The Impact of Physical Education Attendance and Diet on Bone Mineralization in Adolescents
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Basic Characteristics of the Study Group
3.2. Bone Mineralization and Physical Activity
3.3. Bone Mineralization and Diet
3.4. Combined Multivariable Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s Position Statement on Peak Bone Mass Development and Lifestyle Factors: A Systematic Review and Implementation Recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef]
- Otsuka, H.; Tabata, H.; Shi, H.; Kaga, H.; Someya, Y.; Abulaiti, A.; Naito, H.; Umemura, F.; Kakehi, S.; Ishijima, M.; et al. Associations of Exercise Habits in Adolescence and Old Age with Risk of Osteoporosis in Older Adults: The Bunkyo Health Study. J. Clin. Med. 2021, 10, 5968. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cauley, J.A.; Seeley, D.G.; Ensrud, K.E.; Bauer, D.C. Physical Activity and Osteoporotic Fracture Risk in Older Women. Study of Osteoporotic Fractures Research Group. Ann. Intern. Med. 1998, 129, 81–88. [Google Scholar] [CrossRef]
- Lin, Z.; Shi, G.; Liao, X.; Huang, J.; Yu, M.; Liu, W.; Luo, X.; Zhan, H.; Cai, X. Correlation between Sedentary Activity, Physical Activity and Bone Mineral Density and Fat in America: National Health and Nutrition Examination Survey, 2011–2018. Sci. Rep. 2023, 13, 10054. [Google Scholar] [CrossRef]
- Dent, E.; Daly, R.M.; Hoogendijk, E.O.; Scott, D. Exercise to Prevent and Manage Frailty and Fragility Fractures. Curr. Osteoporos. Rep. 2023, 21, 205–215. [Google Scholar] [CrossRef]
- Couce, M.L.; Saenz de Pipaon, M. Bone Mineralization and Calcium Phosphorus Metabolism. Nutrients 2021, 13, 3692. [Google Scholar] [CrossRef]
- Tsugawa, N.; Shiraki, M. Vitamin K Nutrition and Bone Health. Nutrients 2020, 12, 1909. [Google Scholar] [CrossRef]
- Matikainen, N.; Pekkarinen, T.; Ryhänen, E.M.; Schalin-Jäntti, C. Physiology of Calcium Homeostasis: An Overview. Endocrinol. Metab. Clin. N. Am. 2021, 50, 575–590. [Google Scholar] [CrossRef]
- Yao, X.; Hu, J.; Kong, X.; Zhu, Z. Association between Dietary Calcium Intake and Bone Mineral Density in Older Adults. Ecol. Food Nutr. 2021, 60, 89–100. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Bennett, S.; Zou, J.; Xu, J.; Zhang, L. The Effects of Different Dietary Patterns on Bone Health. Nutrients 2024, 16, 2289. [Google Scholar] [CrossRef]
- Di Iorgi, N.; Maruca, K.; Patti, G.; Mora, S. Update on Bone Density Measurements and Their Interpretation in Children and Adolescents. Best. Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 477–498. [Google Scholar] [CrossRef]
- Przytula, A.; Popiolek-Kalisz, J. Bioelectrical Impedance Analysis as a Helpful Tool in Pediatric Obesity Monitoring: A Case Report. Reports 2025, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Colange, G.; Delay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of Body Composition Assessment by DXA and BIA According to the Body Mass Index: A Retrospective Study on 3655 Measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 3548284. [Google Scholar] [CrossRef]
- Falbová, D.; Beňuš, R.; Sulis, S.; Vorobeľová, L. Effect of COVID-19 Pandemic on Bioimpedance Health Indicators in Young Adults. Am. J. Hum. Biol. 2024, 36, e24110. [Google Scholar] [CrossRef]
- Falbová, D.; Kovalčíková, V.; Beňuš, R.; Sulis, S.; Vorobeľová, L. Effect of COVID-19 Pandemic on Lifestyle and Bone Mineral Density in Young Adults. Am. J. Hum. Biol. 2024, 36, e24009. [Google Scholar] [CrossRef] [PubMed]
- Jezewska-Zychowicz, M.; Gawecki, J.; Wadolowska, L.; Czarnocinska, J.; Galinski, G.; Kollajtis-Dolowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybylowicz, K.; Stasiewicz, B. KomPAN® Dietary Habits and Nutrition Beliefs Questionnaire and the Manual for Developing of Nutritional Data; Gawecki, J., Ed.; Committee of Human Nutrition, Polish Academy of Sciences: Olsztyn, Polish, 2020. [Google Scholar]
- Proia, P.; Amato, A.; Drid, P.; Korovljev, D.; Vasto, S.; Baldassano, S. The Impact of Diet and Physical Activity on Bone Health in Children and Adolescents. Front. Endocrinol. 2021, 12, 704647. [Google Scholar] [CrossRef]
- Gracia-Marco, L.; Vicente-Rodríguez, G.; Casajús, J.A.; Molnar, D.; Castillo, M.J.; Moreno, L.A. Effect of Fitness and Physical Activity on Bone Mass in Adolescents: The HELENA Study. Eur. J. Appl. Physiol. 2011, 111, 2671–2680. [Google Scholar] [CrossRef] [PubMed]
- Janz, K.F.; Letuchy, E.M.; Burns, T.L.; Eichenberger Gilmore, J.M.; Torner, J.C.; Levy, S.M. Objectively Measured Physical Activity Trajectories Predict Adolescent Bone Strength: Iowa Bone Development Study. Br. J. Sports Med. 2014, 48, 1032–1036. [Google Scholar] [CrossRef]
- Gabel, L.; Macdonald, H.M.; Nettlefold, L.; McKay, H.A. Physical Activity, Sedentary Time, and Bone Strength From Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT Study. J. Bone Miner. Res. 2017, 32, 1525–1536. [Google Scholar] [CrossRef]
- Rizzoli, R.; Bianchi, M.L.; Garabédian, M.; McKay, H.A.; Moreno, L.A. Maximizing Bone Mineral Mass Gain during Growth for the Prevention of Fractures in the Adolescents and the Elderly. Bone 2010, 46, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, T.; Petit, M.A.; Lin, H.-M.; Beck, T.J. Lifestyle Factors and the Development of Bone Mass and Bone Strength in Young Women. J. Pediatr. 2004, 144, 776–782. [Google Scholar] [CrossRef]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted Exercise against Osteoporosis: A Systematic Review and Meta-Analysis for Optimising Bone Strength throughout Life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional Intake and Bone Health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Orsini, N.; Wolk, A.; Michaëlsson, K. Fruit and Vegetable Intake and Risk of Hip Fracture: A Cohort Study of Swedish Men and Women. J. Bone Min. Res. 2015, 30, 976–984. [Google Scholar] [CrossRef]
- Benetou, V.; Orfanos, P.; Feskanich, D.; Michaëlsson, K.; Pettersson-Kymmer, U.; Byberg, L.; Eriksson, S.; Grodstein, F.; Wolk, A.; Jankovic, N.; et al. Mediterranean Diet and Hip Fracture Incidence among Older Adults: The CHANCES Project. Osteoporos. Int. 2018, 29, 1591–1599. [Google Scholar] [CrossRef]
- Triches, R.M.; Giugliani, E.R.J. Obesity, eating habits and nutritional knowledge among school children. Rev. Saude Publica 2005, 39, 541–547. [Google Scholar] [CrossRef]
- Rusińska, A.; Michałus, I.; Karalus, J.; Golec, J.; Chlebna-Sokół, D. The vitamin D and calcium consumption and bone quality in children of łódŹ (Poland) at the age 9–13 years. Pediatr. Endocrinol. Diabetes Metab. 2011, 17, 82–87. [Google Scholar]
- Kopiczko, A.; Czapla, M.; Juárez-Vela, R.; Ross, C.; Uchmanowicz, B. Dairy Product Consumption, Eating Habits, Sedentary Behaviour and Physical Activity Association with Bone Mineral Density among Adolescent Boys: A Cross-Sectional Observational Study. BMC Pediatr. 2024, 24, 53. [Google Scholar] [CrossRef]
- Sulis, S.; Falbová, D.; Beňuš, R.; Švábová, P.; Hozáková, A.; Vorobeľová, L. Sex and Obesity-Specific Associations of Ultrasound-Assessed Radial Velocity of Sound with Body Composition. Appl. Sci. 2024, 14, 7319. [Google Scholar] [CrossRef]
- Weaver, C.M.; Proulx, W.R.; Heaney, R. Choices for Achieving Adequate Dietary Calcium with a Vegetarian Diet. Am. J. Clin. Nutr. 1999, 70, 543S–548S. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Overall (n = 47) | Participating in PE (n = 38) | Not Participating in PE (n = 9) | p |
|---|---|---|---|---|
| Men [%] | 14% | 18% | 13% | 0.71 |
| BMI [kg/m2] | 20.89 [19.13, 23.11] | 21.20 [19.79, 23.27] | 18.39 [17.91, 20.96] | 0.070 |
| Age [years] | 17.00 [16.00, 17.00] | 17.00 [15.25, 17.00] | 16.50 [16.00, 17.00] | 0.831 |
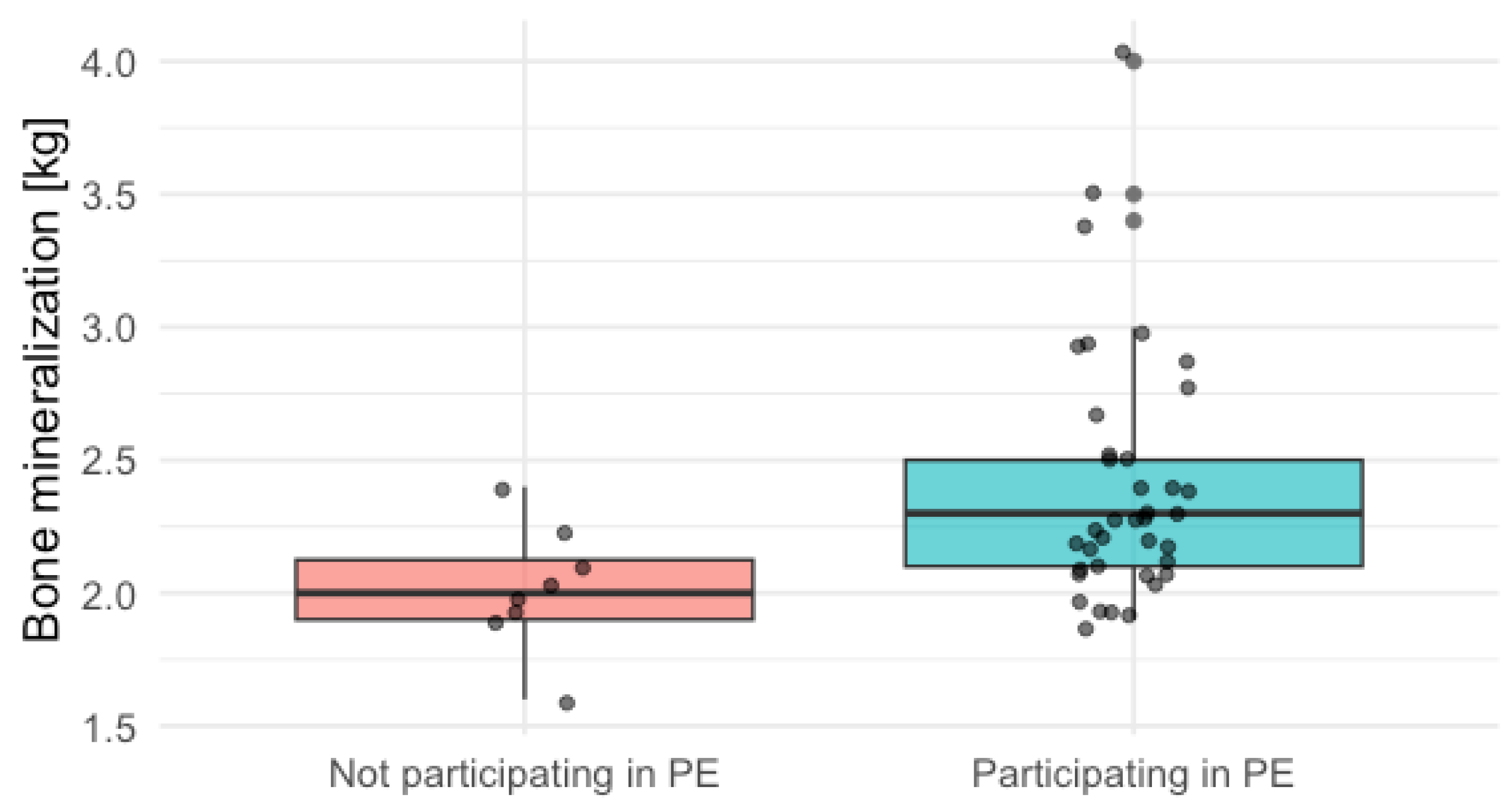
| Bone mineralization [kg] | 2.20 [2.10, 2.48] | 2.30 [2.10, 2.50] | 2.00 [1.90, 2.12] | 0.009 |
| Selected products intake [portion/day] | ||||
| White bread | 0.50 [0.32, 1.00] | 0.50 [0.23, 1.00] | 2.00 [0.50, 2.00] | 0.104 |
| Wholegrain bread | 0.50 [0.06, 0.50] | 0.50 [0.08, 0.50] | 0.50 [0.06, 0.50] | 0.899 |
| Plain pasta | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.50 [0.06, 0.50] | 0.435 |
| Wholegrain pasta | 0.14 [0.06, 0.50] | 0.14 [0.06, 0.50] | 0.06 [0.06, 0.50] | 0.810 |
| Fast food | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.06] | 0.169 |
| Nuts | 0.06 [0.03, 0.06] | 0.06 [0.06, 0.06] | 0.06 [0.00, 0.06] | 0.197 |
| Fried meals | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.14 [0.14, 0.50] | 0.445 |
| Butter | 0.50 [0.14, 1.00] | 0.50 [0.14, 1.00] | 0.50 [0.50, 2.00] | 0.440 |
| Lard | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.985 |
| Margarine | 0.06 [0.00, 0.14] | 0.06 [0.00, 0.14] | 0.00 [0.00, 0.14] | 0.511 |
| Milk | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.50 [0.14, 1.00] | 1.000 |
| Fermented milk products | 0.14 [0.06, 0.50] | 0.14 [0.06, 0.50] | 0.06 [0.06, 0.50] | 0.492 |
| White cottage cheese | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.06] | 0.438 |
| Yellow cheese | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.14 [0.14, 0.50] | 0.569 |
| Processed meat | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.50 [0.50, 1.00] | 0.097 |
| Red meat | 0.06 [0.03, 0.14] | 0.06 [0.01, 0.14] | 0.06 [0.06, 0.06] | 0.536 |
| White meat | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.14 [0.14, 0.50] | 0.150 |
| Fish | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.06] | 0.06 [0.06, 0.14] | 0.610 |
| Eggs | 0.14 [0.06, 0.50] | 0.14 [0.08, 0.50] | 0.14 [0.06, 0.50] | 0.724 |
| Legumes | 0.06 [0.00, 0.06] | 0.06 [0.00, 0.06] | 0.06 [0.00, 0.06] | 0.456 |
| Potatoes | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.50 [0.14, 0.50] | 0.817 |
| Fruits | 0.50 [0.50, 1.00] | 0.50 [0.50, 1.00] | 0.50 [0.14, 0.50] | 0.332 |
| Vegetables | 0.50 [0.50, 2.00] | 0.50 [0.50, 2.00] | 1.00 [0.50, 2.00] | 0.648 |
| Sweets | 0.50 [0.50, 1.00] | 0.50 [0.50, 1.00] | 0.50 [0.50, 1.00] | 0.850 |
| Ready soups | 0.06 [0.00, 0.06] | 0.06 [0.00, 0.06] | 0.06 [0.00, 0.06] | 0.751 |
| Canned meat | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.00] | 0.540 |
| Canned vegetables | 0.06 [0.00, 0.14] | 0.06 [0.00, 0.14] | 0.06 [0.06, 0.14] | 0.198 |
| Fruit juices | 0.14 [0.10, 0.50] | 0.32 [0.14, 0.50] | 0.14 [0.06, 0.50] | 0.899 |
| Vegetable juices | 0.06 [0.00, 0.06] | 0.06 [0.00, 0.06] | 0.00 [0.00, 0.00] | 0.088 |
| Hot drinks | 0.50 [0.06, 1.50] | 0.50 [0.06, 1.00] | 2.00 [0.50, 2.00] | 0.062 |
| Sweetened drink | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.14] | 0.06 [0.06, 0.14] | 0.876 |
| Energy drinks | 0.06 [0.00, 0.14] | 0.00 [0.00, 0.12] | 0.06 [0.00, 0.50] | 0.324 |
| Water | 2.00 [2.00, 2.00] | 2.00 [2.00, 2.00] | 2.00 [2.00, 2.00] | 0.798 |
| Alcohol | 0.00 [0.00, 0.06] | 0.00 [0.00, 0.06] | 0.00 [0.00, 0.00] | 0.465 |
| Diet quality and knowledge [points] | ||||
| pHDI | 19.00 [11.40, 25.25] | 19.20 [11.60, 26.00] | 18.70 [10.10, 23.10] | 0.579 |
| nHDI | 16.21 [12.00, 20.57] | 16.18 [12.36, 19.93] | 17.79 [9.50, 31.29] | 0.561 |
| DQI | 2.88 ± 12.60 | 3.97 ± 10.90 | −1.70 ± 18.27 | 0.394 |
| Dietary knowledge | 10.00 [8.00, 13.00] | 10.00 [8.00, 13.00] | 11.00 [8.00, 16.00] | 0.870 |
| Predictor | B | p | R2 |
|---|---|---|---|
| PE attendance | 0.40 | 0.03 | 0.11 |
| Additional physical activity at school | −0.11 | 0.35 | 0.02 |
| Physical activity at free time | 0.17 | 0.06 | 0.08 |
| Predictor | B | p | R2 | p for Model |
|---|---|---|---|---|
| PE attendance | 0.22353 | 0.0242 | 0.74 | <0.001 |
| Male sex | 0.70967 | <0.001 | ||
| Age | 0.02107 | 0.5360 | ||
| BMI | 0.05635 | <0.001 |
| Predictor | B | p | Age, Sex and BMI—Adjusted | |
|---|---|---|---|---|
| B | p | |||
| White bread | −0.16 | 0.17 | −0.04 | 0.54 |
| Wholegrain bread | 0.22 | 0.15 | −0.16 | 0.09 |
| Plain pasta | 0.67 | 0.02 | 0.26 | 0.12 |
| Wholegrain pasta | 0.81 | <0.001 | 0.03 | 0.87 |
| Fast food | 0.48 | 0.39 | 0.53 | 0.09 |
| Nuts | 0.26 | 0.66 | −0.66 | 0.04 |
| Fried meals | 0.07 | 0.69 | −0.11 | 0.21 |
| Butter | 0.04 | 0.73 | 0.01 | 0.86 |
| Lard | 0.03 | 0.95 | 0.26 | 0.23 |
| Margarine | 0.40 | 0.21 | 0.02 | 0.90 |
| Milk | −0.01 | 0.97 | −0.09 | 0.20 |
| Fermented milk products | 0.43 | 0.04 | −0.03 | 0.80 |
| White cottage cheese | 0.33 | 0.15 | 0.17 | 0.21 |
| Yellow cheese | 0.23 | 0.14 | 0.11 | 0.20 |
| Processed meat | −0.14 | 0.23 | −0.03 | 0.65 |
| Red meat | 0.42 | 0.19 | −0.19 | 0.28 |
| White meat | 0.31 | 0.14 | −0.07 | 0.55 |
| Fish | 0.75 | 0.29 | 0.03 | 0.94 |
| Eggs | 0.25 | 0.21 | −0.14 | 0.21 |
| Legumes | 1.83 | <0.001 | 0.40 | 0.25 |
| Potatoes | 0.12 | 0.71 | −0.43 | 0.01 |
| Fruits | 0.01 | 0.93 | −0.05 | 0.41 |
| Vegetables | −0.04 | 0.66 | −0.08 | 0.14 |
| Sweets | −0.15 | 0.21 | 0.01 | 0.88 |
| Ready soups | −0.02 | 0.95 | 0.16 | 0.42 |
| Canned meat | 3.13 | <0.001 | 1.21 | 0.02 |
| Canned vegetables | 0.38 | 0.08 | 0.15 | 0.22 |
| Fruit juices | 0.05 | 0.75 | 0.00 | 0.98 |
| Vegetable juices | 0.41 | 0.12 | −0.21 | 0.20 |
| Hot drinks | −0.14 | 0.13 | −0.06 | 0.25 |
| Sweetened drink | 0.19 | 0.48 | 0.02 | 0.90 |
| Energy drinks | −0.26 | 0.35 | −0.17 | 0.30 |
| Water | 0.12 | 0.34 | 0.05 | 0.48 |
| Alcohol | 0.83 | 0.66 | 0.94 | 0.41 |
| pHDI | 0.01 | 0.16 | 0.00 | 0.20 |
| nHDI | 0.00 | 0.99 | 0.00 | 0.95 |
| DQI | 0.01 | 0.19 | 0.00 | 0.23 |
| Dietary knowledge | 0.04 | <0.001 | 0.01 | 0.11 |
| Predictor | B | p | R2 | p |
|---|---|---|---|---|
| Milk | −0.11071 | 0.12 | 0.79 | <0.001 |
| Fermented milk products | 0.358265 | 0.04 | ||
| Yellow cheese | 0.246495 | 0.01 | ||
| Eggs | −0.26262 | 0.09 | ||
| Nuts | −0.75367 | 0.02 | ||
| Vegetables | −0.11638 | 0.03 | ||
| Butter | −0.07461 | 0.20 | ||
| BMI | 0.076923 | <0.001 | ||
| Age | 0.044405 | 0.19 | ||
| Male sex | 0.606996 | <0.001 | ||
| PE attendance | 0.34 | 0.03 | 0.34 | <0.001 |
| DQI | 0.01 | 0.16 | ||
| Dietary knowledge | 0.07 | <0.001 | ||
| PE attendance | 0.23 | 0.01 | 0.79 | <0.001 |
| Dietary knowledge | 0.03 | 0.01 | ||
| Male sex | 0.64 | <0.001 | ||
| BMI | 0.05 | <0.001 | ||
| PE attendance | 0.42 | 0.001 | 0.77 | <0.001 |
| White bread | 0.09 | 0.26 | ||
| Wholegrain bread | 0.30 | 0.02 | ||
| Plain pasta | 0.49 | 0.02 | ||
| Wholegrain pasta | 1.19 | <0.001 | ||
| Fast food | 0.77 | 0.049 | ||
| Nuts | −1.07 | 0.01 | ||
| Fermented milk products | 0.60 | 0.001 | ||
| White cottage cheese | −0.37 | 0.04 | ||
| Red meat | 0.69 | 0.01 | ||
| Eggs | −0.62 | 0.004 | ||
| Potatoes | −0.66 | 0.004 | ||
| Fruits | −0.18 | 0.04 | ||
| Ready soups | −0.96 | 0.001 | ||
| Canned meat | 3.74 | <0.001 | ||
| Fruit juices | 0.25 | 0.03 | ||
| Hot drinks | 0.11 | 0.05 | ||
| Sweetened drink | −0.49 | 0.01 | ||
| Butter | 0.08 | 0.28 | ||
| PE attendance | 0.27 | <0.001 | 0.91 | <0.001 |
| Plain pasta | 0.23 | 0.05 | ||
| Wholegrain pasta | 0.43 | <0.001 | ||
| Nuts | −1.05 | <0.001 | ||
| Red meat | 0.22 | 0.10 | ||
| Eggs | −0.17 | 0.04 | ||
| Potatoes | −0.67 | <0.001 | ||
| Fruits | −0.06 | 0.12 | ||
| Ready soups | −0.42 | 0.01 | ||
| Canned meat | 2.10 | <0.001 | ||
| Fruit juices | 0.15 | 0.03 | ||
| Sweetened drink | −0.27 | 0.01 | ||
| Male sex | 0.60 | <0.001 | ||
| BMI | 0.05 | <0.001 | ||
| PE attendance | 0.2 | 0.02 | 0.85 | <0.001 |
| BMI | 0.06 | <0 001 | ||
| Male sex | 0.54 | <0 001 | ||
| Milk | −0.09 | 0.13 | ||
| Fermented milk products | 0.26 | 0.07 | ||
| Yellow cheese | 0.16 | 0.06 | ||
| Eggs | −0.18 | 0.16 | ||
| Nuts | −0.81 | 0.004 | ||
| Vegetables | −0.1 | 0.045 | ||
| Butter | −0.07 | 0.23 | ||
| Legumes | 0.41 | 0.17 | ||
| Dietary knowledge | 0.03 | 0.01 | ||
| PE attendance | 0.25 | 0.004 | 0.81 | <0.001 |
| BMI | 0.06 | <0.001 | ||
| Male sex | 0.65 | <0.001 | ||
| Nuts | −0.69 | 0.01 | ||
| Dietary knowledge | 0.03 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przytula, A.; Popiolek-Kalisz, J. The Impact of Physical Education Attendance and Diet on Bone Mineralization in Adolescents. Nutrients 2025, 17, 3016. https://doi.org/10.3390/nu17183016
Przytula A, Popiolek-Kalisz J. The Impact of Physical Education Attendance and Diet on Bone Mineralization in Adolescents. Nutrients. 2025; 17(18):3016. https://doi.org/10.3390/nu17183016
Chicago/Turabian StylePrzytula, Agata, and Joanna Popiolek-Kalisz. 2025. "The Impact of Physical Education Attendance and Diet on Bone Mineralization in Adolescents" Nutrients 17, no. 18: 3016. https://doi.org/10.3390/nu17183016
APA StylePrzytula, A., & Popiolek-Kalisz, J. (2025). The Impact of Physical Education Attendance and Diet on Bone Mineralization in Adolescents. Nutrients, 17(18), 3016. https://doi.org/10.3390/nu17183016







