Aspergillus oryzae Fermentation of Lophatheri Herba Elevates SCFAs and Transforms Flavonoids to Fortify the Gut Barrier via Microbiota Remodeling in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fermentation of Lophatheri Herba
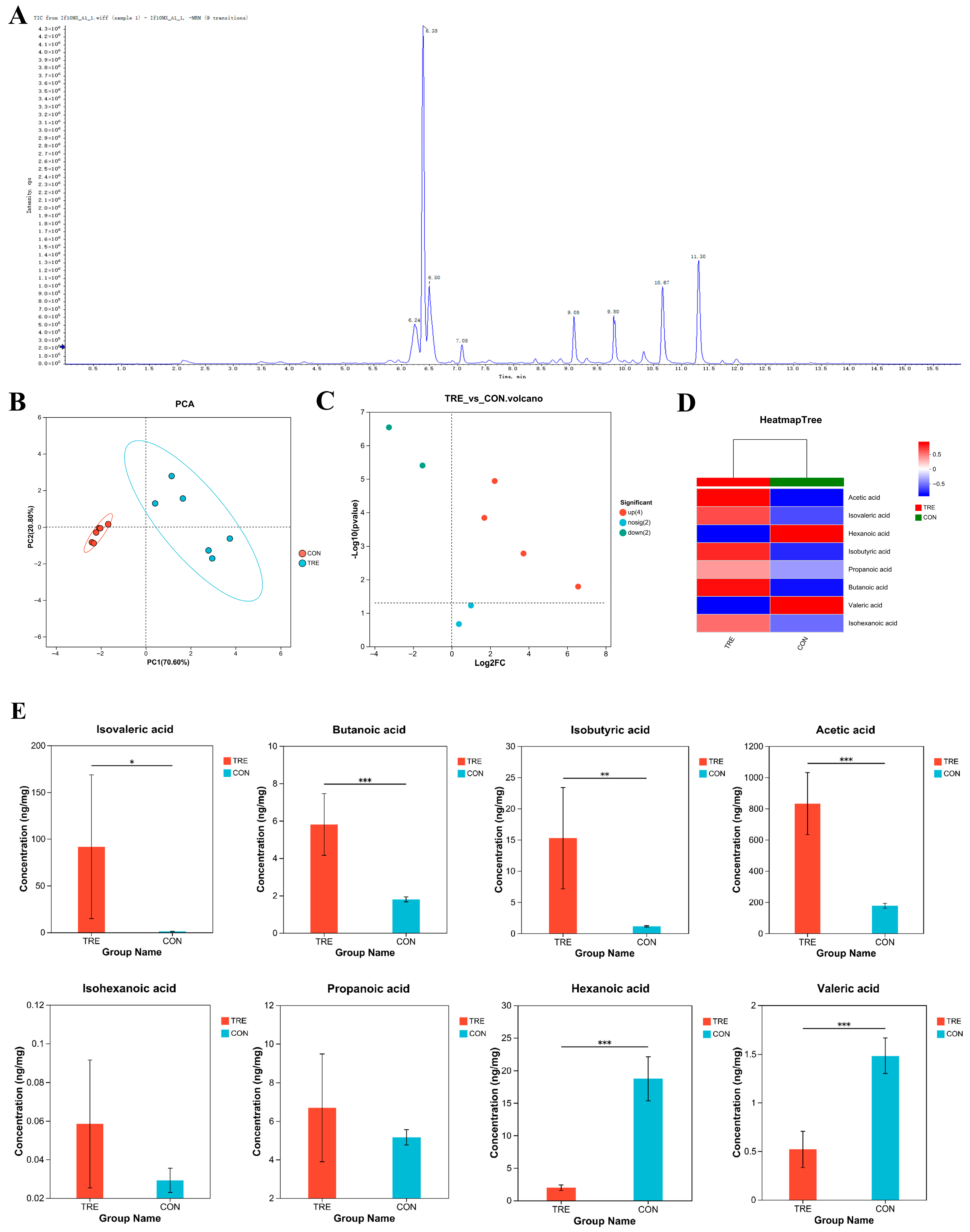
2.3. Detection of Short-Chain Fatty Acids
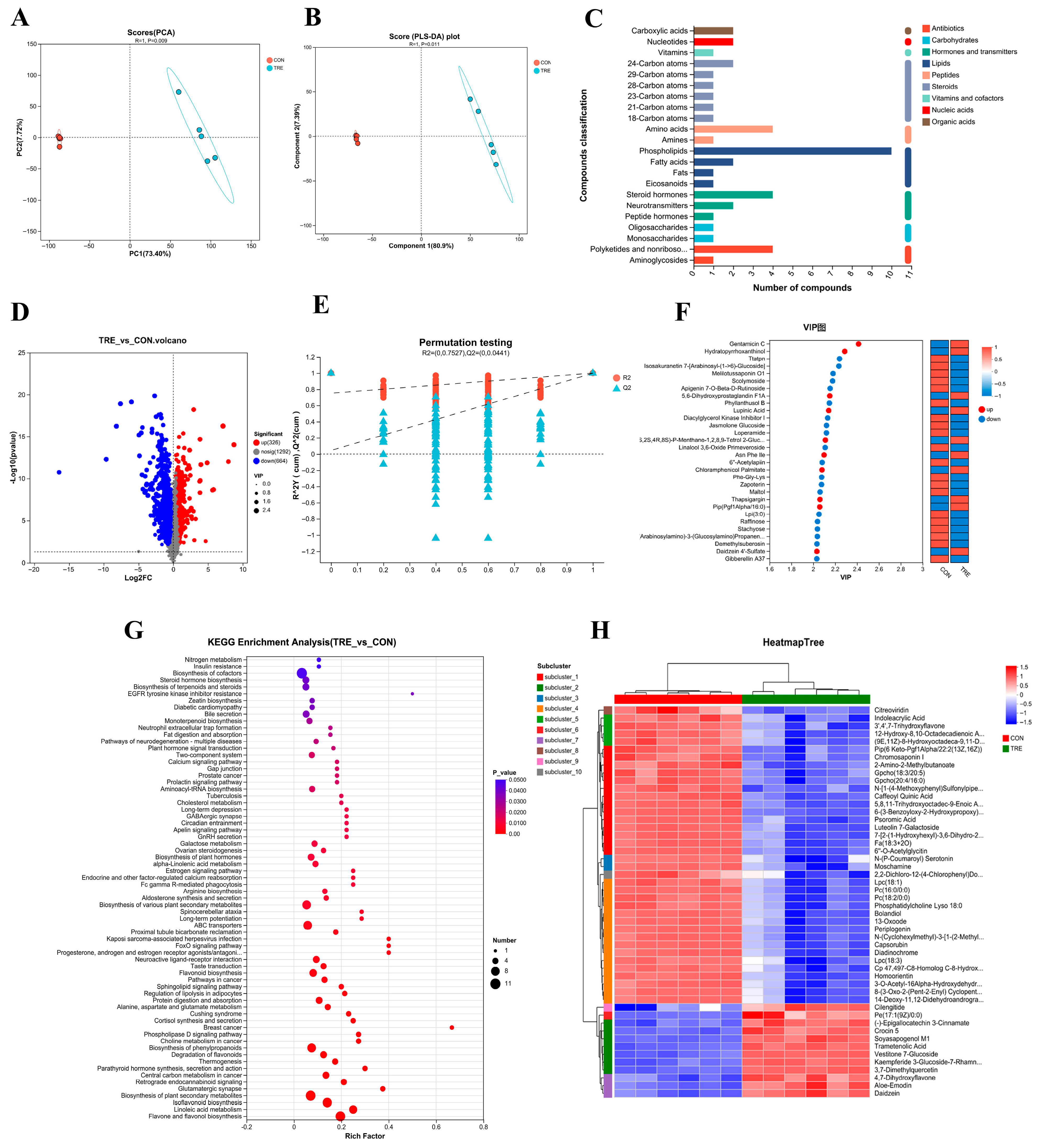
2.4. Untargeted Metabolomics Analysis
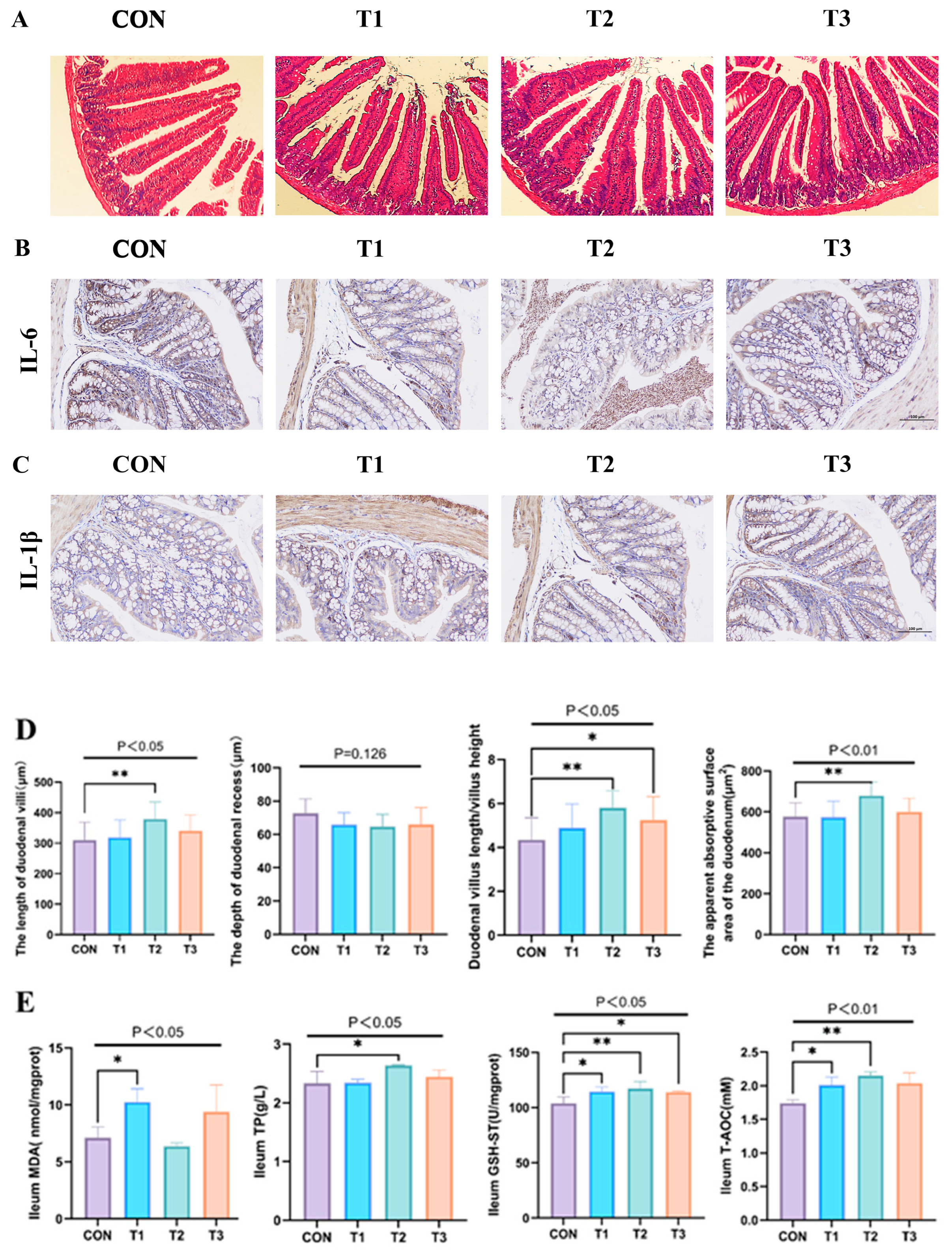
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Ileum Antioxidant Function Assessment
2.7. Immunohistochemistry Staining
2.8. Cecal Microbiota 16S rRNA Sequencing
2.9. Data Analysis
3. Results
3.1. Short-Chain Fatty Acids Detection in Fermented Lophatheri Herba Leaf Products
3.2. Metabolomics of Fermented Lophatheri Herba Leaves
3.3. Impact of Fermented Products on Mouse Growth Performance
3.4. Effect of Fermented Lophatheri Herba Leaves on Intestinal Tissues of Mice
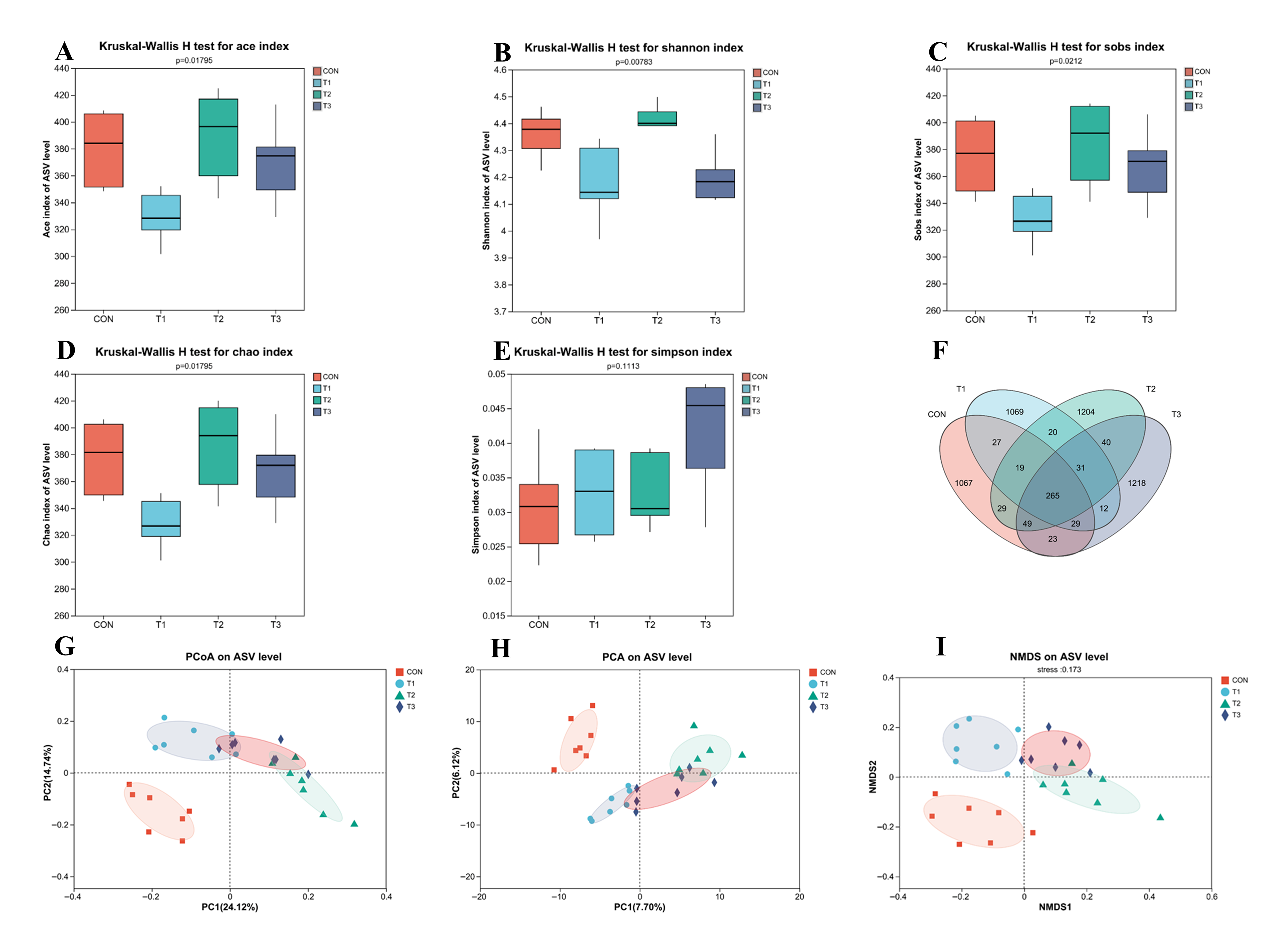
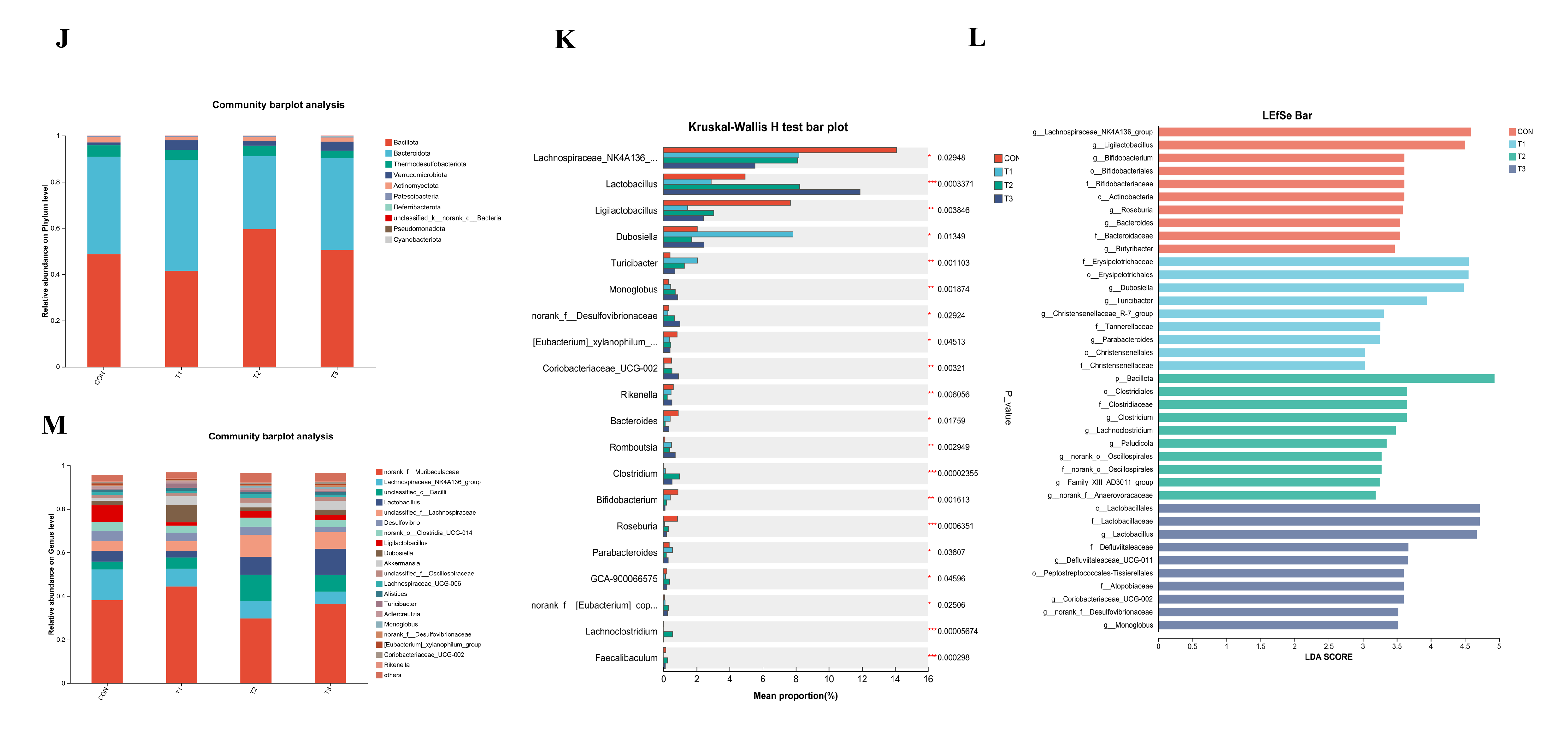
3.5. Effects of Fermented Lophatheri Herba Leaf Products on the Microbiota of Mouse Cecal
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Identification and antioxidant activity of novel chlorogenic acid derivatives from bamboo (Phyllostachys edulis). J. Agric. Food Chem. 2001, 49, 4646–4655. [Google Scholar] [CrossRef]
- Nirmala, C.; Bisht, M.S.; Bajwa, H.K.; Santosh, O. Bamboo: A rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018, 77, 91–99. [Google Scholar] [CrossRef]
- Wang, S.; Yue, Y.; Tang, F.; Sun, J.; Wei, Q.; Yu, J. Chemical Constituents from the Leaves of Dendrocalamus latiflorus. Sci. Silvae Sin. 2013, 49, 135–140. [Google Scholar]
- Fan, J.-S.; Lee, I.J.; Lin, Y.L. Flavone glycosides from commercially available Lophatheri Herba and their chromatographic fingerprinting and quantitation. J. Food Drug Anal. 2015, 23, 821–827. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Wu, S.-X.; Cheng, J.; Saimaiti, A.; Liu, Q.; Shang, A.; Zhou, D.-D.; Huang, S.-Y.; Gan, R.-Y.; Li, H.-B. Antioxidant Activities, Phenolic Compounds, and Sensory Acceptability of Kombucha-Fermented Beverages from Bamboo Leaf and Mulberry Leaf. Antioxidants 2023, 12, 1573. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Zhu, M.-z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.-m.; Xu, W.; Li, J.; Lin, H.-y.; Zhang, Z.; Xiao, J.-b.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Higa, J.K.; Panee, J. Bamboo extract reduces interleukin 6 (IL-6) overproduction under lipotoxic conditions through inhibiting the activation of NF-κB and AP-1 pathways. Cytokine 2011, 55, 18–23. [Google Scholar] [CrossRef]
- Huang, B.-P.; Lin, C.-H.; Chen, H.-M.; Lin, J.-T.; Cheng, Y.-F.; Kao, S.-H. AMPK Activation Inhibits Expression of Proinflammatory Mediators Through Downregulation of PI3K/p38 MAPK and NF-κB Signaling in Murine Macrophages. DNA Cell Biol. 2015, 34, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-l.; Yin, Y.-g. In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by D-galactose. Food Chem. Toxicol. 2012, 50, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, M.; Pan, Q.; Zuo, X.; Guo, A.; Liu, Y. Regulatory effects of lactic acid bacteria co-fermented Dendrocalamus latiflorus shoots on lipid-lowering functions and the gut microbiota in HFD-fed mice. Food Funct. 2025, 16, 3493–3507. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Han, M.; Liang, J.; Zhang, M.; Bai, X.; Yue, T.; Gao, Z. Evaluating the changes in phytochemical composition, hypoglycemic effect, and influence on mice intestinal microbiota of fermented apple juice. Food Res. Int. 2022, 155, 110998. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Lin, H.-T.; Wu, S.-C. Influence of dietary supplementation with Bacillus-fermented adlay on lipid metabolism, antioxidant status and intestinal microflora in hamsters. J. Sci. Food Agric. 2011, 91, 2271–2276. [Google Scholar] [CrossRef]
- Pan, Z.-y.; Wang, Y.-z.; Huang, W.-c.; Liu, Z.; Huang, H.-y.; Sun, J.-a.; Mao, X.-z. Effects of Bacillus subtilis solid-state fermented shrimp head inoculant on growth and intestinal flora of mice. Food Res. Dev. 2022, 43, 191–198. [Google Scholar] [CrossRef]
- Lu, B.; Wu, X.; Tie, X.; Zhang, Y.; Zhang, Y. Toxicology and safety of anti-oxidant of bamboo leaves. Part 1: Acute and subchronic toxicity studies on anti-oxidant of bamboo leaves. Food Chem. Toxicol. 2005, 43, 783–792. [Google Scholar] [CrossRef]
- Bae, J.-W.; Kim, D.-H.; Lee, W.-W.; Kim, H.-Y.; Son, C.-G. Characterizing the human equivalent dose of herbal medicines in animal toxicity studies. J. Ethnopharmacol. 2015, 162, 1–6. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Sun, M.N.; Elmaidomy, A.H.; Youssif, K.A.; Zaki, A.M.M.; Kamal, H.H.; Sayed, A.M.; Abdelmohsen, U.R. Emerging trends and applications of metabolomics in food science and nutrition. Food Funct. 2023, 14, 9050–9082. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Nørgård, M.; Svenningsen, P. Acute Kidney Injury by Ischemia/Reperfusion and Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 15312. [Google Scholar] [CrossRef]
- Andoh, A.; Tsujikawa, T.; Fujiyama, Y. Role of Dietary Fiber and Short-Chain Fatty Acids in the Colon. Curr. Pharm. Des. 2003, 9, 347–358. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, X.; Yang, C.; Gong, W.; Wang, C.; Wu, L.; Wang, D. Short-Chain Fatty Acids Attenuate 5-Fluorouracil-Induced THP-1 Cell Inflammation through Inhibiting NF-κB/NLRP3 Signaling via Glycerolphospholipid and Sphingolipid Metabolism. Molecules 2023, 28, 494. [Google Scholar] [CrossRef]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar] [CrossRef]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Li, Y.; Li, Y.; Feng, C.; Li, Z. Daidzein-rich isoflavones aglycone inhibits lung cancer growth through inhibition of NF-κB signaling pathway. Immunol. Lett. 2020, 222, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Chen, W.N. A metabolomics approach to evaluate post-fermentation enhancement of daidzein and genistein in a green okara extract. J. Sci. Food Agric. 2021, 101, 5124–5131. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Wang, Y.; Xie, J.; Xuan, J.; Geng, J.; Liu, G.; Tu, J.; Xiao, H. Pediococcus pentosaceus JS35 improved flavor, metabolic profile of fermentation supernatant of mulberry leaf powder and increased its antioxidant capacity. Front. Nutr. 2025, 12, 1551689. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, N.-R.; Lee, C.H. Comprehensive Metabolite Profiling of Four Different Beans Fermented by Aspergillus oryzae. Molecules 2022, 27, 7917. [Google Scholar] [CrossRef]
- Pecere, T.; Gazzola, M.V.; Mucignat, C.; Parolin, C.; Dalla Vecchia, F.; Cavaggioni, A.; Basso, G.; Diaspro, A.; Salvato, B.; Carli, M.; et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res. 2000, 60, 2800–2804. [Google Scholar]
- Abdellatef, A.A.; Fathy, M.; Mohammed, A.E.-S.I.; Bakr, M.S.A.; Ahmed, A.H.; Abbass, H.S.; El-Desoky, A.H.; Morita, H.; Nikaido, T.; Hayakawa, Y. Inhibition of cell-intrinsic NF-κB activity and metastatic abilities of breast cancer by aloe-emodin and emodic-acid isolated from Asphodelus microcarpus. J. Nat. Med. 2021, 75, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; He, S.; Yu, Z.; Xu, H.; Wang, Z.; Li, H. Investigation on bowel regulation and constipation relief based on the microbial fermentation solution of cassia seed. J. Ethnopharmacol. 2025, 342, 119412. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhang, X.-f.; Wang, L.-j.; Zheng, Y.-n.; Yuan, C.-c.; Sun, G.-z. Isolation and Characterization of Phenolic Compounds from the Leaves of Salix matsudana. Molecules 2008, 13, 1530–1537. [Google Scholar] [CrossRef]
- Qu, T.; Zhang, N.; Li, C.; Liu, X.; Yun, K.; An, Q. Network pharmacology, molecular docking, molecular dynamics simulation, and experiment verification analysis to reveal the action mechanism of RenShen Guipi Wan in the treatment of anemia. Biotechnol. Lett. 2025, 47, 43. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus Flavanones: What Is Their Role in Cardiovascular Protection? J. Agric. Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-Inflammation Activity of Flavones and Their Structure–Activity Relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- Jing, T.; Yunlong, L.; Wenzhe, Y.; Yueliang, Z.; Xiaoqian, H.; Ning-Ping, T.; Mingfu, W. Naringenin, a common flavanone, inhibits the formation of AGEs in bread and attenuates AGEs-induced oxidative stress and inflammation in RAW264.7 cells. Food Chem. 2018, 269, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Claude, S.; Boby, C.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Gérard, N.; Morand, C.; Milenkovic, D. Flavanol metabolites reduce monocyte adhesion to endothelial cells through modulation of expression of genes via p38-MAPK and p65-Nf-kB pathways. Mol. Nutr. Food Res. 2014, 58, 1016–1027. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, S.-M.; Ko, D.-B.; Jeong, J.-J.; Hwang, Y.-H.; Kim, D.-H. Soyasapogenol B and Genistein Attenuate Lipopolysaccharide-Induced Memory Impairment in Mice by the Modulation of NF-κB-Mediated BDNF Expression. J. Agric. Food Chem. 2017, 65, 6877–6885. [Google Scholar] [CrossRef]
- Khaksari, Z.; Mehri, F.; Abdolvahab, M.H.; Manavi, M.A.; Nasab, M.H.F.; Karbasi, A.; Baeeri, M.; Ranjbar, A. Crocin and Nano-Crocin Mitigate Paraquat Hepatotoxicity by Modulating Expression of Genes Involved in Oxidative Stress and Inflammation. Pharm. Nanotechnol. 2025. ahead of print. [Google Scholar] [CrossRef]
- Bharrhan, S.; Chopra, K.; Arora, S.K.; Toor, J.S.; Rishi, P. Down-regulation of NF-κB signalling by polyphenolic compounds prevents endotoxin-induced liver injury in a rat model. Innate Immun. 2012, 18, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Varela-Rodriguez, L.; Sanchez-Ramirez, B.; Stephanie Rodriguez-Reyna, I.; Juan Ordaz-Ortiz, J.; Chavez-Flores, D.; Salas-Munoz, E.; Carlos Osorio-Trujillo, J.; Ramos-Martinez, E.; Talamas-Rohana, P. Biological and toxicological evaluation of Rhus trilobata Nutt. (Anacardiaceae) used traditionally in mexico against cancer. BMC Complement. Altern. Med. 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Meydani, M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am. J. Clin. Nutr. 2001, 73, 941–948. [Google Scholar] [CrossRef]
- Reardon, D.A.; Neyns, B.; Weller, M.; Tonn, J.C.; Nabors, L.B.; Stupp, R. Cilengitide: An RGD pentapeptide ανβ3 and ανβ5 integrin inhibitor in development for glioblastoma and other malignancies. Future Oncol. 2011, 7, 339–354. [Google Scholar] [CrossRef]
- Martinez, R.M.; Hulten, K.G.; Bui, U.; Clarridge, J.E. Molecular Analysis and Clinical Significance of Lactobacillus spp. Recovered from Clinical Specimens Presumptively Associated with Disease. J. Clin. Microbiol. 2014, 52, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Cheng, S.; Li, H.; Huang, Y.; Su, Y.; Li, Y.; Jia, A.; Jiang, Y.; Zhang, Y.; Man, C. Lactobacillus gasseri JM1 Isolated from Infant Feces Alleviates Colitis in Mice via Protecting the Intestinal Barrier. Nutrients 2023, 15, 139. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Liu, J.; Chang, G.; Huang, J.; Wang, Y.; Ma, N.; Roy, A.C.; Shen, X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor κB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1674–1682. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, M.; Wen, Q.; Xie, Y.; Ding, Q.; Xie, X.; Xie, Q.; Chen, M. Effect of Artemisia selengensis Turcz extract on lipid metabolism and gut microbiota in high-fat diet-induced C57BL/6J obese mice. J. Food Sci. 2025, 90, e70162. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Li, S.; Chang, Z.; Liu, H.; Zhang, D.; Wang, S.; Zhang, X.; Wang, J. Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy. Antioxidants 2024, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zu, M.; Jiang, A.; Cao, Y.; Wu, J.; Shahbazi, M.-A.; Shi, X.; Reis, R.L.; Kundu, S.C.; Xiao, B. Magnetic natural lipid nanoparticles for oral treatment of colorectal cancer through potentiated antitumor immunity and microbiota metabolite regulation. Biomaterials 2024, 307, 122530. [Google Scholar] [CrossRef]
- Li, X.; Sun, B.; Qin, Y.; Yue, F.; Lu, X. Amelioration of Obesity-Related Disorders in High-Fat Diet-Fed C57BL/6 Mice Following Fecal Microbiota Transplantation From DL-Norvaline-Dosed Mice. Mol. Nutr. Food Res. 2025, 69, e202400577. [Google Scholar] [CrossRef]
- Zhang, T.; Chang, M.; Hou, X.; Yan, M.; Zhang, S.; Song, W.; Sheng, Q.; Yuan, Y.; Yue, T. Apple polyphenols prevent patulin-induced intestinal damage by modulating the gut microbiota and metabolism of the gut-liver axis. Food Chem. 2025, 463, 141049. [Google Scholar] [CrossRef]
- Mo, Z.; Zhan, M.; Yang, X.; Xie, P.; Xiao, J.; Cao, Y.; Xiao, H.; Song, M. Fermented dietary fiber from soy sauce residue exerts antidiabetic effects through regulating the PI3K/AKT signaling pathway and gut microbiota-SCFAs-GPRs axis in type 2 diabetic mellitus mice. Int. J. Biol. Macromol. 2024, 270, 132251. [Google Scholar] [CrossRef]
- Lynch, J.B.; Gonzalez, E.L.; Choy, K.; Faull, K.F.; Jewell, T.; Arellano, A.; Liang, J.; Yu, K.B.; Paramo, J.; Hsiao, E.Y. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat. Commun. 2023, 14, 3669. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Zhang, B.; Yin, J.; Liuqi, S.; Wang, J.; Peng, B.; Wang, S. Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating Gut Microbiota and Bile Acid Metabolism. J. Agric. Food Chem. 2022, 70, 14864–14876. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, H.; Hu, C.; Wen, Y.; Shang, D.; Gan, T.; Guo, Z.; Dai, L.; Luo, Y. Inulin alleviates chronic ketamine-induced impairments in memory and prepulse inhibition by regulating the gut microbiota, inflammation, and kynurenine pathway. Int. J. Biol. Macromol. 2025, 294, 139503. [Google Scholar] [CrossRef]
- Wu, X.; Huang, X.; Ma, W.; Li, M.; Wen, J.; Chen, C.; Liu, L.; Nie, S. Bioactive polysaccharides promote gut immunity via different ways. Food Funct. 2023, 14, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Zhu, Z.; Zeng, J.; Xia, Y.; Zhang, H.; Wu, Y.; Song, Z.; Ai, L. Fabrication of zein-tamarind seed polysaccharide-curcumin nanocomplexes: Their characterization andimpact on alleviating colitis andgut microbiota dysbiosis in mice. Food Funct. 2024, 15, 2563–2576. [Google Scholar] [CrossRef]
- Hu, S.; Yang, H.; Gao, X.; Li, S.; Jiang, W.; Liu, Y. Egg oil from Portunus trituberculatus alleviated obesity and regulated gut microbiota in mice. Sci. Rep. 2020, 10, 8454. [Google Scholar] [CrossRef]
- Gao, X.; Du, L.; Randell, E.; Zhang, H.; Li, K.; Li, D. Effect of different phosphatidylcholines on high fat diet-induced insulin resistance in mice. Food Funct. 2021, 12, 1516–1528. [Google Scholar] [CrossRef]
- Wu, Y.; Shao, Y.; Shao, X.; Yu, H.; Wang, M.; Wang, J.; She, Y.; Liu, J.; Zhang, T.; Li, Z.; et al. Qingke beta-glucan and Lactobacillus mitigate neuroinflammation and enhance cognitive function in an Alzheimer’s disease mouse model. Int. J. Biol. Macromol. 2025, 319, 145427. [Google Scholar] [CrossRef]
- Song, B.; Sun, P.; Kong, L.; Xiao, C.; Pan, X.; Song, Z. The improvement of immunity and activation of TLR2/NF-κB signaling pathway by Romboutsia ilealis in broilers. J. Anim. Sci. 2024, 102, skae286. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, P.; Cui, Y.; Hu, X.; Chen, F.; Ma, C. Protective Effects of Hydroxyphenyl Propionic Acids on Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 1043. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Xu, Z.; Qu, H.; Liu, H. Dendrobium officinale leaf phenolic extracts alleviate diabetes mellitus in mice via modulating metabolism and reshaping gut microbiota. J. Sci. Food Agric. 2025, 105, 5377–5387. [Google Scholar] [CrossRef]
- Fei, Z.; Xie, D.; Wang, M.; Zhang, Y.; Zhang, H.; Du, Q.; Jin, P. Enhanced biotransformation of bioactive components and volatile compounds of bamboo (Phyllostachys glauca McClure) leaf juice fermented by probiotic Streptococcus thermophiles. LWT-Food Sci. Technol. 2023, 173, 114363. [Google Scholar] [CrossRef]
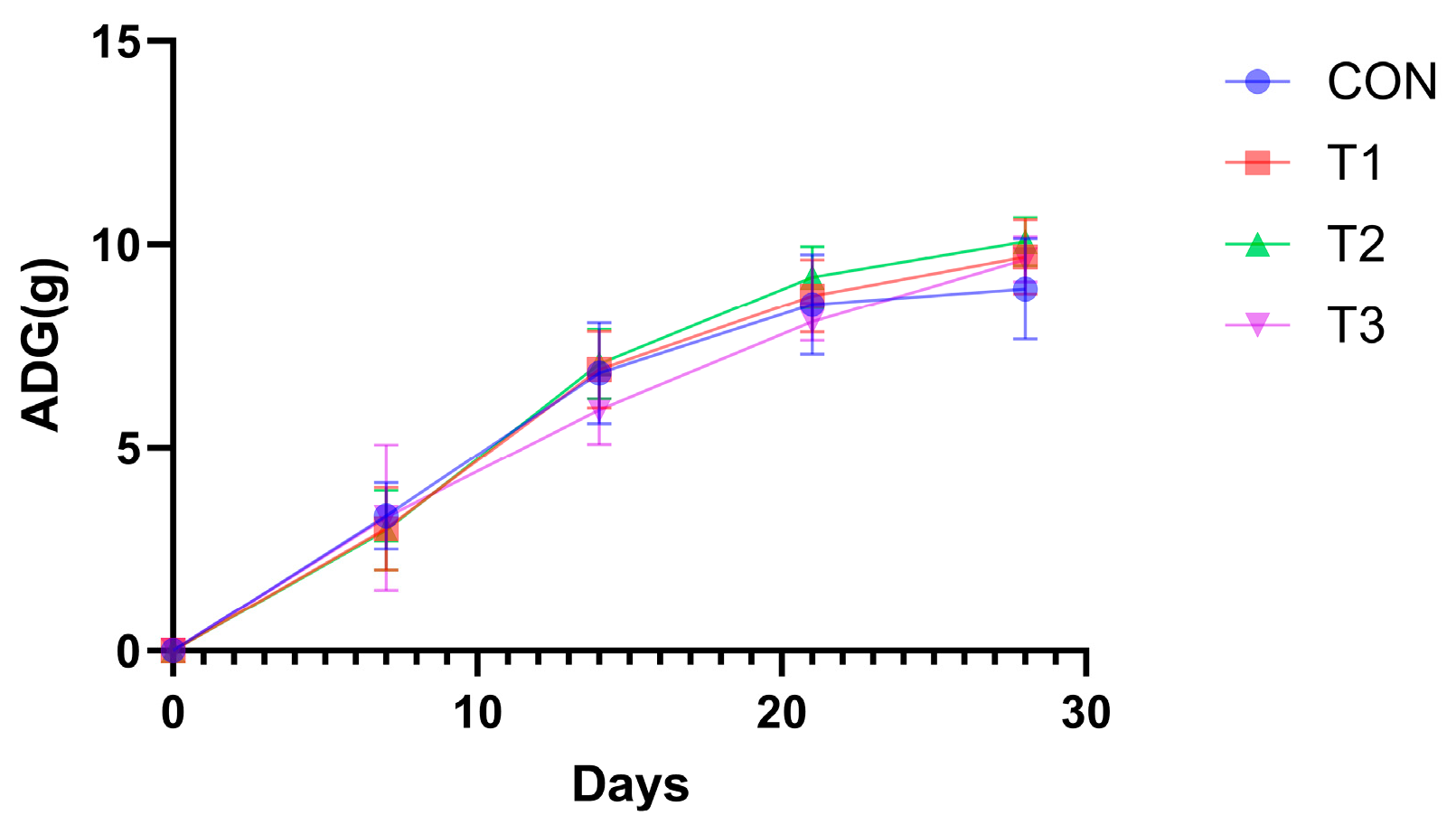
| Items | CON | T1 | T2 | T3 | SEM | p |
|---|---|---|---|---|---|---|
| 0–7 d ADG | 3.32 | 3.00 | 2.97 | 3.28 | 0.17 | 0.839 |
| 0–14 d ADG | 6.83 | 6.92 | 7.06 | 5.94 | 0.15 | 0.041 |
| 0–21 d ADG | 8.52 | 8.73 | 9.19 | 8.10 | 0.14 | 0.046 |
| 0–28 d ADG | 8.91 | 9.70 | 10.07 | 9.63 | 0.15 | 0.044 |
| FCR (F/G) | 14.60 | 11.49 | 10.46 | 11.63 | 0.33 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Chen, J.; Chen, R.; Liang, W.; Huang, R.; Tang, L.; Qian, L. Aspergillus oryzae Fermentation of Lophatheri Herba Elevates SCFAs and Transforms Flavonoids to Fortify the Gut Barrier via Microbiota Remodeling in Mice. Nutrients 2025, 17, 2996. https://doi.org/10.3390/nu17182996
Ma X, Chen J, Chen R, Liang W, Huang R, Tang L, Qian L. Aspergillus oryzae Fermentation of Lophatheri Herba Elevates SCFAs and Transforms Flavonoids to Fortify the Gut Barrier via Microbiota Remodeling in Mice. Nutrients. 2025; 17(18):2996. https://doi.org/10.3390/nu17182996
Chicago/Turabian StyleMa, Xin, Jiaxuan Chen, Rui Chen, Wenjiao Liang, Rui Huang, Lishiyuan Tang, and Lichun Qian. 2025. "Aspergillus oryzae Fermentation of Lophatheri Herba Elevates SCFAs and Transforms Flavonoids to Fortify the Gut Barrier via Microbiota Remodeling in Mice" Nutrients 17, no. 18: 2996. https://doi.org/10.3390/nu17182996
APA StyleMa, X., Chen, J., Chen, R., Liang, W., Huang, R., Tang, L., & Qian, L. (2025). Aspergillus oryzae Fermentation of Lophatheri Herba Elevates SCFAs and Transforms Flavonoids to Fortify the Gut Barrier via Microbiota Remodeling in Mice. Nutrients, 17(18), 2996. https://doi.org/10.3390/nu17182996






