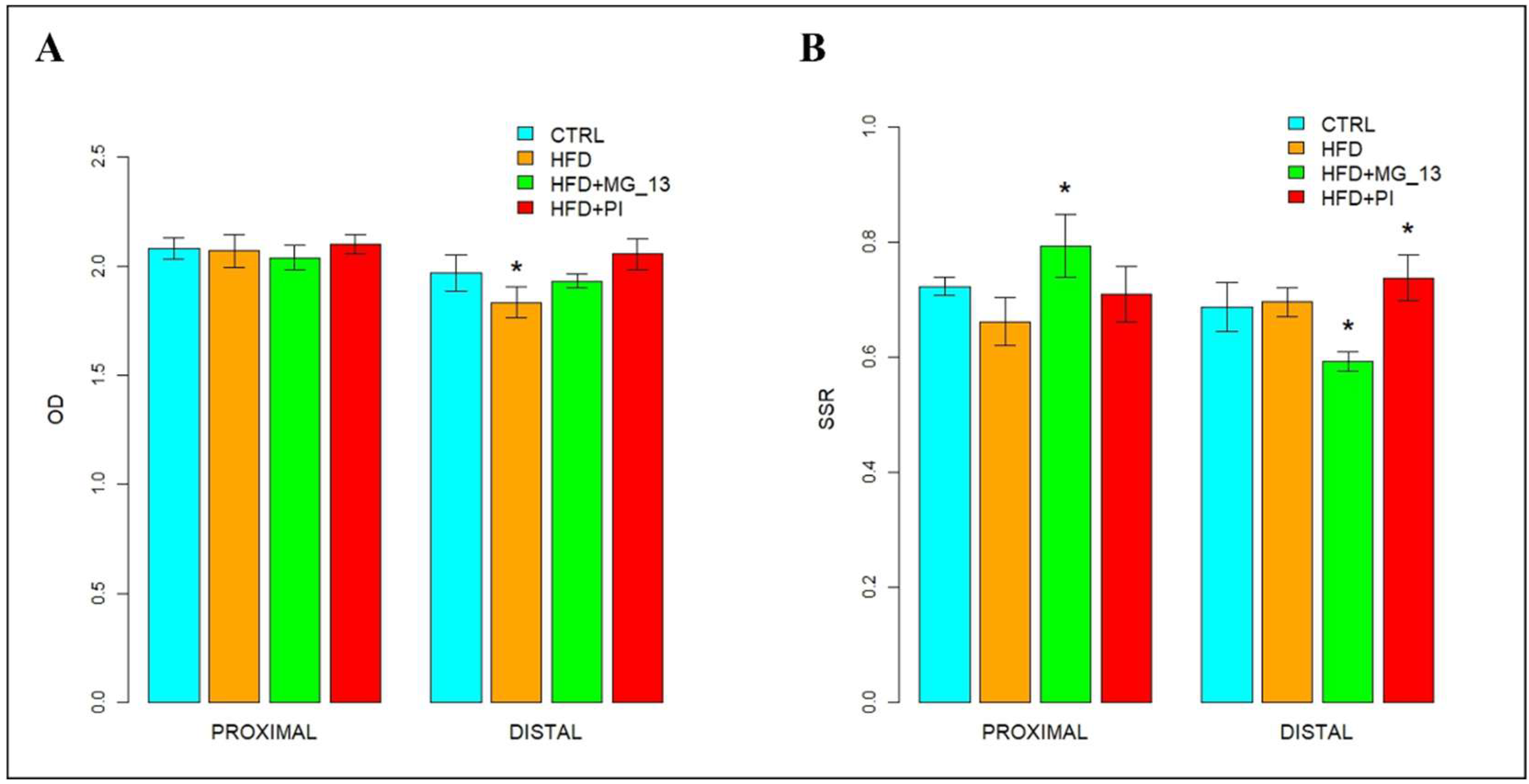
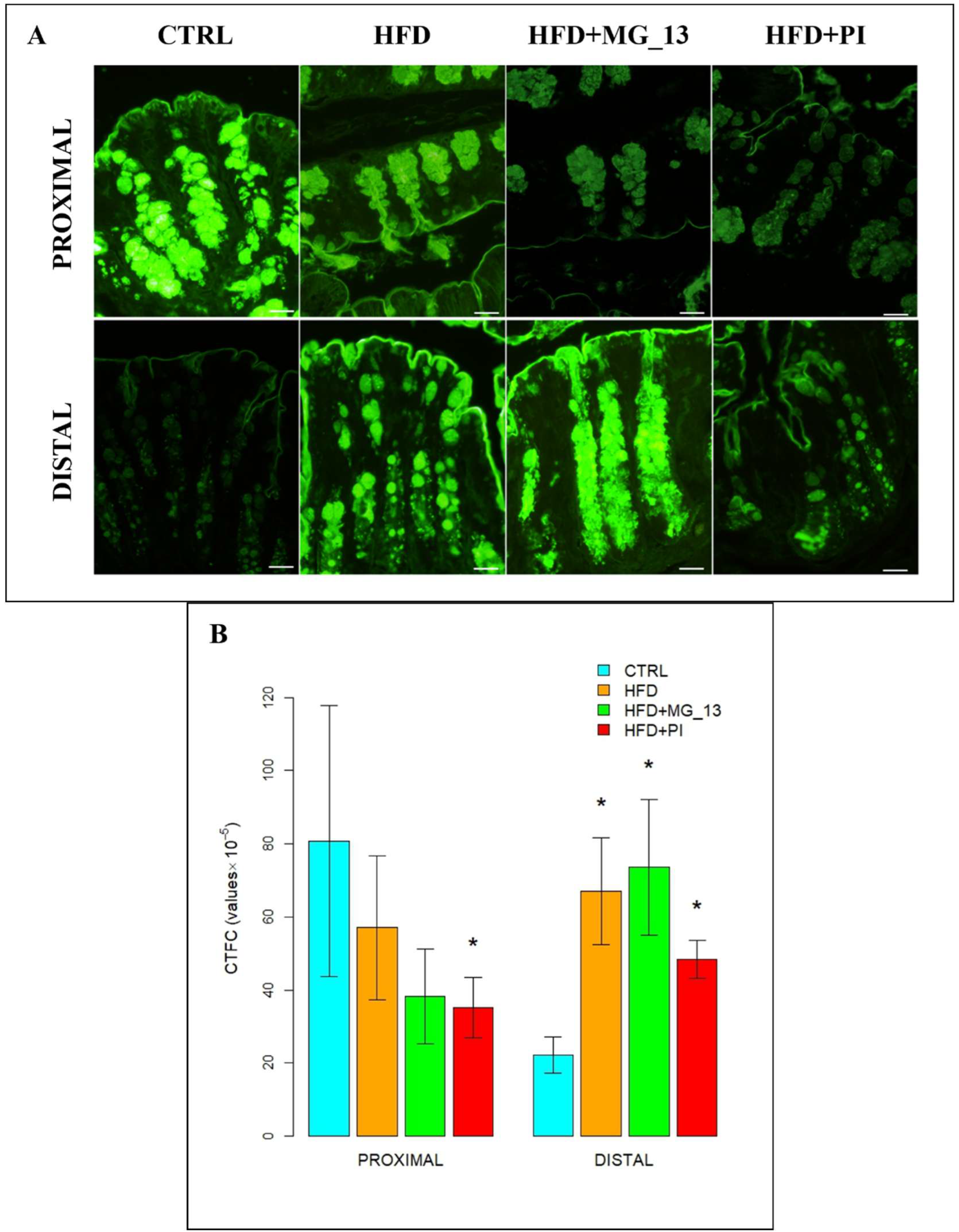
3.1. Conventional Histochemistry
3.1.1. PAS, HID-Alcian Blue pH 2.5 to Characterize Acidic and Neutral Glycoconjugates
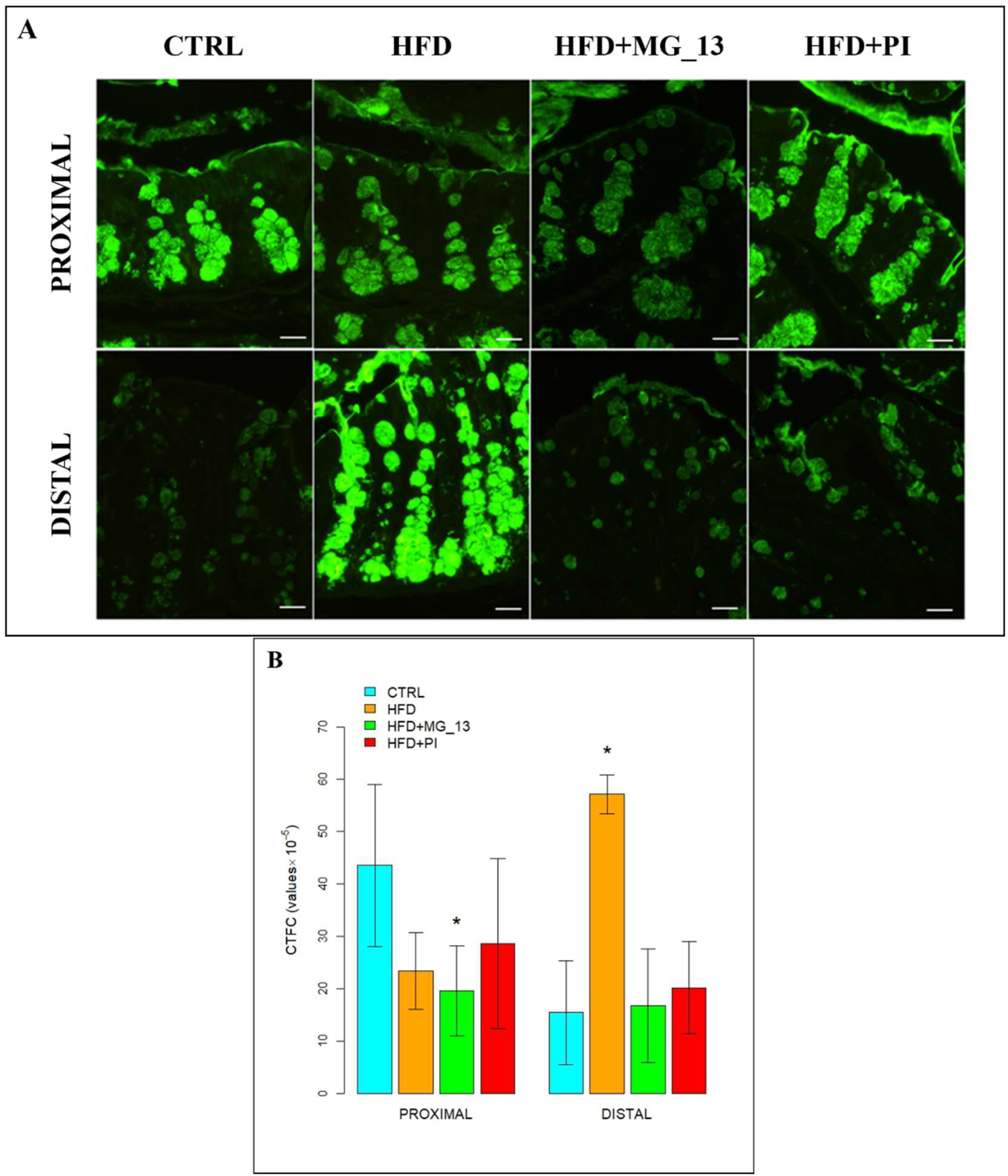
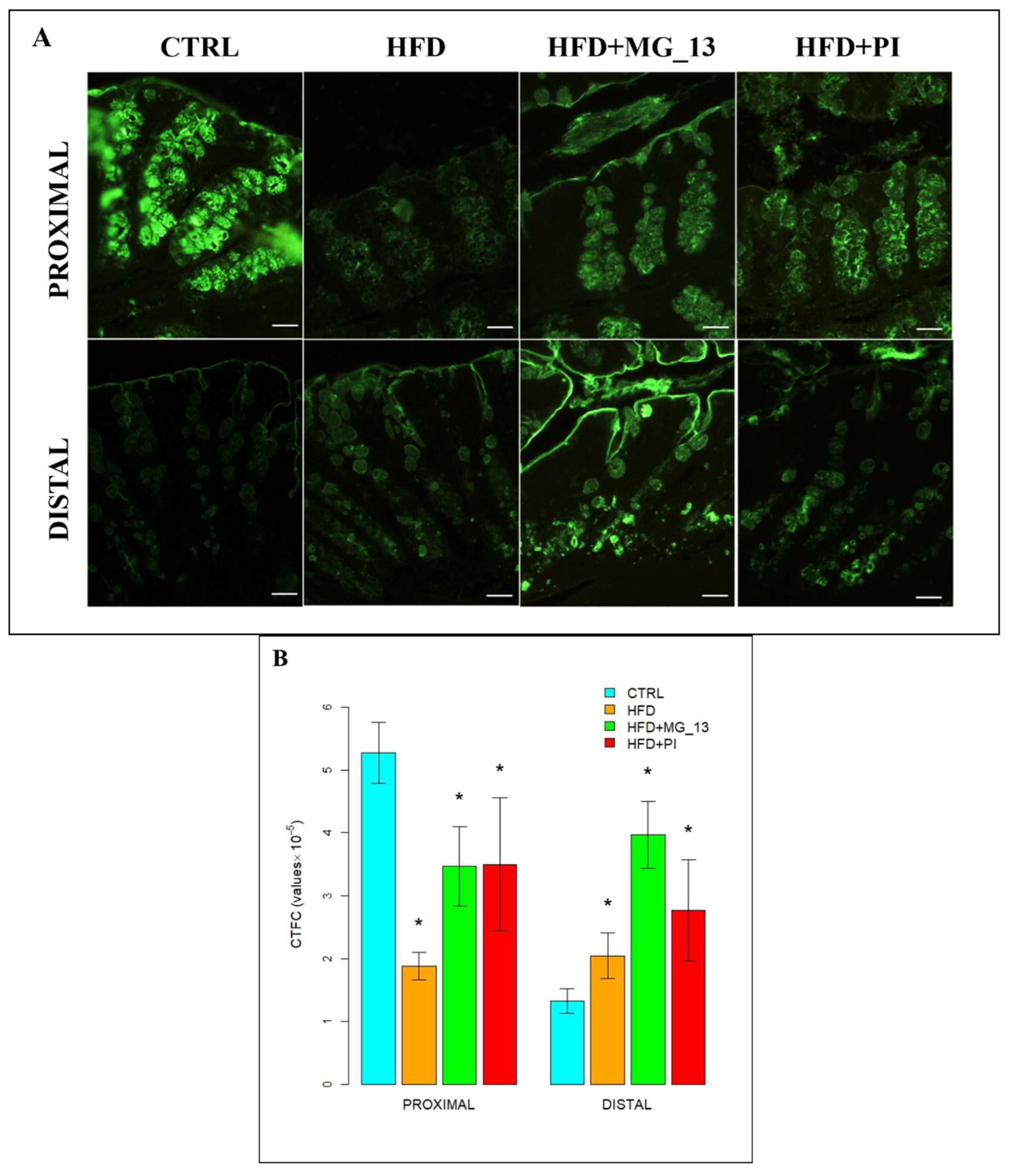
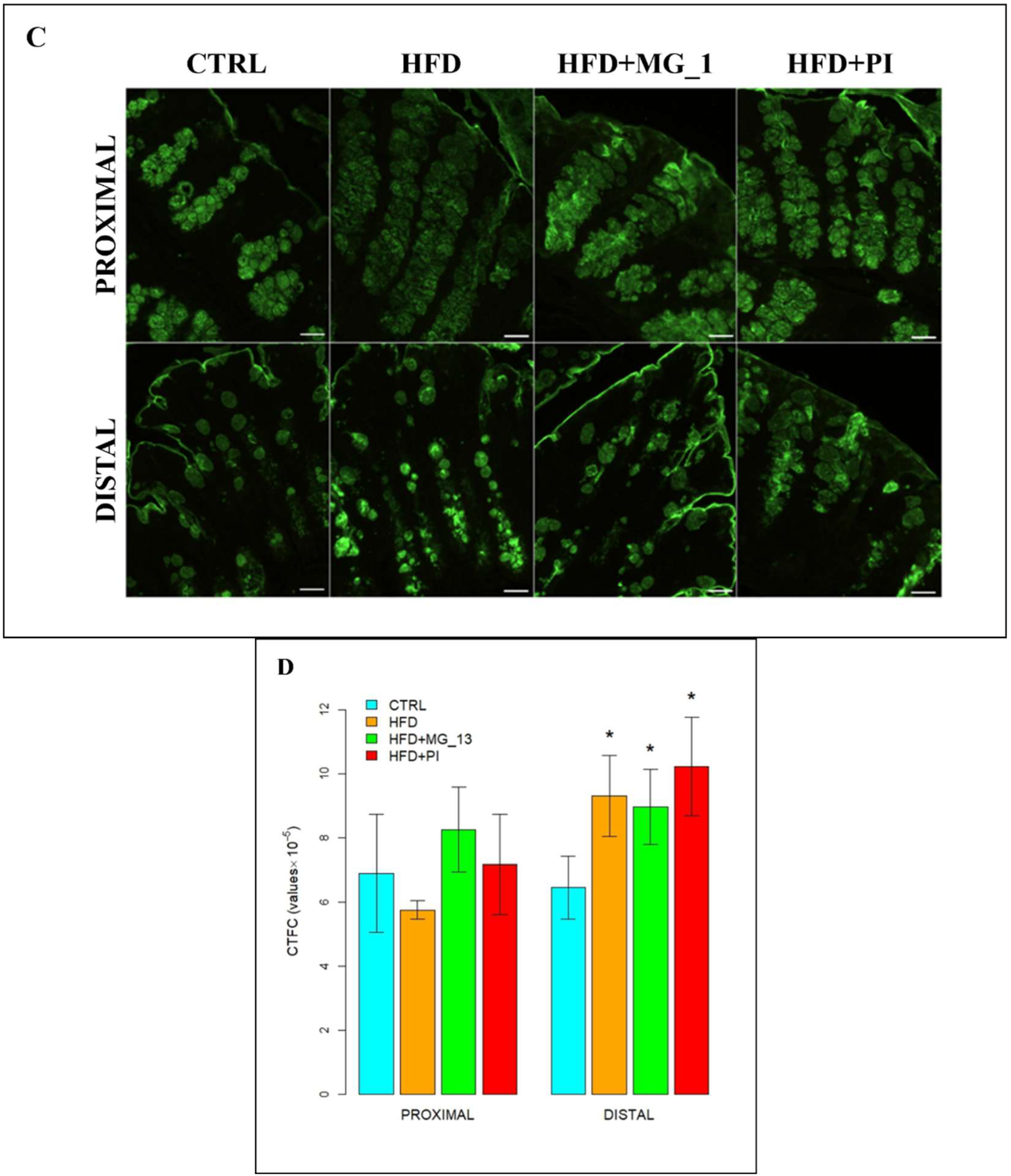
3.1.2. Lectin Histochemistry
UEA-I for the Identification of α (1,2)-Linked Fucose in O-Linked Glycans
AAL for the Identification of α (1,6)-Linked Fucose in O-Linked Glycans
WGA for the Identification of N-Acetylglucosamine in O-Linked Glycans
SBA for the Identification of N-Acetylgalactosamine in O-Linked Glycans
PNA for the Identification of Terminal Galactose β1,3N-Acetylgalactosamine in O-Linked Glycans
ConA for the Identification of D-Mannose in O-Linked Glycans
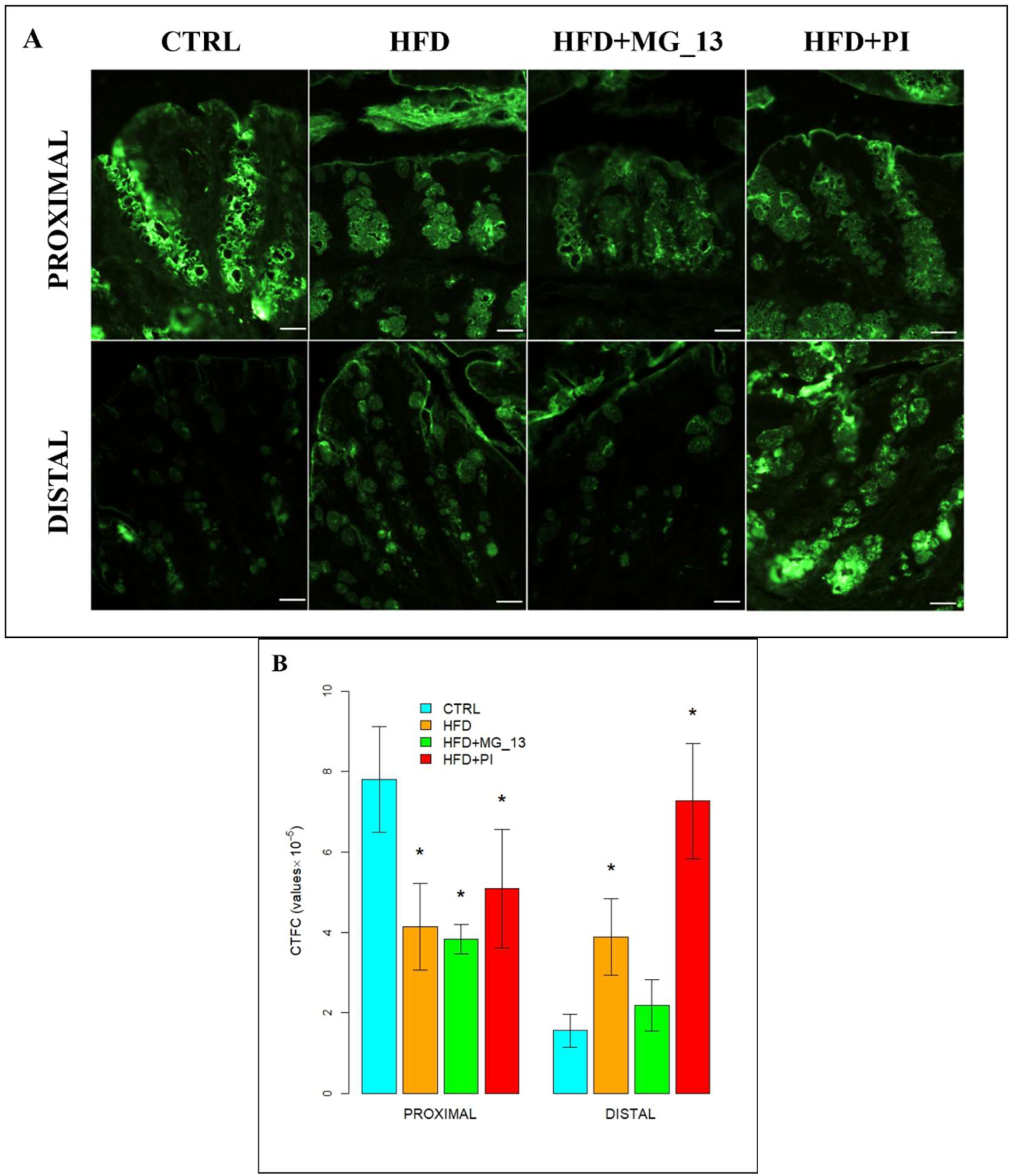
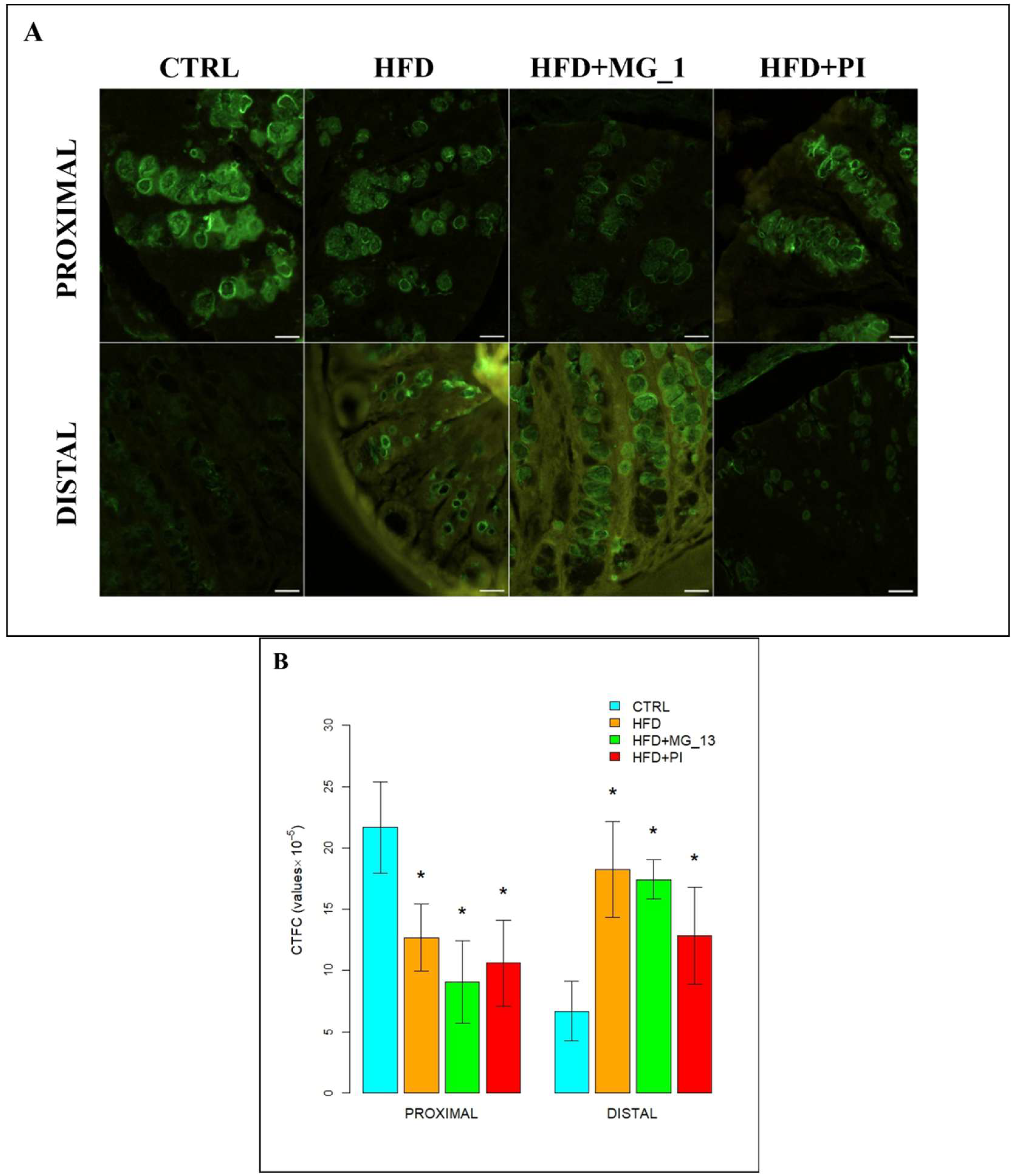
3.2. Immunohistochemistry Anti-Muc2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemente-Suarez, V.J.; Beltran-Velasco, A.I.; Redondo-Florez, L.; Martin-Rodriguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Rosi, A.; Scazzina, F.; Frias-Toral, E.; Abdelkarim, O.; Aly, M.; Zambrano-Villacres, R.; Pons, J.; Vazquez-Araujo, L.; Sumalla Cano, S.; et al. Youth Healthy Eating Index (YHEI) and Diet Adequacy in Relation to Country-Specific National Dietary Recommendations in Children and Adolescents in Five Mediterranean Countries from the DELICIOUS Project. Nutrients 2024, 16, 3907. [Google Scholar] [CrossRef] [PubMed]
- Noye Tuplin, E.W.; Alukic, E.; Lowry, D.E.; Chleilat, F.; Wang, W.; Cho, N.A.; Sampsell, K.; Sales, K.M.; Mayengbam, S.; McCoy, K.D.; et al. Dietary fiber combinations to mitigate the metabolic, microbial, and cognitive imbalances resulting from diet-induced obesity in rats. FASEB J. 2022, 36, e22269. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Shin, Y.C.; Lee, J.S.; Yoon, Y.C.; Kim, J.M.; Sung, M.K. N-Acetylglucosamine and its dimer ameliorate inflammation in murine colitis by strengthening the gut barrier function. Food Funct. 2023, 14, 8533–8544. [Google Scholar] [CrossRef]
- Mastrodonato, M.; Mentino, D.; Portincasa, P.; Calamita, G.; Liquori, G.E.; Ferri, D. High-fat diet alters the oligosaccharide chains of colon mucins in mice. Histochem. Cell Biol. 2014, 142, 449–459. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Hansson, G.C.; Johansson, M.E. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Gut Microbes 2010, 1, 51–54. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Vrinda, A.M.; Thangaraju, M.; Nair, S.A.; Howarth, G.S.; Pothuraju, R. Unraveling the role of mucins and gut microbiota in gastrointestinal cancers chemoresistance. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189385. [Google Scholar] [CrossRef]
- Ajay, A.; Gaur, S.S.; Shams, R.; Dash, K.K.; Mukarram, S.A.; Kovacs, B. Chickpeas and gut microbiome: Functional food implications for health. Heliyon 2024, 10, e39314. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. J. Funct. Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Disca, V.; Carrà, F.; Stampini, M.; Arlorio, M.; Locatelli, M. Treatments of food and food by-products to enhance gut microbiota short-chain fatty acids production. Food Biosci. 2025, 72, 107435. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108 (Suppl. S1), S11–S26. [Google Scholar] [CrossRef]
- De Giovanni, C.; Pavan, S.; Taranto, F.; Di Rienzo, V.; Miazzi, M.M.; Marcotrigiano, A.R.; Mangini, G.; Montemurro, C.; Ricciardi, L.; Lotti, C. Genetic variation of a global germplasm collection of chickpea (Cicer arietinum L.) including Italian accessions at risk of genetic erosion. Physiol. Mol. Biol. Plants 2017, 23, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Centrone, M.; Gena, P.; Ranieri, M.; Di Mise, A.; D’Agostino, M.; Mastrodonato, M.; Venneri, M.; De Angelis, D.; Pavan, S.; Pasqualone, A.; et al. In Vitro and In Vivo Nutraceutical Characterization of Two Chickpea Accessions: Differential Effects on Hepatic Lipid Over-Accumulation. Antioxidants 2020, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Summo, C.; Centrone, M.; Rybicka, I.; D’Agostino, M.; Annicchiarico, P.; Caponio, F.; Pavan, S.; Tamma, G.; Pasqualone, A. Macro-and micro-nutrient composition and antioxidant activity of chickpea and pea accessions. Pol. J. Food Nutr. Sci. 2021, 71, 177–185. [Google Scholar] [CrossRef]
- Mentino, D.; Nicchia, G.P.; Frigeri, A.; Desantis, S.; Guglielmi, M.V.; Semeraro, D.; Scillitani, G.; Mastrodonato, M. Altered glycosylation in secreting cells of the gastric glands of aquaporin-4-deficient mice. Microsc. Res. Tech. 2024, 87, 1836–1848. [Google Scholar] [CrossRef]
- Spicer, S.; Leppi, T.; Stoward, P. Suggestions for a histochemical terminology of carbohydrate-rich tissue components. J. Histochem. Cytochem. 1965, 13, 599–603. [Google Scholar] [CrossRef]
- Lueth, M.; Sturegård, E.; Sjunnesson, H.; Wadström, T.; Schumacher, U. Lectin histochemistry of the gastric mucosa in normal and Helicobacter pylori infected guinea-pigs. J. Mol. Histol. 2005, 36, 51–58. [Google Scholar] [CrossRef]
- Oue, N.; Sentani, K.; Sakamoto, N.; Uraoka, N.; Yasui, W. Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells. Int. J. Clin. Oncol. 2019, 24, 771–778. [Google Scholar] [CrossRef]
- Phillipson, M.; Johansson, M.E.; Henriksnas, J.; Petersson, J.; Gendler, S.J.; Sandler, S.; Persson, A.E.; Hansson, G.C.; Holm, L. The gastric mucus layers: Constituents and regulation of accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G806–G812. [Google Scholar] [CrossRef]
- Mastrodonato, M.; Calamita, G.; Mentino, D.; Scillitani, G. High-fat Diet Alters the Glycosylation Patterns of Duodenal Mucins in a Murine Model. J. Histochem. Cytochem. 2020, 68, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, R.; Cipriano, G.; Fanizza, C.; Gerussi, T.; Maglietta, R.; Petrella, A.; Pietroluongo, G.; Ricci, P.; Semeraro, D.; Guglielmi, M.V. Glycopatterns of the foregut in the striped dolphin Stenella coeruleoalba, Meyen 1833 from the Mediterranean Sea. Mar. Mammal Sci. 2022, 38, 190–211. [Google Scholar] [CrossRef]
- Carlucci, R.; Mentino, D.; Semeraro, D.; Ricci, P.; Sion, L.; Scillitani, G. Comparative histochemical analysis of intestinal glycoconjugates in the blunthead pufferfish Sphoeroides pachygaster and grey triggerfish Balistes capriscus (Teleostei: Tetraodontiformes). J. Fish Biol. 2019, 94, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Lotan, R.; Sharon, N. Peanut (Arachis hypogaea) agglutinin. Methods Enzymol. 1978, 50, 361–367. [Google Scholar]
- Bhattacharyya, L.; Haraldsson, M.; Brewer, C.F. Precipitation of galactose-specific lectins by complex-type oligosaccharides and glycopeptides: Studies with lectins from Ricinus communis (agglutinin I), Erythrina indica, Erythrina arborescens, Abrus precatorius (agglutinin), and Glycine max (soybean). Biochemistry 1988, 27, 1034–1041. [Google Scholar] [CrossRef]
- Gallagher, J.T.; Morris, A.; Dexter, T.M. Identification of two binding sites for wheat-germ agglutinin on polylactosamine-type oligosaccharides. Biochem. J. 1985, 231, 115–122. [Google Scholar] [CrossRef]
- Debray, H.; Decout, D.; Strecker, G.; Spik, G.; Montreuil, J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur. J. Biochem. 1981, 117, 41–55. [Google Scholar] [CrossRef]
- Sughii, S.; Kabat, E.A.; Baer, H.H. Further immunochemical studies on the combining sites of Lotus tetragonolobus and Ulex europaeus I and II lectins. Carbohydr. Res. 1982, 99, 99–101. [Google Scholar] [CrossRef]
- Debray, H.; Montreuil, J. Aleuria aurantia agglutinin. A new isolation procedure and further study of its specificity towards various glycopeptides and oligosaccharides. Carbohydr. Res. 1989, 185, 15–26. [Google Scholar] [CrossRef]
- Alonso, E.; Saez, F.J.; Madrid, J.F.; Hernandez, F. Lectin histochemistry shows fucosylated glycoconjugates in the primordial germ cells of Xenopus embryos. J. Histochem. Cytochem. 2003, 51, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Parillo, F.; Diverio, S.; Todini, L.; Fagioli, O. Histochemical detection of the lectin-binding carbohydrates in the zona pellucida during oocyte growth in the wild boar (Sus scrofa scrofa). Vet. Res. 2001, 32, 581–590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rasband, W. ImageJ, US National Institutes of Health. 2007. Available online: http://imagej.nih.gov/ij/ (accessed on 7 August 2025).[Green Version]
- Landini, G.; Martinelli, G.; Piccinini, F. Colour deconvolution: Stain unmixing in histological imaging. Bioinformatics 2021, 37, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Ruifrok, A. Quantification of histochemical staining by color deconvolution. Anal. Quant. Cytol. Histol./Int. Acad. Cytol. Am. Soc. Cytol. 2001, 23, 291–299. [Google Scholar]
- Guglielmi, M.V.; Semeraro, D.; Ricci, P.; Mastrodonato, M.; Mentino, D.; Carlucci, R.; Mastrototaro, F.; Scillitani, G. First data on the effect of Aluminium intake in Chamelea gallina of exploited stocks in the southern Adriatic Sea (Central Mediterranean Sea). Reg. Stud. Mar. Sci. 2023, 63, 103025. [Google Scholar] [CrossRef]
- Bankole, E.; Read, E.; Curtis, M.A.; Neves, J.F.; Garnett, J.A. The Relationship between Mucins and Ulcerative Colitis: A Systematic Review. J. Clin. Med. 2021, 10, 1935. [Google Scholar] [CrossRef]
- Kawashima, H. Roles of the gel-forming MUC2 mucin and its O-glycosylation in the protection against colitis and colorectal cancer. Biol. Pharm. Bull. 2012, 35, 1637–1641. [Google Scholar] [CrossRef]
- Dawson, P.A.; Huxley, S.; Gardiner, B.; Tran, T.; McAuley, J.L.; Grimmond, S.; McGuckin, M.A.; Markovich, D. Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut 2009, 58, 910–919. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Wang, X.; Qi, G.; Wei, L.; Zhang, D. Sialylation in the gut: From mucosal protection to disease pathogenesis. Carbohydr. Polym. 2024, 343, 122471. [Google Scholar] [CrossRef] [PubMed]
- Putera, H.D.; Doewes, R.I.; Shalaby, M.N.; Ramirez-Coronel, A.A.; Clayton, Z.S.; Abdelbasset, W.K.; Murtazaev, S.S.; Jalil, A.T.; Rahimi, P.; Nattagh-Eshtivani, E.; et al. The effect of conjugated linoleic acids on inflammation, oxidative stress, body composition and physical performance: A comprehensive review of putative molecular mechanisms. Nutr. Metab. 2023, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.J.; Lowe, J.B. Fucose: Biosynthesis and biological function in mammals. Glycobiology 2003, 13, 41R–53R. [Google Scholar] [CrossRef] [PubMed]
- Saldova, R.; Thomsson, K.A.; Wilkinson, H.; Chatterjee, M.; Singh, A.K.; Karlsson, N.G.; Knaus, U.G. Characterization of intestinal O-glycome in reactive oxygen species deficiency. PLoS ONE 2024, 19, e0297292. [Google Scholar] [CrossRef]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Inoue, T.; Iijima, H.; Tajiri, M.; Shinzaki, S.; Shiraishi, E.; Hiyama, S.; Mukai, A.; Nakajima, S.; Iwatani, H.; Nishida, T.; et al. Deficiency of N-acetylgalactosamine in O-linked oligosaccharides of IgA is a novel biologic marker for Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 1723–1734. [Google Scholar] [CrossRef]
- Sanz-Martinez, I.; Pereira, S.; Merino, P.; Corzana, F.; Hurtado-Guerrero, R. Molecular recognition of GalNAc in mucin-type O-glycosylation. Acc. Chem. Res. 2023, 56, 548–560. [Google Scholar] [CrossRef]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. d-Mannose: Properties, Production, and Applications: An Overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef]
- Xiao, P.; Hu, Z.; Lang, J.; Pan, T.; Mertens, R.T.; Zhang, H.; Guo, K.; Shen, M.; Cheng, H.; Zhang, X. Mannose metabolism normalizes gut homeostasis by blocking the TNF-α-mediated proinflammatory circuit. Cell. Mol. Immunol. 2023, 20, 119–130. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
| Lectin | Origin | Buffer | Dilution | Inhibitory Sugar Conc. |
|---|---|---|---|---|
| PNA | Arachis hypogaea | Hepes | 10 mg/mL | 0.2 M Gal |
| SBA | Glycine max | Hepes | 20 mg/mL | 0.2 M GalNAc |
| WGA | Triticum vulgare | Hepes | 20 mg/mL | 0.5 M GlcNAc |
| UEA-I | Ulex europaeus | Hepes | 10 mg/mL | 0.2 M L-Fuc |
| AAL | Aleuria aurantia | Hepes | 10 mg/mL | 0.2 M L-Fuc |
| ConA | Canavalia ensiformis | Hepes | 20 mg/mL | 0.1 M MαM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mentino, D.; Semeraro, D.; Taldone, N.; Pavan, S.; Caponio, F.; Gena, P.; Ranieri, M.; Tamma, G.; Guglielmi, M.V.; Scillitani, G.; et al. Mucin Alterations in Response to High-Fat Diet and the Potential Protective Role of Chickpea Accessions. Nutrients 2025, 17, 3035. https://doi.org/10.3390/nu17193035
Mentino D, Semeraro D, Taldone N, Pavan S, Caponio F, Gena P, Ranieri M, Tamma G, Guglielmi MV, Scillitani G, et al. Mucin Alterations in Response to High-Fat Diet and the Potential Protective Role of Chickpea Accessions. Nutrients. 2025; 17(19):3035. https://doi.org/10.3390/nu17193035
Chicago/Turabian StyleMentino, Donatella, Daniela Semeraro, Nastasia Taldone, Stefano Pavan, Francesco Caponio, Patrizia Gena, Marianna Ranieri, Grazia Tamma, Marco Vito Guglielmi, Giovanni Scillitani, and et al. 2025. "Mucin Alterations in Response to High-Fat Diet and the Potential Protective Role of Chickpea Accessions" Nutrients 17, no. 19: 3035. https://doi.org/10.3390/nu17193035
APA StyleMentino, D., Semeraro, D., Taldone, N., Pavan, S., Caponio, F., Gena, P., Ranieri, M., Tamma, G., Guglielmi, M. V., Scillitani, G., Fensore, S., & Mastrodonato, M. (2025). Mucin Alterations in Response to High-Fat Diet and the Potential Protective Role of Chickpea Accessions. Nutrients, 17(19), 3035. https://doi.org/10.3390/nu17193035











