Unlocking Hopeaphenol: A Potent Ally Against Cardiac Hypertrophy via AMPK Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.1.1. Experimental Animals
2.1.2. Cell Line
2.1.3. Main Reagents, Consumables, and Instruments
2.2. Experimental Methods
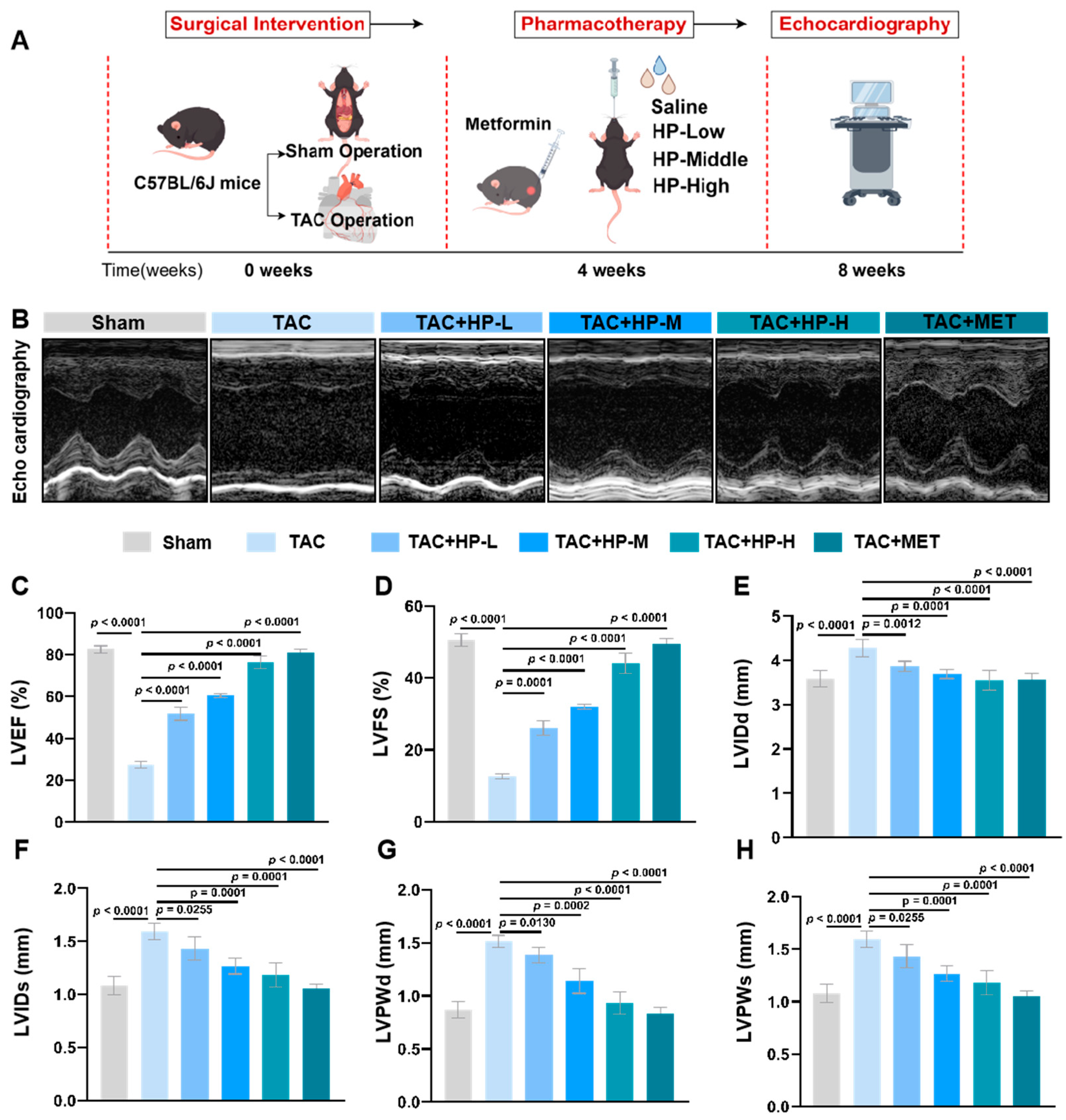
2.2.1. Animal Model Construction and Grouping
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Hopeaphenol Solution Preparation
2.2.4. Cardiac Function Detection
2.2.5. Tissue Staining
2.2.6. Western Blot
2.2.7. qRT-PCR Detection
2.2.8. CCK-8 Assay for Cell Viability
2.2.9. Cell Modeling, Grouping, and Administration
2.2.10. Mitochondrial ROS Detection
2.2.11. Mitochondrial Membrane Potential Detection
2.2.12. Cell Thermal Shift Assay (CETSA)
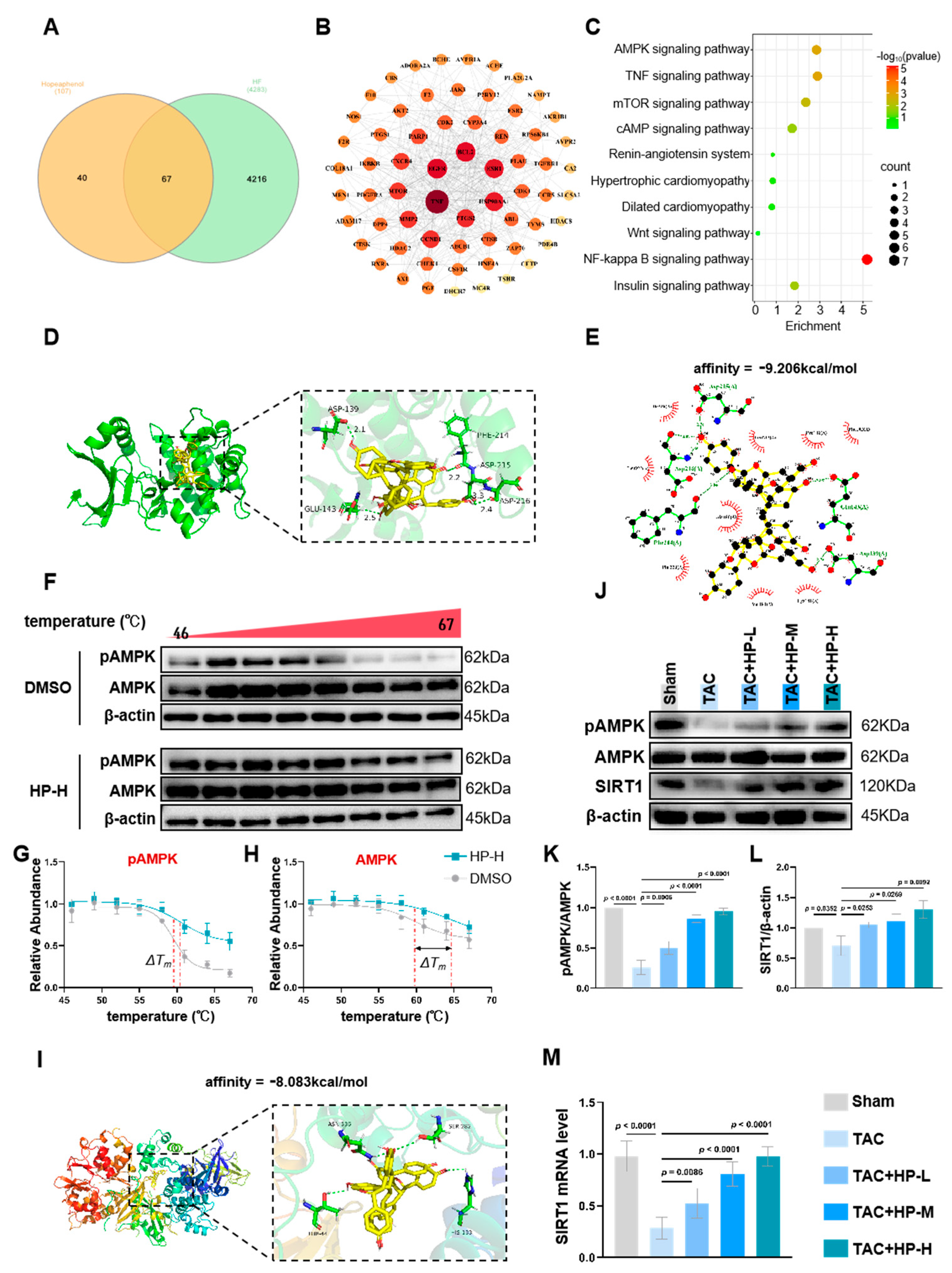
2.3. Network Pharmacology Analysis
2.3.1. Screening of Active Components and Targets
2.3.2. Acquisition of Heart Failure Target Genes
2.3.3. Screening of Intersection Targets
2.3.4. Construction of Hopeaphenol-Heart Failure Target Network
2.3.5. Construction of PPI Network and Screening of Core Targets
2.3.6. GO and KEGG Enrichment Analyses
2.3.7. Molecular Docking Analysis
2.4. Statistical Analysis
3. Results
3.1. Hopeaphenol Significantly Enhances Cardiac Function in TAC Mice
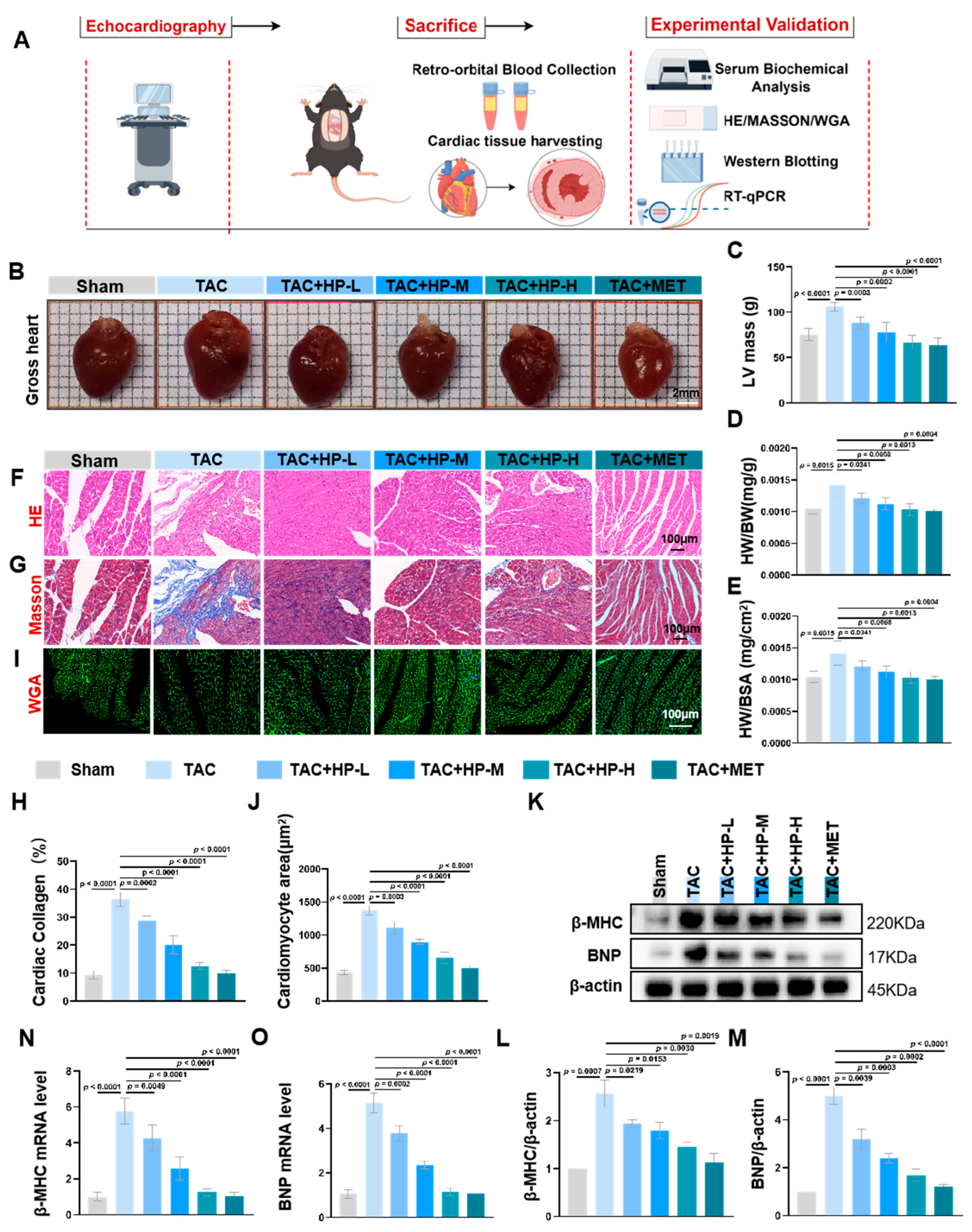
3.2. Hopeaphenol Mitigates Cardiac Hypertrophy and Fibrosis in TAC Mice
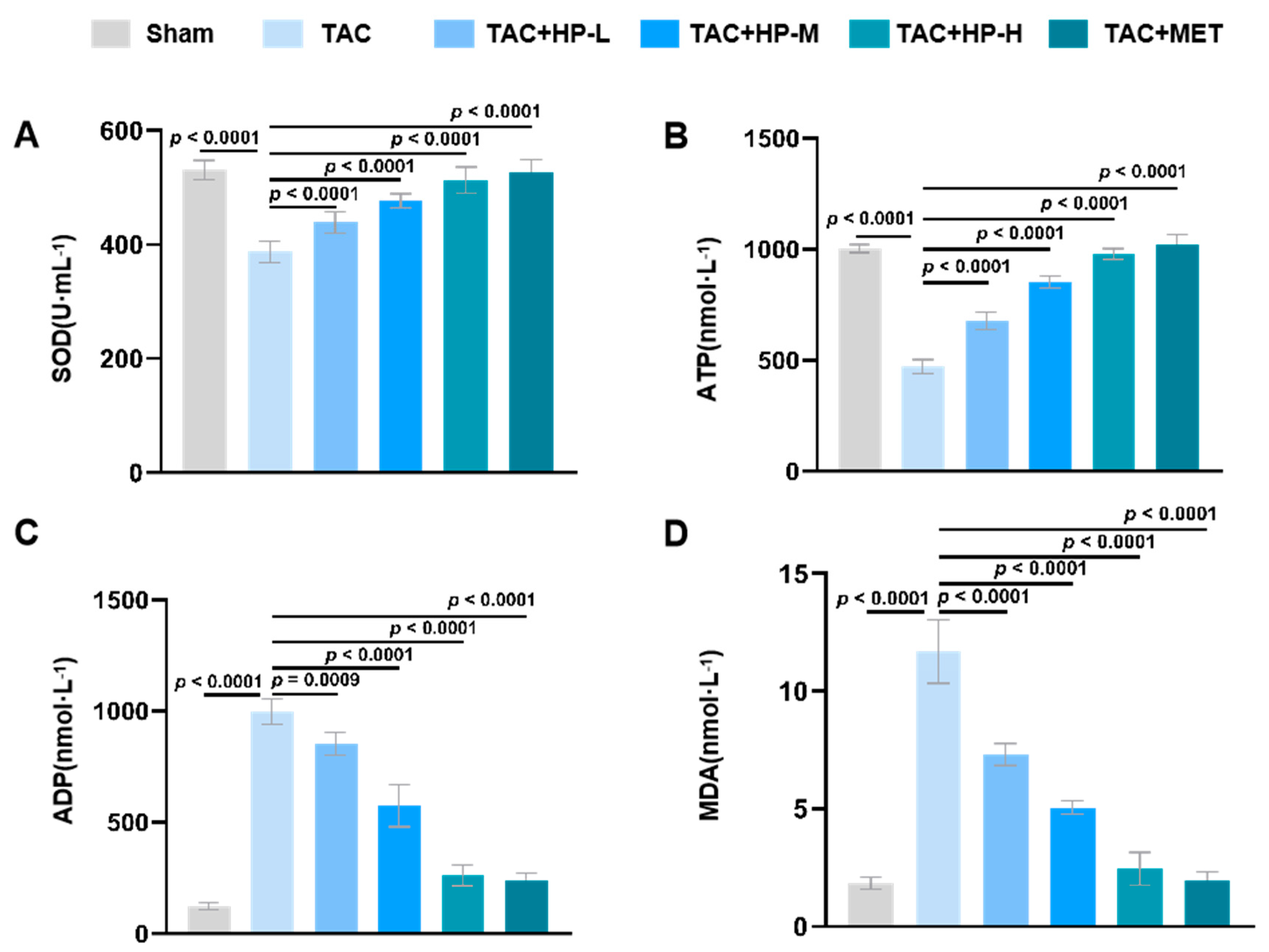
3.3. Hopeaphenol Alleviates Oxidative Stress and Metabolic Issues
3.4. Hopeaphenol Exerts Cardioprotective Effects by Targeting the AMPK Pathway
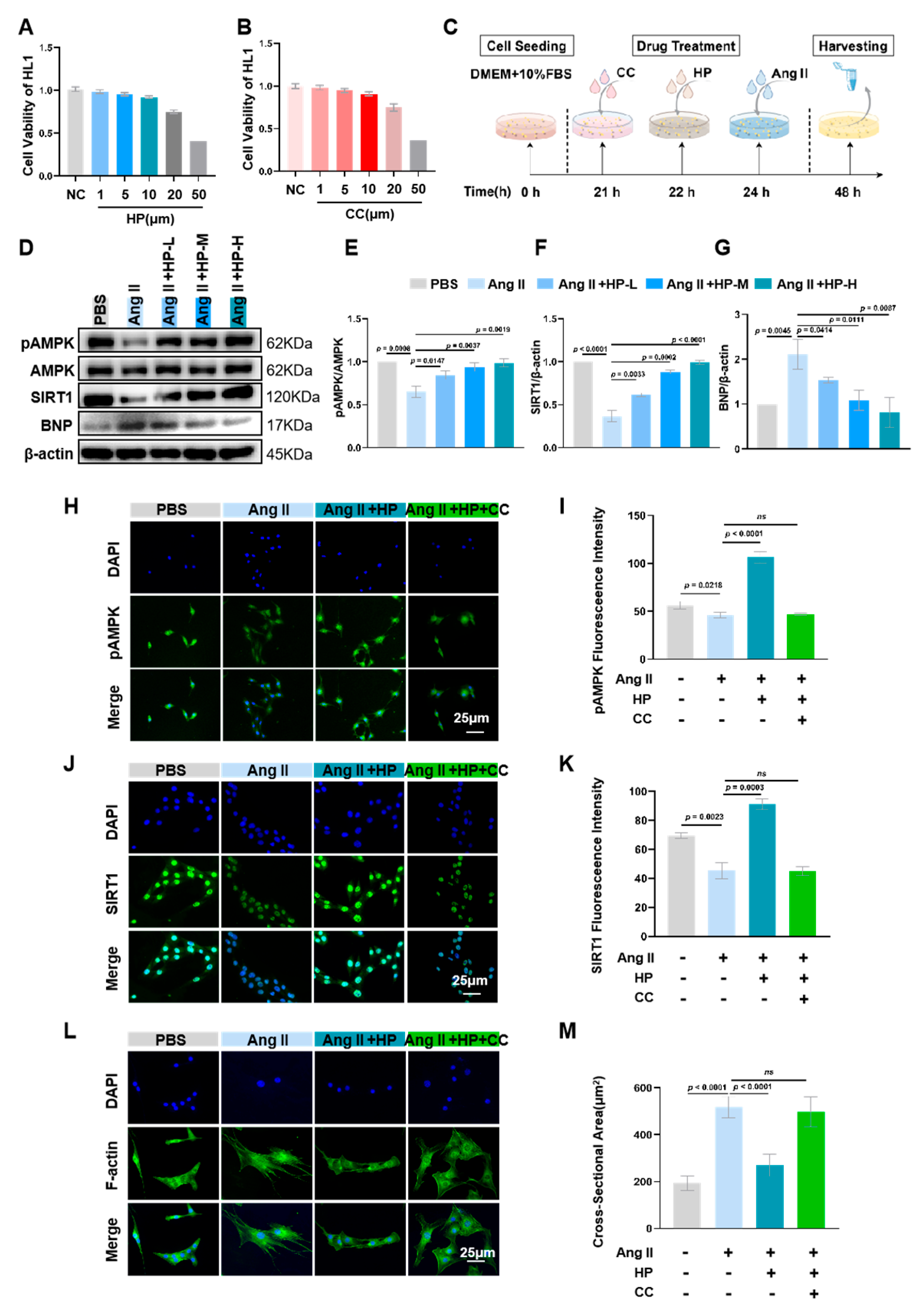
3.5. Hopeaphenol Mitigates Ang II-Induced Cardiomyocyte Hypertrophy Via Activation of the AMPK/SIRT1 Pathway
3.6. Hopeaphenol Mitigates Ang II-Induced Mitochondrial Dysfunction in HL-1 Cardiomyocytes Via the AMPK Pathway
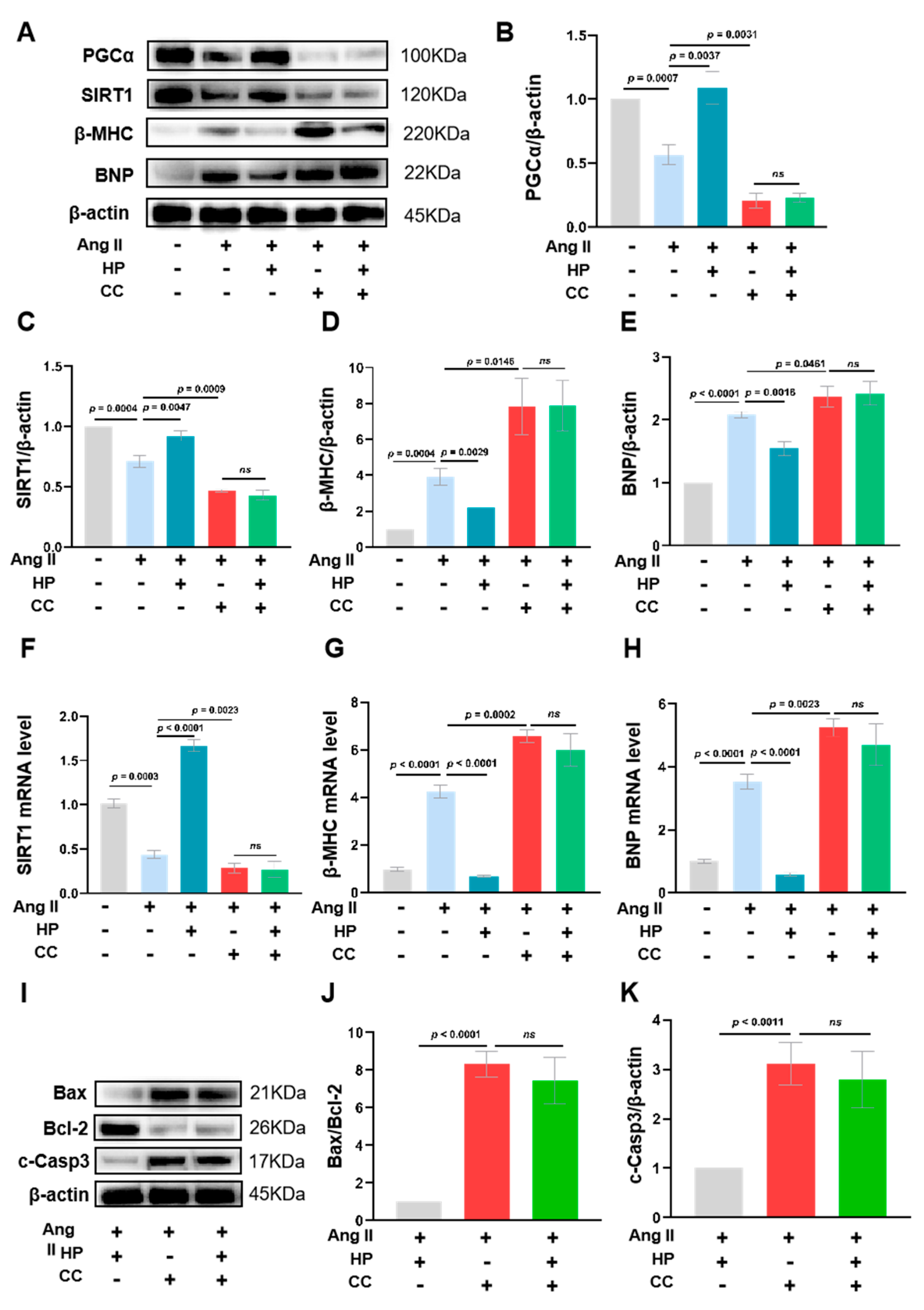
3.7. Inhibition of AMPK Activity Partially Blocks the Protective Effects of Hopeaphenol on Cardiomyocytes
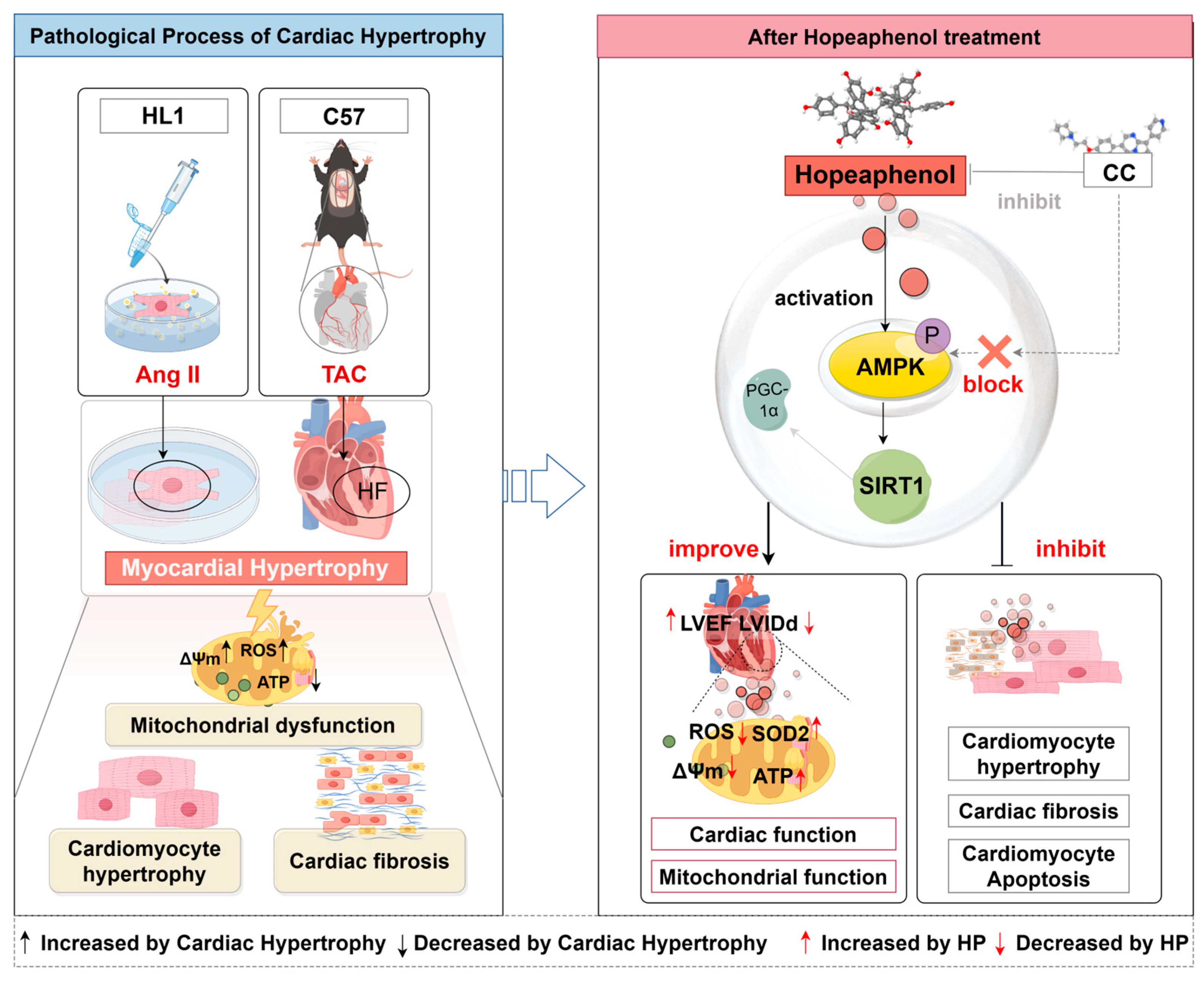
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Ye, B.; Zhou, H.; Chen, Y.; Luo, W.; Lin, W.; Zhao, Y.; Han, J.; Han, X.; Huang, W.; Wu, G.; et al. USP25 Ameliorates Pathological Cardiac Hypertrophy by Stabilizing SERCA2a in Cardiomyocytes. Circ. Res. 2023, 132, 465–480. [Google Scholar] [CrossRef]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy Nature reviews. Cardiology 2018, 15, 387–407. [Google Scholar] [CrossRef]
- Barry, S.P.; Davidson, S.M.; Townsend, P.A. Molecular regulation of cardiac hypertrophy. Int. J. Biochem. Cell Biol. 2008, 40, 2023–2039. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Jaswal, J.S. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ussher, J.R.; Sankaralingam, S.; Wagg, C.; Zaugg, M.; Lopaschuk, G.D. Cardiac insulin-resistance and decreased mitochondrial energy production precede the development of systolic heart failure after pressure-overload hypertrophy. Circ. Heart Fail. 2013, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liang, T.; Lu, Y.W.; Fu, X.; Dong, X.; Pu, L.; Hong, T.; Zhou, Y.; Zhang, Y.; Liu, N.; et al. A defect in mitochondrial protein translation influences mitonuclear communication in the heart. Nat. Commun. 2023, 14, 1595. [Google Scholar] [CrossRef]
- Ritterhoff, J.T.R. Metabolic mechanisms in physiological and pathological cardiac hypertrophy: New paradigms and challenges. Nat. Rev. Cardiol. 2023, 20, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef]
- Peng, S.; Lu, X.F.; Qi, Y.D.; Li, J.; Xu, J.; Yuan, T.Y.; Wu, X.Y.; Ding, Y.; Li, W.H.; Zhou, G.-Q.; et al. LCZ696 Ameliorates Oxidative Stress and Pressure Overload-Induced Pathological Cardiac Remodeling by Regulating the Sirt3/MnSOD Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 9815039. [Google Scholar] [CrossRef]
- O’brien, C.M.; Mulukutla, B.C.; Mashek, D.G.; Hu, W.-S. Regulation of Metabolic Homeostasis in Cell Culture Bioprocesses. Trends Biotechnol. 2020, 38, 1113–1127. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, M.; Yu, X.; Shui, X.; Tang, L.; Chen, Z.; Xiong, Z. lncRNA NBR2 attenuates angiotensin II-induced myocardial hypertrophy through repressing ER stress via activating LKB1/AMPK/Sirt1 pathway. Bioengineered 2022, 13, 13667–13679. [Google Scholar] [CrossRef]
- Dolinsky, V.W.S.C.; Rogan, K.J.; Chan, A.Y.; Nagendran, J.; Wang, S.; Dyck, J.R.B. Resveratrol prevents pathological but not physiological cardiac hypertrophy. J. Mol. Med. 2015, 93, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef]
- Giuliani, C.; Iezzi, M.; Ciolli, L.; Hysi, A.; Bucci, I.; Di Santo, S.; Rossi, C.; Zucchelli, M.; Napolitano, G. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 107 Pt A, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed]
- Seya, K.; Kanemaru, K.; Sugimoto, C.; Suzuki, M.; Takeo, T.; Motomura, S.; Kitahara, H.; Niwa, M.; Oshima, Y.; Furukawa, K.-I. Opposite effects of two resveratrol (trans-3,5,4′-trihydroxystilbene) tetramers, vitisin A and hopeaphenol, on apoptosis of myocytes isolated from adult rat heart. J. Pharmacol. Exp. Ther. 2009, 328, 90–98. [Google Scholar] [CrossRef]
- Dealmeida, A.C.; Van Oort, R.J.; Wehrens, X.H. Transverse aortic constriction in mice. J. Vis. Exp. JoVE 2010, 38, e1729. [Google Scholar] [CrossRef]
- Fu, Y.N.; Xiao, H.; Ma, X.W.; Jiang, S.-Y.; Xu, M.; Zhang, Y.-Y. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol. Sin. 2011, 32, 879–887. [Google Scholar] [CrossRef]
- Hwangbo, D.S.; Lee, H.Y.; Abozaid, L.S.; Min, K.-J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, C.; Yu, T.; Zhu, J.; Pan, Y.; Shuai, W.; Kong, B.; Huang, H. Ubiquitin specific protease 38 aggravates pathological cardiac remodeling by stabilizing phospho-TBK1. Int. J. Biol. Sci. 2024, 20, 1815–1832. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Gallo, G.; Rubattu, S.; Volpe, M. Mitochondrial Dysfunction in Heart Failure: From Pathophysiological Mechanisms to Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 2667. [Google Scholar] [CrossRef] [PubMed]
- Nollet, E.E.; Duursma, I.; Rozenbaum, A.; Eggelbusch, M.; I Wüst, R.C.; Schoonvelde, S.A.C.; Michels, M.; Jansen, M.; van der Wel, N.N.; Bedi, K.C.; et al. Mitochondrial dysfunction in human hypertrophic cardiomyopathy is linked to cardiomyocyte architecture disruption and corrected by improving NADH-driven mitochondrial respiration. Eur. Heart J. 2023, 44, 1170–1185. [Google Scholar] [CrossRef]
- Lyon, R.C.; Zanella, F.; Omens, J.H.; Sheikh, F. Mechanotransduction in cardiac hypertrophy and failure. Circ. Res. 2015, 116, 1462–1476. [Google Scholar] [CrossRef]
- Ingwall, J.S. Energy metabolism in heart failure and remodelling. Cardiovasc. Res. 2009, 81, 412–419. [Google Scholar] [CrossRef]
- Dai, D.F.; Hsieh, E.J.; Liu, Y.; Chen, T.; Beyer, R.P.; Chin, M.T.; MacCoss, M.J.; Rabinovitch, P.S. Mitochondrial proteome remodelling in pressure overload-induced heart failure: The role of mitochondrial oxidative stress. Cardiovasc. Res. 2012, 93, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Smyrnias, I.; Gray, S.P.; Okonko, D.O.; Sawyer, G.; Zoccarato, A.; Catibog, N.; López, B.; González, A.; Ravassa, S.; Díez, J.; et al. Cardioprotective Effect of the Mitochondrial Unfolded Protein Response During Chronic Pressure Overload. J. Am. Coll. Cardiol. 2019, 73, 1795–1806. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.Y.; He, L.; Song, J.; Jinno, M.; Rogers, A.J.; Sethu, P.; Halade, G.V.; Rajasekaran, N.S.; Liu, X.; Prabhu, S.D.; et al. Mitoquinone ameliorates pressure overload-induced cardiac fibrosis and left ventricular dysfunction in mice. Redox Biol. 2019, 21, 101100. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Xiao, H. AMPK and cardiac remodelling. Sci. China Life Sci. 2018, 61, 14–23. [Google Scholar] [CrossRef]
- Dong, H.W.; Zhang, L.F.; Bao, S.L. AMPK regulates energy metabolism through the SIRT1 signaling pathway to improve myocardial hypertrophy. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2757–2766. [Google Scholar]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-S.; Doerksen, R.J.; Chen, S.-H.; Sung, W.-C.; Juan, C.-C.; Rawendra, R.D.; Chen, C.-R.; Li, J.-W.; Aisha; Huang, T.-C.; et al. Screening and profiling stilbene-type natural products with angiotensin-converting enzyme inhibitory activity from Ampelopsis brevipedunculata var. hancei (Planch.) Rehder. J. Pharm. Biomed. Anal. 2015, 108, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Häusl, A.S.; Bajaj, T.; Brix, L.M.; Pöhlmann, M.L.; Hafner, K.; De Angelis, M.; Nagler, J.; Dethloff, F.; Balsevich, G.; Schramm, K.-W.; et al. Mediobasal hypothalamic FKBP51 acts as a molecular switch linking autophagy to whole-body metabolism. Sci. Adv. 2022, 8, eabi4797. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, Y.; Wang, G.; Feng, S.; Ge, X.; Ye, W.; Wang, Z.; Zhu, Y.; Cai, W.; Bai, J.; et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022, 53, 102344. [Google Scholar] [CrossRef]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Zinellu, A.; Sotgia, S.; Vardeu, A.; Hoa, P.T.; Van Nguyen, L.H.; Carru, C.; Pintus, G. Resveratrol alters human endothelial cells redox state and causes mitochondrial-dependent cell death. Food Chem. Toxicol. 2015, 78, 10–16. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef]
- Hadi, S.M.; Ullah, M.F.; Azmi, A.S.; Ahmad, A.; Shamim, U.; Zubair, H.; Khan, H.Y. Resveratrol mobilizes endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for chemoprevention of cancer. Pharm. Res. 2010, 27, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Sakanashi, Y.; Matsui, H.; Oyama, T.; Nishimura, Y.; Masuda, T.; Oyama, Y. Cytometric analysis of cytotoxicity of polyphenols and related phenolics to rat thymocytes: Potent cytotoxicity of resveratrol to normal cells. Basic. Clin. Pharmacol. Toxicol. 2009, 104, 455–462. [Google Scholar] [CrossRef]
- Berardi, V.; Ricci, F.; Castelli, M.; Galati, G.; Risuleo, G. Resveratrol exhibits a strong cytotoxic activity in cultured cells and has an antiviral action against polyomavirus: Potential clinical use. J. Exp. Clin. Cancer Res. 2009, 28, 96. [Google Scholar] [CrossRef]
- Deng, R.; Xu, C.; Chen, X.; Chen, P.; Wang, Y.; Zhou, X.; Jin, J.; Niu, L.; Ying, M.; Huang, M.; et al. Resveratrol suppresses the inducible expression of CYP3A4 through the pregnane X receptor. J. Pharmacol. Sci. 2014, 126, 146–154. [Google Scholar] [CrossRef]
- Detampel, P.B.M.; Krähenbühl, S.; Huwyler, J. Drug interaction potential of resveratrol. Drug Metab. Rev. 2012, 44, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, N.Y.E.; Güner, B.; Kabakçi, R.; Bilmen, F.S. Effect of resveratrol on hematological and biochemical alterations in rats exposed to fluoride. BioMed Res. Int. 2014, 2014, 698628. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Niu, F.; Liu, S.-L.; Li, Y.; Zhang, L.-M.; Du, H.-B.; Zhao, Z.-G.; Niu, C.-Y. Effect of Resveratrol on Blood Rheological Properties in LPS-Challenged Rats. Front. Physiol. 2018, 9, 1202. [Google Scholar] [CrossRef]
- Crowell, J.A.; Korytko, P.J.; Morrissey, R.L.; Booth, T.D.; Levine, B.S. Resveratrol-associated renal toxicity. Toxicol. Sci. 2004, 82, 614–619. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| AMPK | GTCAAAGCCGACCCAATGATA | CGTACACGCAAATAATAGGGGTT |
| SIRT1 | TGATTGGCACCGATCCTCG | CCACAGCGTCATATCATCCAG |
| β-MHC | CCTGCGGAAGTCTGAGAAGG | CTCGGGACACGATCTTGGC |
| BNP | GAGGTCACTCCTATCCTCTGG | GAGGTCACTCCTATCCTCTGG |
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, M.; Zhang, Z.; Fang, C.; Zhuang, H.; Zhao, J.; Wang, T.; Wang, J.; Li, C.; Fang, C. Unlocking Hopeaphenol: A Potent Ally Against Cardiac Hypertrophy via AMPK Activation. Nutrients 2025, 17, 3025. https://doi.org/10.3390/nu17183025
Chen J, Wang M, Zhang Z, Fang C, Zhuang H, Zhao J, Wang T, Wang J, Li C, Fang C. Unlocking Hopeaphenol: A Potent Ally Against Cardiac Hypertrophy via AMPK Activation. Nutrients. 2025; 17(18):3025. https://doi.org/10.3390/nu17183025
Chicago/Turabian StyleChen, Jinhong, Mengyuan Wang, Zhongzheng Zhang, Chongkai Fang, Haowen Zhuang, Jiaqi Zhao, Tianyu Wang, Junyan Wang, Chun Li, and Chunping Fang. 2025. "Unlocking Hopeaphenol: A Potent Ally Against Cardiac Hypertrophy via AMPK Activation" Nutrients 17, no. 18: 3025. https://doi.org/10.3390/nu17183025
APA StyleChen, J., Wang, M., Zhang, Z., Fang, C., Zhuang, H., Zhao, J., Wang, T., Wang, J., Li, C., & Fang, C. (2025). Unlocking Hopeaphenol: A Potent Ally Against Cardiac Hypertrophy via AMPK Activation. Nutrients, 17(18), 3025. https://doi.org/10.3390/nu17183025






