Early Oral Administration of D-Chiro-Inositol Reverses Hippocampal Insulin and Glutamate Signaling Deficits in the 3×Tg Humanized Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics Statement
2.2. Pharmacological Treatment with Pure DCI and Monitorization of Its Plasma Levels
2.2.1. Pharmacological Treatment with Pure DCI
2.2.2. Measurement of Plasma Levels of DCI
- (a)
- Methoximation—Carbonyl groups were protected by adding 10 μL of methoxyamine hydrochloride solution (40 mg/mL in pyridine) and incubating at 30 °C for 90 min.
- (b)
- Silylation—Volatility was enhanced by adding 80 μL of N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) containing 1% trimethylchlorosilane (TMCS, Pierce) and incubating at 37 °C for 30 min.
2.3. Tissue Sampling and Biochemical Procedures
2.3.1. Sample Collection
2.3.2. Proteomic Analysis of Dorsal Hippocampus
Sample Preparation
LC-MS and Data Analysis
Data Processing: Selection of Normalization Methods
Fold Change Calculation
Results Visualization
Complexity Reduction and Candidate Selection
Functional Analysis
2.3.3. RT-qPCR
2.3.4. Western Blot Analysis
2.4. Statistical Analyses
3. Results
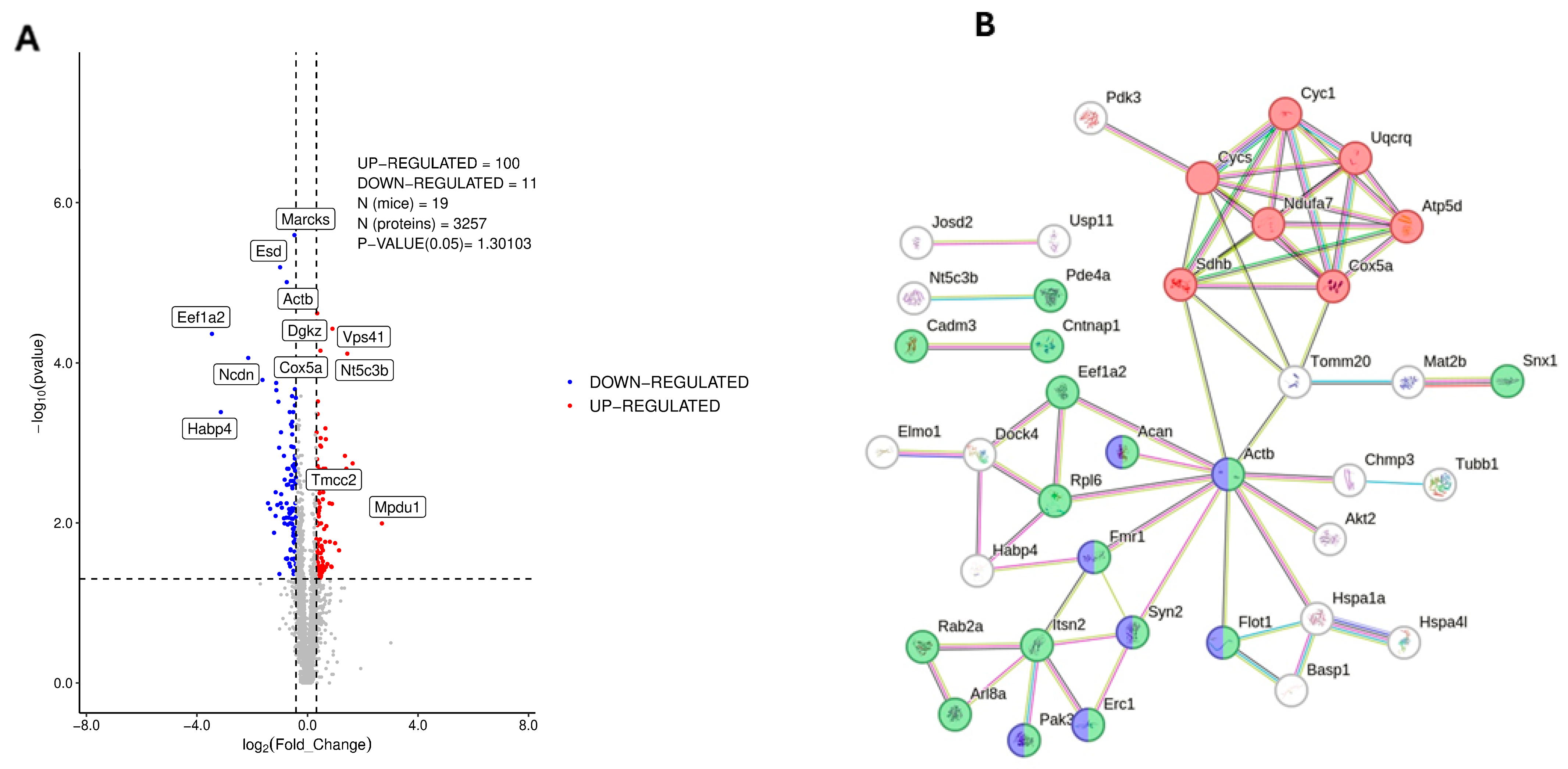
3.1. Proteomic Analysis of Dorsal Hippocampus in 3×Tg-AD Mice
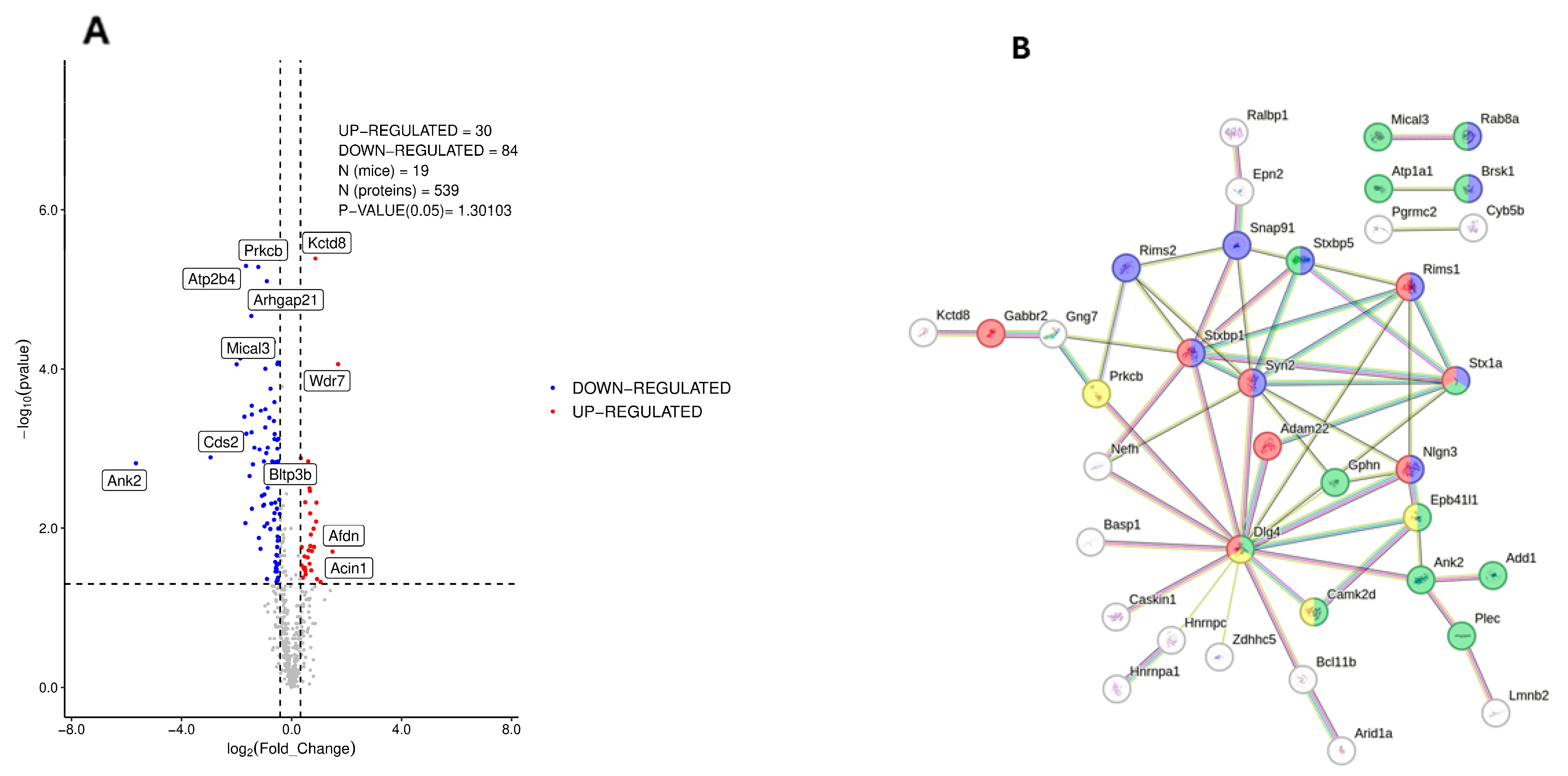
3.2. Phosphoproteomic Analysis of Dorsal Hippocampus in 3×Tg-AD Mice
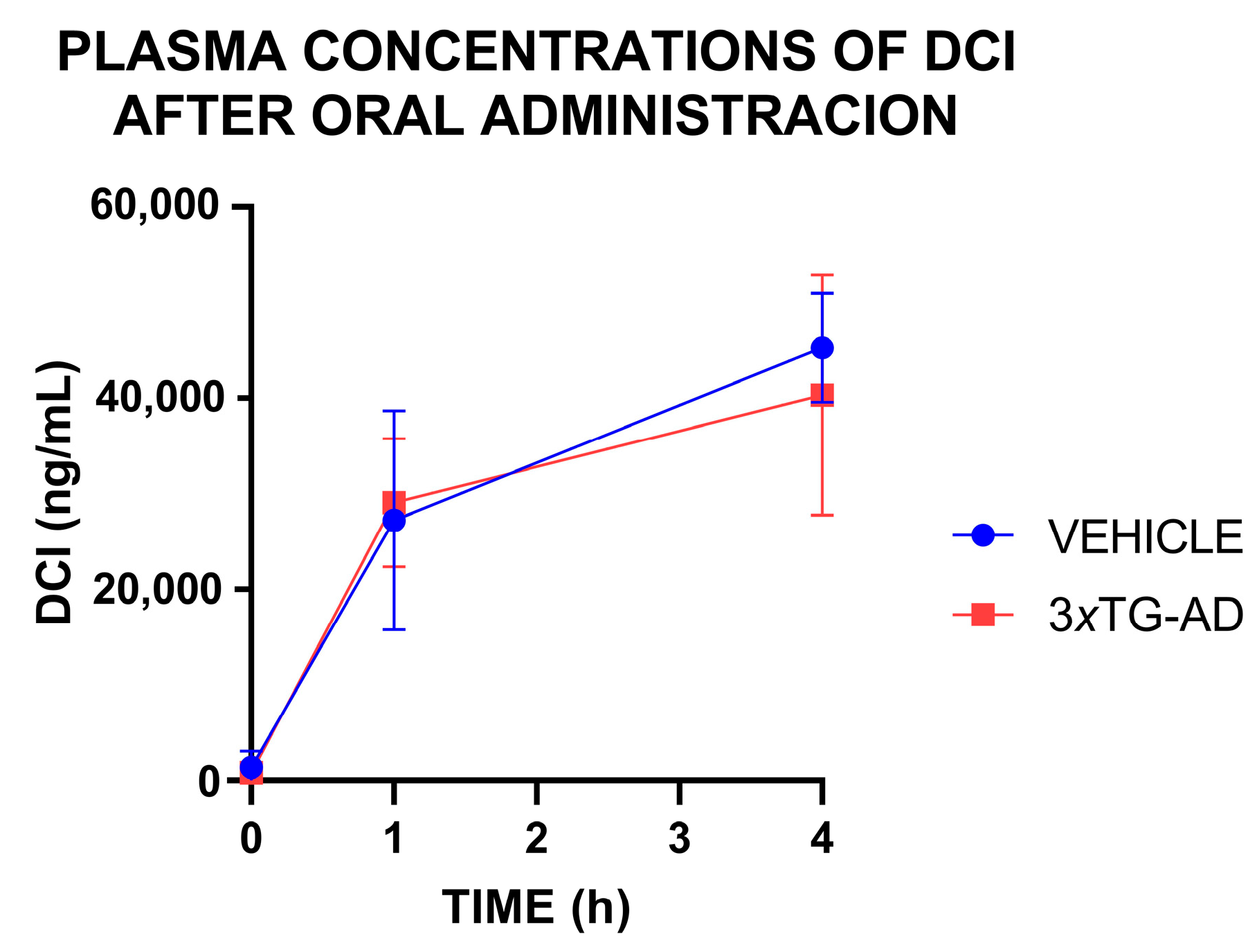
3.3. Plasma Concentrations of DCI After an Oral Administration
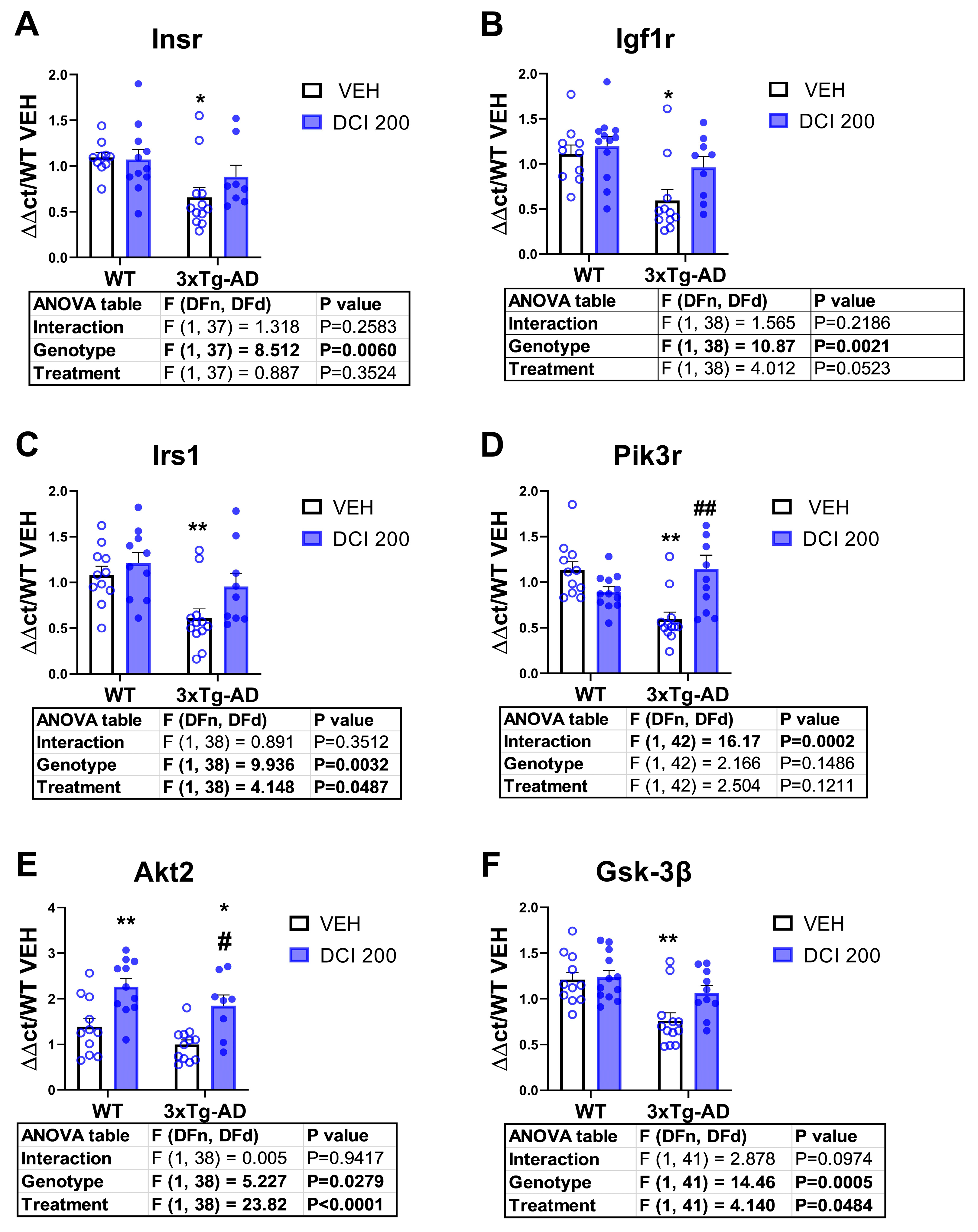
3.4. Effects of DCI on the mRNA Expression of Insulin Receptor-PI3K/AKT Pathway in the Hippocampus of 3×Tg-AD Mice
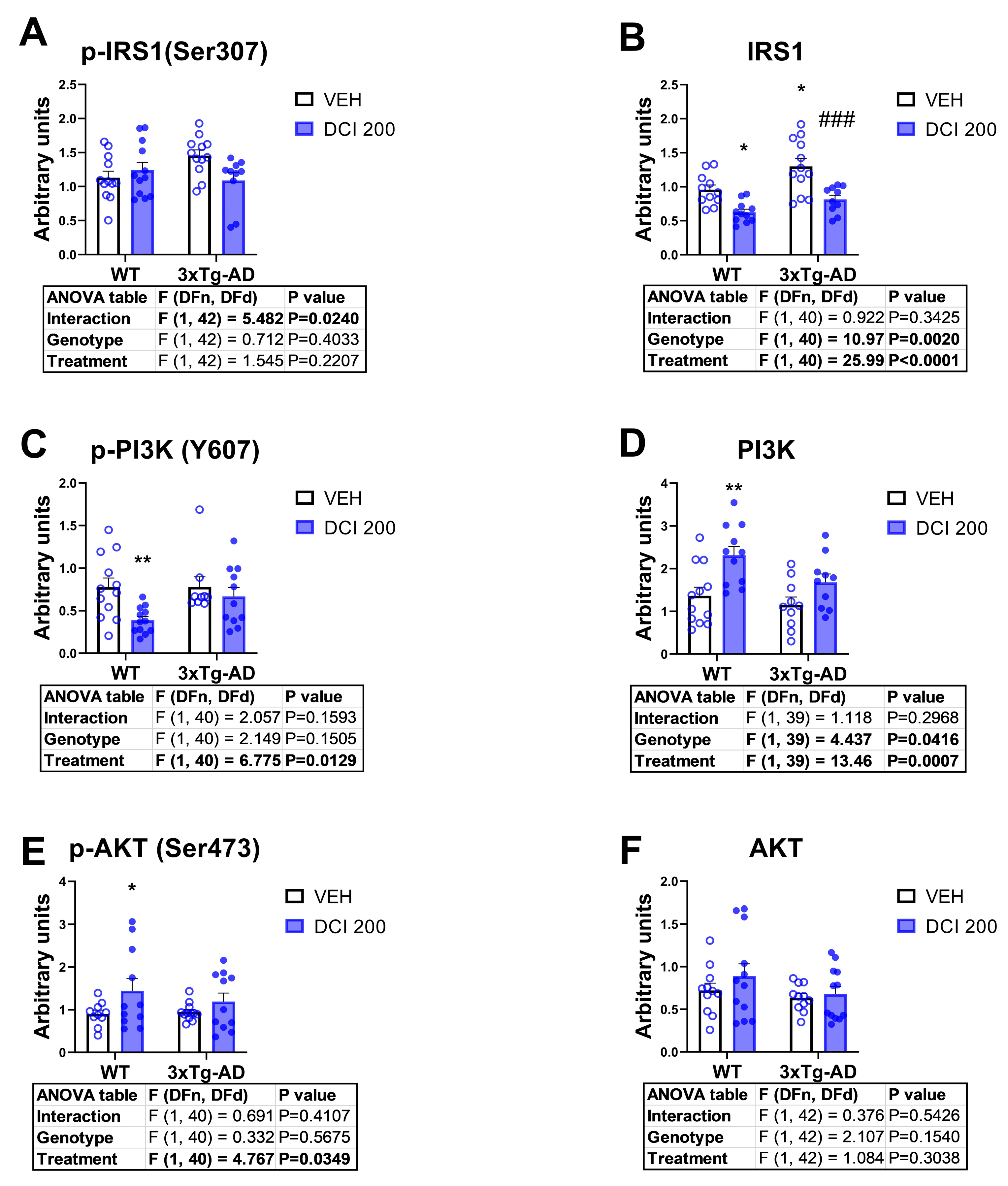
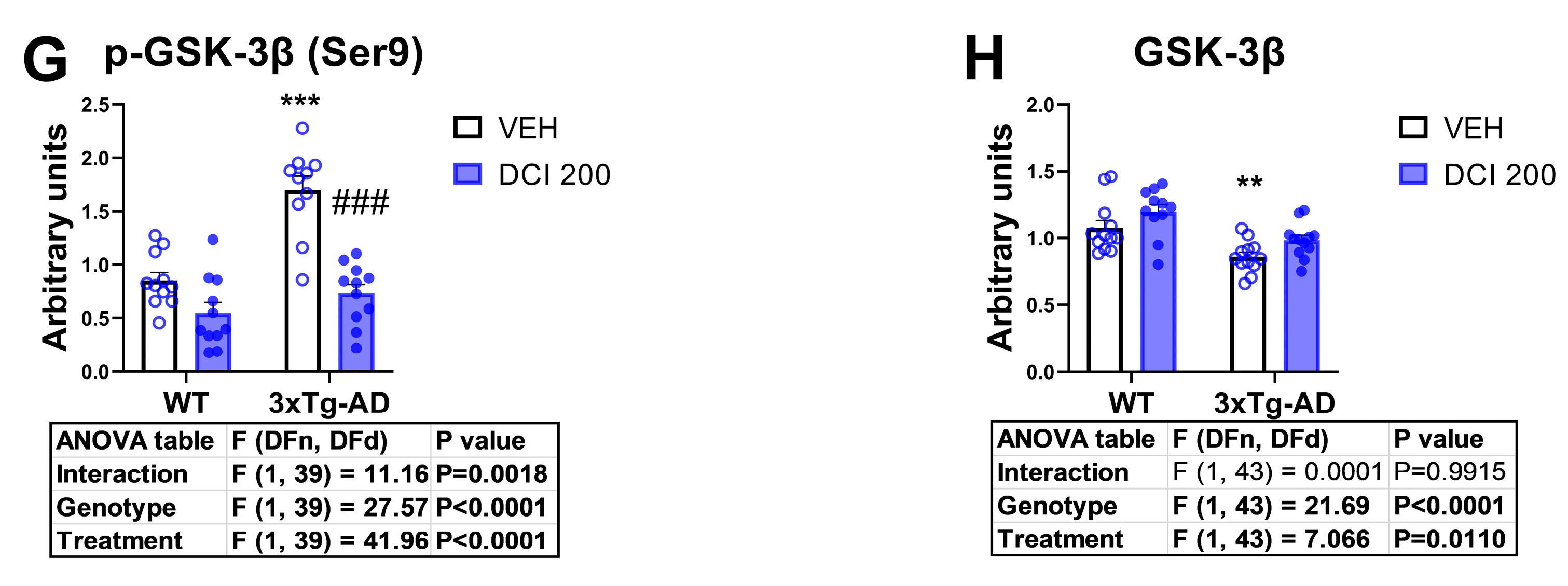
3.5. Effects of DCI on the Phosphorylation Status of the Main Proteins of the Canonical Insulin Signaling Pathway in the Hippocampus of 3×Tg-AD Mice
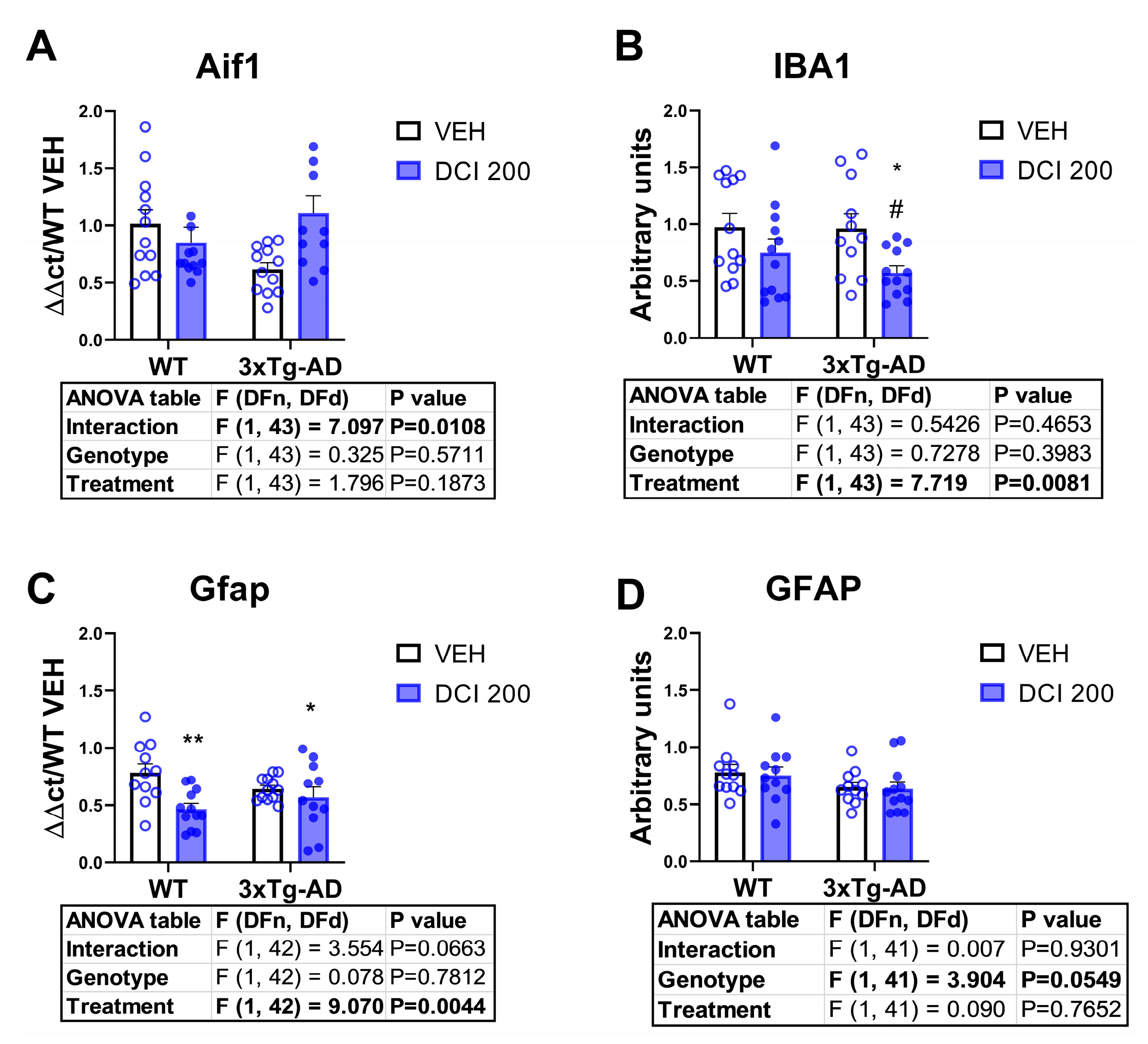
3.6. Effects of DCI on Glial Markers in the Hippocampus of 3×Tg-AD Mice
3.7. Effects of DCI on the mRNA Expression of Glutamate Receptors in the Hippocampus of 3×Tg-AD Mice
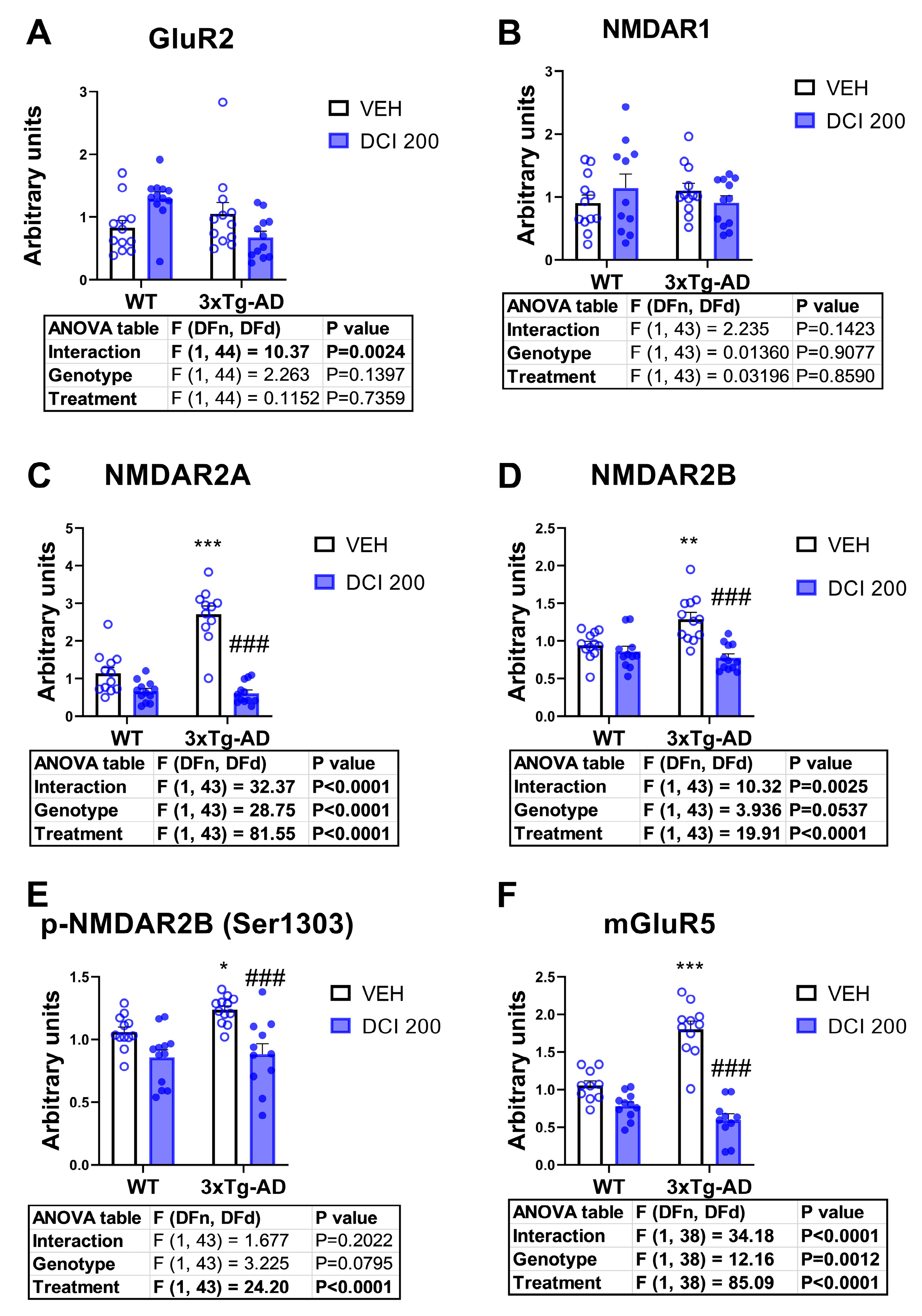
3.8. Effects of DCI on the Protein Expression of Glutamate Receptors in the Hippocampus of 3×Tg-AD Mice
3.9. Effects of DCI on the mRNA Expression of the Endocannabinoid System in the Hippocampus of 3×Tg-AD Mice
3.10. Sex-Dependent Effects of DCI on the Hippocampus of 3×Tg-AD Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 2 September 2025).
- Kumar, A.; Singh, A.; Ekavali. A Review on Alzheimer’s Disease Pathophysiology and Its Management: An Update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid Deposition as the Central Event in the Aetiology of Alzheimer’s Disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Tung, Y.C.; Quinlan, M.; Wisniewski, H.M.; Binder, L.I. Abnormal Phosphorylation of the Microtubule-Associated Protein Tau (Tau) in Alzheimer Cytoskeletal Pathology. Proc. Natl. Acad. Sci. USA 1986, 83, 4913–4917. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain Insulin Resistance in Alzheimer’s Disease and Related Disorders: Mechanisms and Therapeutic Approaches. Lancet Neurol. 2020, 19, 758–766. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Ritter, A.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2018. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 195–214. [Google Scholar] [CrossRef]
- Huynh, Q.S.; Elangovan, S.; Holsinger, R.M.D. Non-Pharmacological Therapeutic Options for the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 11037. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; De La Monte, S.M. Impaired Insulin and Insulin-like Growth Factor Expression and Signaling Mechanisms in Alzheimer’s Disease—Is This Type 3 Diabetes? J. Alzheimer’s Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Guillén-Nieto, G.; Rodríguez-Rodríguez, N.; Bringas-Vega, M.L.; García-del-Barco-Herrera, D.; Berlanga-Saez, J.O.; García-Ojalvo, A.; Valdés-Sosa, M.J.; Valdés-Sosa, P.A. Insulin Resistance at the Crossroad of Alzheimer Disease Pathology: A Review. Front. Endocrinol. 2020, 11, 560375. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Gonçalves, R.A.; Ferreira, S.T. Impaired Insulin Signalling and Allostatic Load in Alzheimer Disease. Nat. Rev. Neurosci. 2022, 23, 215–230. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Lourenco, M.V.; Ferreira, S.T. How Does Brain Insulin Resistance Develop in Alzheimer’s Disease? Alzheimer’s Dement. 2014, 10, S26–S32. [Google Scholar]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskaine, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain-Special Emphasis on Pi3k-Akt Pathway. Front. Neurosci. 2019, 13, 102416. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Yu, Z.L.; Chung, S.K.; Xu, B. The Roles of Dietary Polyphenols at Crosstalk between Type 2 Diabetes and Alzheimer’s Disease in Ameliorating Oxidative Stress and Mitochondrial Dysfunction via PI3K/Akt Signaling Pathways. Ageing Res. Rev. 2024, 99, 102416. [Google Scholar] [CrossRef]
- Hristov, M.; Nankova, A.; Andreeva-Gateva, P. Alterations of the Glutamatergic System in Diabetes Mellitus. Metab. Brain Dis. 2024, 39, 321–333. [Google Scholar] [CrossRef]
- Martín, E.D.; Sánchez-Perez, A.; Trejo, J.L.; Martin-Aldana, J.A.; Cano Jaimez, M.; Pons, S.; Acosta Umanzor, C.; Menes, L.; White, M.F.; Burks, D.J. IRS-2 Deficiency Impairs NMDA Receptor-Dependent Long-Term Potentiation. Cereb. Cortex 2012, 22, 1717–1727. [Google Scholar] [CrossRef]
- Grillo, C.A.; Piroli, G.G.; Lawrence, R.C.; Wrighten, S.A.; Green, A.J.; Wilson, S.P.; Sakai, R.R.; Kelly, S.J.; Wilson, M.A.; Mott, D.D.; et al. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes 2015, 64, 3927–3936. [Google Scholar] [CrossRef] [PubMed]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-Chiro-Inositol Glycans in Insulin Signaling and Insulin Resistance. Mol. Med. 2010, 16, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Filippello, A.; Scamporrino, A.; Di Mauro, S.; Malaguarnera, R.; Di Pino, A.; Scicali, R.; Purrello, F.; Piro, S. Direct Effects of D-Chiro-Inositol on Insulin Signaling and Glucagon Secretion of Pancreatic Alpha Cells. Biomolecules 2020, 10, 1404. [Google Scholar] [CrossRef]
- Shi, L.; Yu, X.T.; Li, H.; Wu, G.S.; Luo, H.R. D-Chiro-Inositol Increases Antioxidant Capacity and Longevity of Caenorhabditis Elegans via Activating Nrf-2/SKN-1 and FOXO/DAF-16. Exp. Gerontol. 2023, 175, 112145. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Fonseca, F.R. De The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar]
- Zhang, B.; Guo, X.; Li, Y.; Peng, Q.; Gao, J.; Liu, B.; Wang, M. D-Chiro Inositol Ameliorates Endothelial Dysfunction via Inhibition of Oxidative Stress and Mitochondrial Fission. Mol. Nutr. Food Res. 2017, 61, 1600710. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Navarro, J.A.; Tovar, R.; Rosell-Valle, C.; Gutiérrez-Adan, A.; Ledesma, J.C.; Sanjuan, C.; Pavón, F.J.; Baixeras, E.; Rodríguez de Fonseca, F.; et al. Activation of Pi3k/Akt Signaling Pathway in Rat Hypothalamus Induced by an Acute Oral Administration of d-Pinitol. Nutrients 2021, 13, 2268. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Pacheco-Sánchez, B.; Rosell-Valle, C.; Medina-Vera, D.; Navarro, J.A.; Fernández-Arjona, M.d.M.; de Ceglia, M.; Sanjuan, C.; Simon, V.; Cota, D.; et al. Dietary Administration of D-Chiro-Inositol Attenuates Sex-Specific Metabolic Imbalances in the 5xFAD Mouse Model of Alzheimer’s Disease. Biomed. Pharmacother. 2022, 150, 112994. [Google Scholar] [CrossRef]
- Pitt, J.; Thorner, M.; Brautigan, D.; Larner, J.; Klein, W.L. Protection against the Synaptic Targeting and Toxicity of Alzheimer’s-Associated Aβ Oligomers by Insulin Mimetic Chiro-Inositols. FASEB J. 2013, 27, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Velazquez, R.; Tran, A.; Ishimwe, E.; Denner, L.; Dave, N.; Oddo, S.; Dineley, K.T. Central Insulin Dysregulation and Energy Dyshomeostasis in Two Mouse Models of Alzheimer’s Disease. Neurobiol. Aging 2017, 58, 1–13. [Google Scholar] [CrossRef]
- Mota, S.I.; Ferreira, I.L.; Valero, J.; Ferreiro, E.; Carvalho, A.L.; Oliveira, C.R.; Rego, A.C. Impaired Src Signaling and Post-Synaptic Actin Polymerization in Alzheimer’s Disease Mice Hippocampus—Linking NMDA Receptors and the Reelin Pathway. Exp. Neurol. 2014, 261, 698–709. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The Arrive Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Medina-Vera, D.; López-Gambero, A.J.; Verheul-Campos, J.; Navarro, J.A.; Morelli, L.; Galeano, P.; Suárez, J.; Sanjuan, C.; Pacheco-Sánchez, B.; Rivera, P.; et al. Therapeutic Efficacy of the Inositol D-Pinitol as a Multi-Faceted Disease Modifier in the 5×FAD Humanized Mouse Model of Alzheimer’s Amyloidosis. Nutrients 2024, 16, 4186. [Google Scholar] [CrossRef]
- Käll, L.; Canterbury, J.D.; Weston, J.; Noble, W.S.; MacCoss, M.J. Semi-Supervised Learning for Peptide Identification from Shotgun Proteomics Datasets. Nat. Methods 2007, 4, 923–925. [Google Scholar] [CrossRef]
- Medina-Vera, D.; Navarro, J.A.; Rivera, P.; Rosell-Valle, C.; Gutiérrez-Adán, A.; Sanjuan, C.; López-Gambero, A.J.; Tovar, R.; Suárez, J.; Pavón, F.J.; et al. D-Pinitol Promotes Tau Dephosphorylation through a Cyclin-Dependent Kinase 5 Regulation Mechanism: A New Potential Approach for Tauopathies? Br. J. Pharmacol. 2022, 179, 4655–4672. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar]
- Yao, J.; Irwin, R.W.; Zhao, L.; Nilsen, J.; Hamilton, R.T.; Brinton, R.D. Mitochondrial Bioenergetic Deficit Precedes Alzheimer’s Pathology in Female Mouse Model of Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 14670–14675. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic Energy Use and Supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef]
- Kittler, J.T.; Moss, S.J. Neurotransmitter Receptor Trafficking and the Regulation of Synaptic Strength. Traffic 2001, 2, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.A.; Schuman, E.M. Local Translational Control in Dendrites and Its Role in Long-Term Synaptic Plasticity. J. Neurobiol. 2005, 64, 116–131. [Google Scholar] [PubMed]
- Selkoe, D.J. Alzheimer’s Disease Is a Synaptic Failure. Science 2002, 298, 789–791. [Google Scholar]
- Huganir, R.L.; Nicoll, R.A. AMPARs and Synaptic Plasticity: The Last 25 Years. Neuron 2013, 80, 704–717. [Google Scholar] [CrossRef]
- Moloney, A.M.; Griffin, R.J.; Timmons, S.; O’Connor, R.; Ravid, R.; O’Neill, C. Defects in IGF-1 Receptor, Insulin Receptor and IRS-1/2 in Alzheimer’s Disease Indicate Possible Resistance to IGF-1 and Insulin Signalling. Neurobiol. Aging 2010, 31, 224–243. [Google Scholar] [CrossRef]
- Talbot, K.; Wang, H.Y.; Kazi, H.; Han, L.Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated Brain Insulin Resistance in Alzheimer’s Disease Patients Is Associated with IGF-1 Resistance, IRS-1 Dysregulation, and Cognitive Decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef]
- Plum, L.; Schubert, M.; Brüning, J.C. The Role of Insulin Receptor Signaling in the Brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef]
- Duka, T.; Duka, V.; Joyce, J.N.; Sidhu, A. α-Synuclein Contributes to GSK-3β-catalyzed Tau Phosphorylation in Parkinson’s Disease Models. FASEB J. 2009, 23, 2820–2830. [Google Scholar] [CrossRef]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and Tau: Two Convergence Points in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, S141–S144. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Network Abnormalities and Interneuron Dysfunction in Alzheimer Disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-Mediated Excitotoxicity and Neurodegeneration in Alzheimer’s Disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Terry, R.D.; DeTeresa, R.M.; Hansen, L.A. Immunohistochemical Quantification of the Synapse-Related Protein Synaptophysin in Alzheimer Disease. Neurosci. Lett. 1989, 103, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Rissman, R.A.; De Blas, A.L.; Armstrong, D.M. GABAA Receptors in Aging and Alzheimer’s Disease. J. Neurochem. 2007, 103, 1285–1292. [Google Scholar] [CrossRef]
- Sze, C.I.; Bi, H.; Kleinschmidt-DeMasters, B.K.; Filley, C.M.; Martin, L.J. N-Methyl-D-Aspartate Receptor Subunit Proteins and Their Phosphorylation Status Are Altered Selectively in Alzheimer’s Disease. J. Neurol. Sci. 2001, 182, 151–159. [Google Scholar] [CrossRef]
- Ribeiro, F.M.; Vieira, L.B.; Pires, R.G.W.; Olmo, R.P.; Ferguson, S.S.G. Metabotropic Glutamate Receptors and Neurodegenerative Diseases. Pharmacol. Res. 2017, 115, 179–191. [Google Scholar] [CrossRef]
- Rammes, G.; Hasenjäger, A.; Sroka-Saidi, K.; Deussing, J.M.; Parsons, C.G. Therapeutic Significance of NR2B-Containing NMDA Receptors and MGluR5 Metabotropic Glutamate Receptors in Mediating the Synaptotoxic Effects of β-Amyloid Oligomers on Long-Term Potentiation (LTP) in Murine Hippocampal Slices. Neuropharmacology 2011, 60, 982–990. [Google Scholar] [CrossRef]
- Xu, L.Z.; Li, B.Q.; Li, F.Y.; Li, Y.; Qin, W.; Zhao, Y.; Jia, J.P. NMDA Receptor GluN2B Subunit Is Involved in Excitotoxicity Mediated by Death-Associated Protein Kinase 1 in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 91, 877–893. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Goodell, D.J.; Zaegel, V.; Coultrap, S.J.; Hell, J.W.; Bayer, K.U. DAPK1 Mediates LTD by Making CaMKII/GluN2B Binding LTP Specific. Cell Rep. 2017, 19, 2231–2243. [Google Scholar] [CrossRef]
- Gong, B.; Vitolo, O.V.; Trinchese, F.; Liu, S.; Shelanski, M.; Arancio, O. Persistent Improvement in Synaptic and Cognitive Functions in an Alzheimer Mouse Model after Rolipram Treatment. J. Clin. Investig. 2004, 114, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Endocannabinoid Signaling as a Synaptic Circuit Breaker in Neurological Disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.; Zilberter, M.; Pasquaré, S.J.; Alpár, A.; Schulte, G.; Ferreira, S.G.; Köfalvi, A.; Martín-Moreno, A.M.; Keimpema, E.; Tanila, H.; et al. Molecular Reorganization of Endocannabinoid Signalling in Alzheimer’s Disease. Brain 2011, 134, 1041–1060. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Fan, N.; Teng, Z.Q.; Wu, Y.; Yang, H.; Tang, Y.P.; Sun, H.; Song, Y.; Chen, C. XΔ9-THC-Caused Synaptic and Memory Impairments Are Mediated through COX-2 Signaling. Cell 2013, 155, 1154–1165. [Google Scholar] [CrossRef]
- Monory, K.; Massa, F.; Egertová, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Stella, N. Cannabinoid Signaling in Glial Cells. Glia 2004, 48, 267–277. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.J.; Rueda-Zubiaurre, A.; Ortega-Gutiérrez, S.; de Sola, R.G.; Guaza, C. Endocannabinoids Drive the Acquisition of an Alternative Phenotype in Microglia. Brain Behav. Immun. 2015, 49, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Vasilyev, D.V.; Goncalves, M.B.; Howell, F.V.; Hobbs, C.; Reisenberg, M.; Shen, R.; Zhang, M.Y.; Strassle, B.W.; Lu, P.; et al. Loss of Retrograde Endocannabinoid Signaling and Reduced Adult Neurogenesis in Diacylglycerol Lipase Knock-out Mice. J. Neurosci. 2010, 30, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascioll, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The Endogenous Cannabinoid System Controls Extinction of Aversive Memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Sánchez, B.; Verheul-Campos, J.; Vargas, A.; Tovar, R.; Rodríguez-Pozo, M.; Navarro, J.A.; López-Gambero, A.J.; Baixeras, E.; Serrano-Castro, P.J.; Suárez, J.; et al. Early Oral Administration of D-Chiro-Inositol Reverses Hippocampal Insulin and Glutamate Signaling Deficits in the 3×Tg Humanized Mouse Model of Alzheimer’s Disease. Nutrients 2025, 17, 3024. https://doi.org/10.3390/nu17183024
Pacheco-Sánchez B, Verheul-Campos J, Vargas A, Tovar R, Rodríguez-Pozo M, Navarro JA, López-Gambero AJ, Baixeras E, Serrano-Castro PJ, Suárez J, et al. Early Oral Administration of D-Chiro-Inositol Reverses Hippocampal Insulin and Glutamate Signaling Deficits in the 3×Tg Humanized Mouse Model of Alzheimer’s Disease. Nutrients. 2025; 17(18):3024. https://doi.org/10.3390/nu17183024
Chicago/Turabian StylePacheco-Sánchez, Beatriz, Julia Verheul-Campos, Antonio Vargas, Rubén Tovar, Miguel Rodríguez-Pozo, Juan A. Navarro, Antonio J. López-Gambero, Elena Baixeras, Pedro J. Serrano-Castro, Juan Suárez, and et al. 2025. "Early Oral Administration of D-Chiro-Inositol Reverses Hippocampal Insulin and Glutamate Signaling Deficits in the 3×Tg Humanized Mouse Model of Alzheimer’s Disease" Nutrients 17, no. 18: 3024. https://doi.org/10.3390/nu17183024
APA StylePacheco-Sánchez, B., Verheul-Campos, J., Vargas, A., Tovar, R., Rodríguez-Pozo, M., Navarro, J. A., López-Gambero, A. J., Baixeras, E., Serrano-Castro, P. J., Suárez, J., Sanjuan, C., Rivera, P., & Rodríguez de Fonseca, F. (2025). Early Oral Administration of D-Chiro-Inositol Reverses Hippocampal Insulin and Glutamate Signaling Deficits in the 3×Tg Humanized Mouse Model of Alzheimer’s Disease. Nutrients, 17(18), 3024. https://doi.org/10.3390/nu17183024







