Impact of Dietitian-Guided Individualized Nutrition (DGIN) on ICU Outcomes in Critically Ill Patients: A Retrospective Cohort Study in Taiwan
Highlights
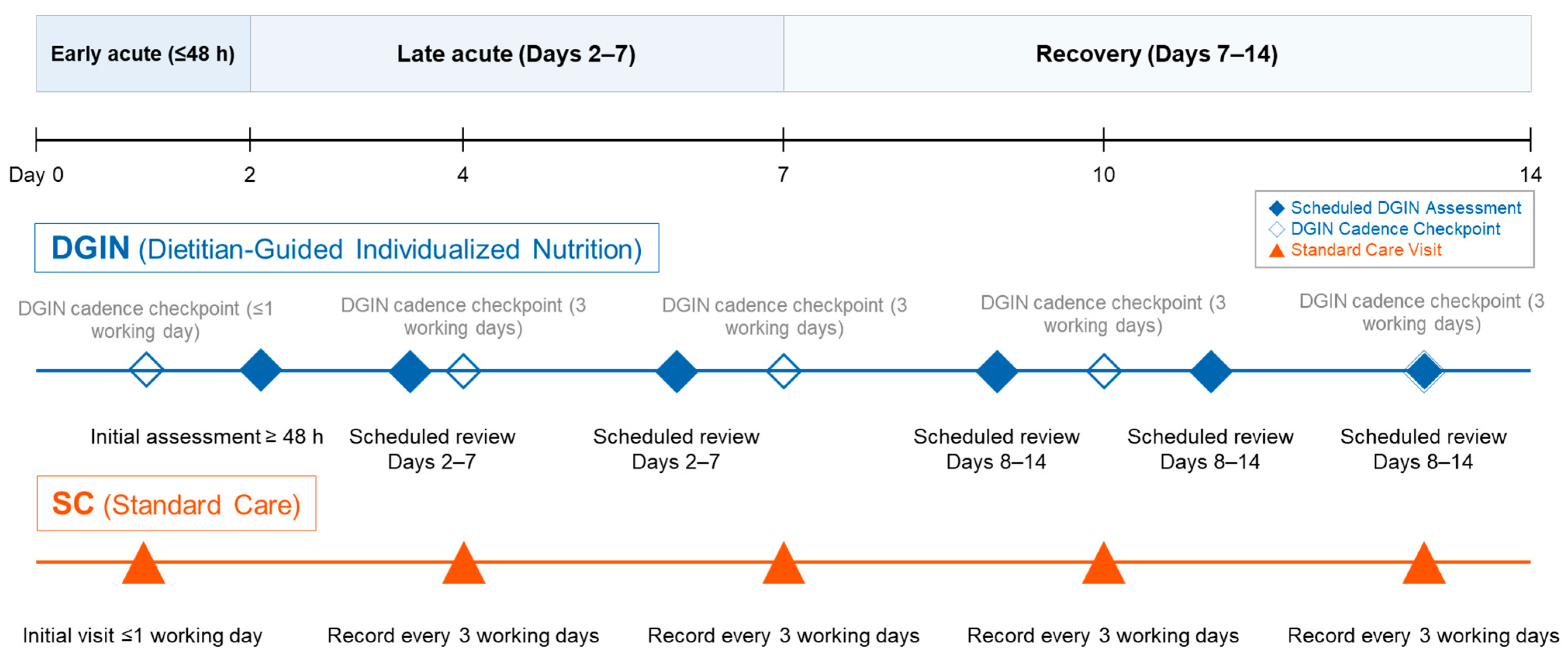
- In an ICU cohort of 2381 adults, we compared dietitian-guided individualized nutrition (DGIN; prespecified reassessment cadence ≤ 48 h, two visits in days 2–7, three in days 8–14) with standard care (SC).
- DGIN was associated with a shorter ICU length of stay, with no difference in in-hospital mortality versus SC.
- Early readmission (14-/30-day) was higher with DGIN; causes were not adjudicated, and findings were associative.
- Results were consistent after multivariable adjustment and a prespecified 1:1 propensity-score-matched analysis; protocolized, dietitian-guided care may standardize ICU nutrition delivery, while post-ICU transitional strategies may help address readmissions.
Abstract
1. Introduction
1.1. Critical Care Nutrition
1.2. The Taiwan National Health Insurance (NHI) Program
2. Materials and Methods
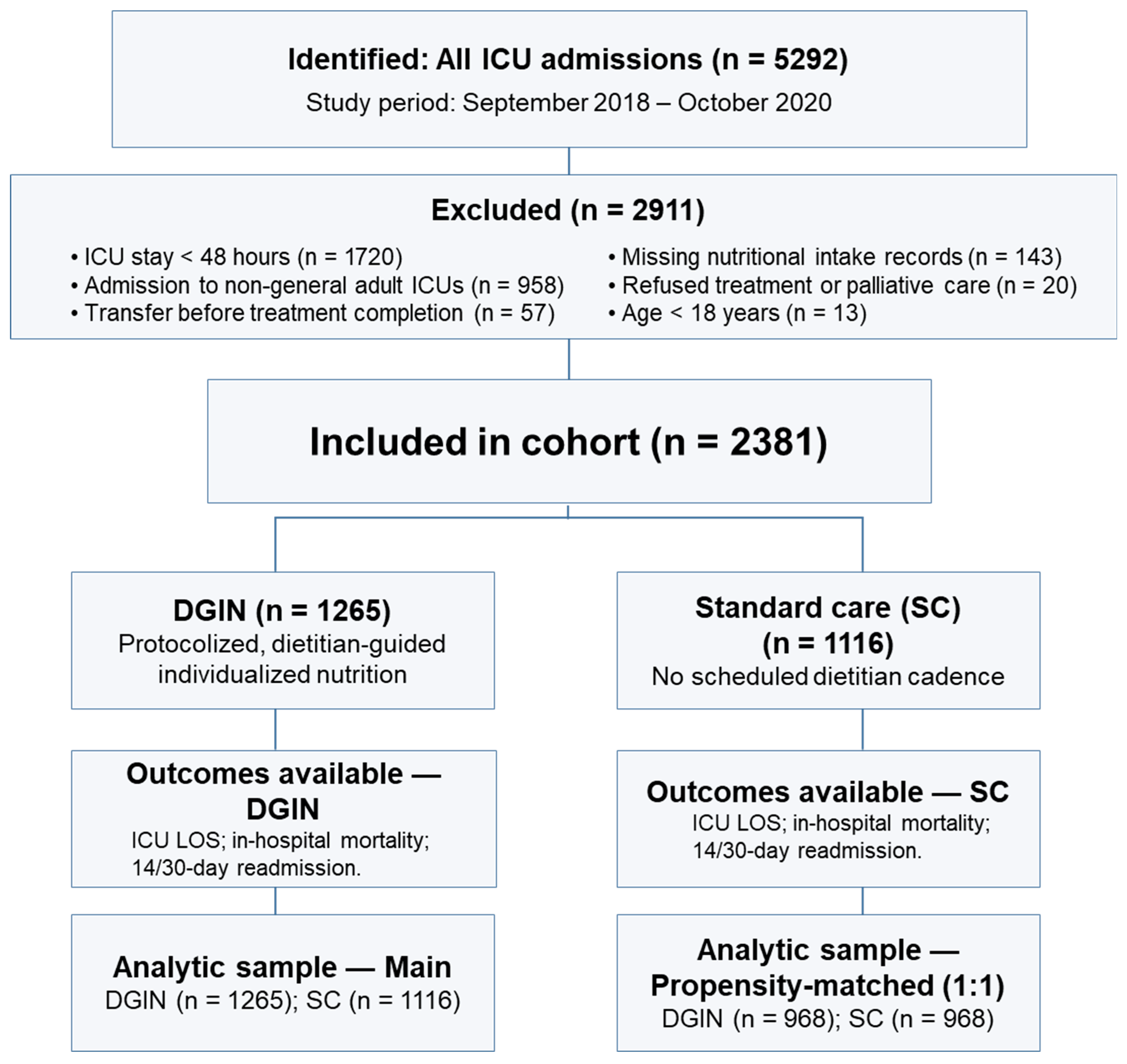
2.1. Study Population and Setting
2.2. Study Groups and Nutrition Protocols
2.3. Statistical Analysis
2.4. Propensity Score Matching Analysis
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Nutritional Intake and Biochemical Indicators
3.3. Clinical Outcomes
4. Discussion
4.1. Summary of Key Findings
4.2. ICU Length of Stay and Nutritional Stabilization
4.3. Unexpectedly Higher Readmission Rates
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DGIN | Dietitian-Guided Individualized Nutrition |
| SC | Standard Care |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| ICU | Intensive Care Unit |
| NHI | National Health Insurance |
| CCU | Coronary Care Unit |
| MICU | Medical Intensive Care Unit |
| PICU | Pediatric Intensive Care Unit |
| RCC | Respiratory Care Center |
| SICU | Surgical Intensive Care Unit |
References
- Van Dyck, L.; Casaer, M.P. Nutrition in the ICU: Sometimes route does matter. Lancet 2018, 391, 98–100. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; Steingrub, J.; Hite, R.D.; Moss, M.; Morris, A.; Dong, N.; Rock, P. Initial trophic vs full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA 2012, 307, 795–803. [Google Scholar] [CrossRef]
- Chapman, M.; Peake, S.L.; Bellomo, R.; Davies, A.; Deane, A.; Horowitz, M.; Hurford, S.; Lange, K.; Little, L.; Mackle, D.; et al. Energy-Dense versus Routine Enteral Nutrition in the Critically Ill. N. Engl. J. Med. 2018, 379, 1823–1834. [Google Scholar] [CrossRef]
- Deane, A.M.; Little, L.; Bellomo, R.; Chapman, M.J.; Davies, A.R.; Ferrie, S.; Horowitz, M.; Hurford, S.; Lange, K.; Litton, E.; et al. Outcomes Six Months after Delivering 100% or 70% of Enteral Calorie Requirements during Critical Illness (TARGET). A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 814–822. [Google Scholar] [CrossRef]
- Marik, P.E. Enteral nutrition in the critically ill: Myths and misconceptions. Crit. Care Med. 2014, 42, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y.; et al. Obesity in the critically ill: A narrative review. Intensive Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, C.; Lin, Q.; Li, T. The obesity paradox for survivors of critically ill patients. Crit. Care 2022, 26, 198. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Heyland, D.K.; Cahill, N.E.; Dhaliwal, R.; Sun, X.; Day, A.G.; McClave, S.A. Impact of enteral feeding protocols on enteral nutrition delivery: Results of a multicenter observational study. JPEN J. Parenter. Enter. Nutr. 2010, 34, 675–684. [Google Scholar] [CrossRef]
- Peev, M.P.; Yeh, D.D.; Quraishi, S.A.; Osler, P.; Chang, Y.; Gillis, E.; Albano, C.E.; Darak, S.; Velmahos, G.C. Causes and consequences of interrupted enteral nutrition: A prospective observational study in critically ill surgical patients. JPEN J. Parenter. Enter. Nutr. 2015, 39, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, M.; Sanui, M.; Komuro, T.; Iizuka, Y.; Kamio, T.; Koyama, H.; Mouri, H.; Masuyama, T.; Ono, K.; Lefor, A.K. Interruption of enteral nutrition in the intensive care unit: A single-center survey. J. Intensive Care 2017, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Heyland, D.K.; Weijs, P.J.; Coss-Bu, J.A.; Taylor, B.; Kristof, A.S.; O’Keefe, G.E.; Martindale, R.G. Protein Delivery in the Intensive Care Unit: Optimal or Suboptimal? Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2017, 32, 58s–71s. [Google Scholar] [CrossRef]
- Heyland, D.K.; Schroter-Noppe, D.; Drover, J.W.; Jain, M.; Keefe, L.; Dhaliwal, R.; Day, A. Nutrition support in the critical care setting: Current practice in canadian ICUs--opportunities for improvement? JPEN J. Parenter. Enter. Nutr. 2003, 27, 74–83. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Huang, Y.-T.; Tsai, Y.-W.; Huang, S.-M.; Kuo, K.N.; McKee, M.; Nolte, E. The impact of universal National Health Insurance on population health: The experience of Taiwan. BMC Health Serv. Res. 2010, 10, 225. [Google Scholar] [CrossRef]
- Owili, P.O.; Muga, M.A.; Yang, Y.T.; Hsu, Y.E. Perceived Impact of Taiwan’s National Health Insurance Allocation Strategy: Health Professionals’ Perspective. Int. J. Environ. Res. Public Health 2019, 16, 467. [Google Scholar] [CrossRef]
- Tsuei, S.H.-T. Reflection on 30 years of Taiwanese national health insurance: Analysis of Taiwanese health system progress, challenges, and opportunities. J. Formos. Med. Assoc. 2024, 123, S180–S187. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lee, P.-C.; Lo, W.-C. Real-world trends and reform imperatives for Taiwan National Health Insurance. J. Formos. Med. Assoc. 2025. [Google Scholar] [CrossRef]
- Jan, C.-F.J.; Chang, C.-J.J.; Hwang, S.-J.; Chen, T.-J.; Yang, H.-Y.; Chen, Y.-C.; Huang, C.-K.; Chiu, T.-Y. Impact of team-based community healthcare on preventable hospitalisation: A population-based cohort study in Taiwan. BMJ Open 2021, 11, e039986. [Google Scholar] [CrossRef]
- Sulo, S.; Vargas, J.; Gomez, G.; Misas, J.D.; Serralde-Zúñiga, A.E.; Correia, M. Hospital nutrition care informs potential cost-savings for healthcare: A budget impact analysis. Clin. Nutr. ESPEN 2021, 42, 195–200. [Google Scholar] [CrossRef]
- Buitrago, G.; Vargas, J.; Sulo, S.; Partridge, J.S.; Guevara-Nieto, M.; Gomez, G.; Misas, J.D.; Correia, M. Targeting malnutrition: Nutrition programs yield cost savings for hospitalized patients. Clin. Nutr. 2020, 39, 2896–2901. [Google Scholar] [CrossRef]
- Lo, S.C.; Ma, K.S.; Li, Y.R.; Li, Z.Y.; Lin, C.H.; Lin, H.C.; Yang, S.F. Nutritional support for successful weaning in patients undergoing prolonged mechanical ventilation. Sci. Rep. 2022, 12, 12044. [Google Scholar] [CrossRef]
- Koekkoek, K.; van Zanten, A.R.H. Nutrition in the ICU: New trends versus old-fashioned standard enteral feeding? Curr. Opin. Anaesthesiol. 2018, 31, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Munk, T.; Svendsen, J.A.; Knudsen, A.W.; Østergaard, T.B.; Thomsen, T.; Olesen, S.S.; Rasmussen, H.H.; Beck, A.M. A multimodal nutritional intervention after discharge improves quality of life and physical function in older patients—A randomized controlled trial. Clin. Nutr. 2021, 40, 5500–5510. [Google Scholar] [CrossRef] [PubMed]
- Cramon, M.Ø.; Raben, I.; Beck, A.M.; Andersen, J.R. Individual nutritional intervention for prevention of readmission among geriatric patients—A randomized controlled pilot trial. Pilot Feasibility Stud. 2021, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- MacFarling Meure, C.; Steer, B.; Porter, J. Interrelationships between Dietary Outcomes, Readmission Rates and Length of Stay in Hospitalised Oncology Patients: A Scoping Review. Nutrients 2023, 15, 400. [Google Scholar] [CrossRef]
- Ridley, E.J.; Bailey, M.; Chapman, M.J.; Chapple, L.-a.S.; Deane, A.M.; Gojanovic, M.; Higgins, A.M.; Hodgson, C.L.; King, V.L.; Marshall, A.P.; et al. The impact of a tailored nutrition intervention delivered for the duration of hospitalisation on daily energy delivery for patients with critical illness (INTENT): A phase II randomised controlled trial. Crit. Care 2025, 29, 8. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.-C.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef]
- Baik, S.M.; Kim, M.; Lee, J.G. Comparison of Early Enteral Nutrition Versus Early Parenteral Nutrition in Critically Ill Patients: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 10. [Google Scholar] [CrossRef]
- Bels, J.L.M.; Thiessen, S.; van Gassel, R.J.J.; Beishuizen, A.; De Bie Dekker, A.; Fraipont, V.; Lamote, S.; Ledoux, D.; Scheeren, C.; De Waele, E.; et al. Effect of high versus standard protein provision on functional recovery in people with critical illness (PRECISe): An investigator-initiated, double-blinded, multicentre, parallel-group, randomised controlled trial in Belgium and the Netherlands. Lancet 2024, 404, 659–669. [Google Scholar] [CrossRef]
- Reignier, J.; Plantefeve, G.; Mira, J.P.; Argaud, L.; Asfar, P.; Aissaoui, N.; Badie, J.; Botoc, N.V.; Brisard, L.; Bui, H.N.; et al. Low versus standard calorie and protein feeding in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet. Respir. Med. 2023, 11, 602–612. [Google Scholar] [CrossRef]
- Heyland, D.K.; Patel, J.; Compher, C.; Rice, T.W.; Bear, D.E.; Lee, Z.Y.; González, V.C.; O’Reilly, K.; Regala, R.; Wedemire, C.; et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): An international, multicentre, pragmatic, registry-based randomised trial. Lancet 2023, 401, 568–576. [Google Scholar] [CrossRef]
- Summers, M.J.; Chapple, L.-a.S.; Karahalios, A.; Bellomo, R.; Chapman, M.J.; Ferrie, S.; Finnis, M.E.; French, C.; Hurford, S.; Kakho, N.; et al. Augmented Enteral Protein During Critical Illness: The TARGET Protein Randomized Clinical Trial. JAMA 2025, 334, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, I.; Yatabe, T.; Kuroiwa, H.; Tamura, T.; Yokoyama, M. Influence of nutrition support therapy on readmission among patients with acute heart failure in the intensive care unit: A single-center observational study. Clin. Nutr. 2020, 39, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.F.; Lucania, S.; Fadeur, M.; Verbrugge, A.M.; Cavalier, E.; Colson, C.; Misset, B. Adequacy of Nutritional Intakes during the Year after Critical Illness: An Observational Study in a Post-ICU Follow-Up Clinic. Nutrients 2022, 14, 3797. [Google Scholar] [CrossRef]
- Sharma, Y.; Miller, M.; Kaambwa, B.; Shahi, R.; Hakendorf, P.; Horwood, C.; Thompson, C. Malnutrition and its association with readmission and death within 7 days and 8-180 days postdischarge in older patients: A prospective observational study. BMJ Open 2017, 7, e018443. [Google Scholar] [CrossRef]

| Variable | SC (n = 1116) | DGIN (n = 1265) | p Value |
|---|---|---|---|
| Age, years | 66.0 ± 16.4 | 65.7 ± 16.6 | 0.629 |
| Sex, Male, n (%) | 432 (38.7) | 488 (38.6) | 0.947 |
| Admission type, n (%) | 0.247 | ||
| - Medical | 678 (60.8) | 739 (58.4) | |
| - Surgical | 438 (39.2) | 526 (41.6) | |
| Weight, kg | 60.9 ± 14.0 | 62.5 ± 15.3 | 0.021 |
| BMI, kg/m2 | 23.5 ± 6.7 | 24.3 ± 9.1 | 0.005 |
| APACHE II score | 18.3 ± 7.0 | 17.3 ± 7.3 | 0.004 |
| Energy at ICU admission, kcal/kg/day | 14.7 ± 11.1 | 13.4 ± 10.5 | 0.005 |
| Protein at ICU admission, g/kg/day | 0.6 ± 0.5 | 0.5 ± 0.4 | <0.001 |
| Laboratory Values | |||
| Albumin, g/dL | 3.1 ± 0.6 | 3.2 ± 0.7 | 0.001 |
| Prealbumin, mg/dL | 13.8 ± 7.2 | 13.3 ± 7.8 | 0.557 |
| Hemoglobin, g/dL | 10.1 ± 2.1 | 10.0 ± 2.0 | 0.620 |
| BUN, mg/dL | 36.9 ± 37.7 | 36.9 ± 36.1 | 0.749 |
| Creatinine, mg/dL | 1.9 ± 2.1 | 1.9 ± 2.2 | 0.818 |
| Potassium, mmol/L | 3.8 ± 0.8 | 3.9 ± 0.7 | 0.012 |
| Calcium, mg/dL | 8.2 ± 0.8 | 8.2 ± 0.8 | 0.642 |
| Magnesium, mg/dL | 2.1 ± 0.4 | 2.1 ± 0.4 | 0.558 |
| Phosphorus, mg/dL | 4.0 ± 1.9 | 4.1 ± 2.0 | 0.380 |
| C-reactive protein, mg/L | 6.2 ± 6.9 | 6.4 ± 7.1 | 0.781 |
| Outcome | SC | DGIN | Adjusted OR (95% CI) † | p Value |
|---|---|---|---|---|
| Panel A. Overall cohort | ||||
| In-hospital mortality, n (%) | 241/1116 (21.6) | 241/1265 (19.1) | aOR 0.99 (0.75–1.30) | 0.955 |
| 14-day readmission, n (%) | 90/752 (12.0) | 97/401 (24.2) | aOR 2.25 (1.52–3.32) | <0.001 |
| 30-day readmission, n (%) | 90/659 (13.7) | 97/302 (32.1) | aOR 3.32 (2.23–4.95) | <0.001 |
| ICU length of stay, days (mean ± SD) | 8.1 ± 6.7 | 7.1 ± 7.4 | — | <0.001 |
| Panel B. Propensity-matched cohort (1:1) | ||||
| In-hospital mortality, n (%) | 198/968 (20.5) | 199/968 (20.6) | aOR 1.05 (0.79–1.40) | 0.123 |
| 14-day readmission, n (%) | 73/664 (11.0) | 77/306 (25.2) | aOR 2.42 (1.61–3.63) | <0.001 |
| 30-day readmission, n (%) | 84/588 (14.3) | 70/229 (30.6) | aOR 3.05 (2.01–4.62) | <0.001 |
| ICU length of stay, days (mean ± SD) | 8.1 ± 6.8 | 7.4 ± 7.6 | — | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, S.-C.; Lin, H.-C.; Wang, Y.-H.; Chen, Y.-R.; Yang, S.-F. Impact of Dietitian-Guided Individualized Nutrition (DGIN) on ICU Outcomes in Critically Ill Patients: A Retrospective Cohort Study in Taiwan. Nutrients 2025, 17, 2995. https://doi.org/10.3390/nu17182995
Lo S-C, Lin H-C, Wang Y-H, Chen Y-R, Yang S-F. Impact of Dietitian-Guided Individualized Nutrition (DGIN) on ICU Outcomes in Critically Ill Patients: A Retrospective Cohort Study in Taiwan. Nutrients. 2025; 17(18):2995. https://doi.org/10.3390/nu17182995
Chicago/Turabian StyleLo, Shih-Ching, Hsing-Chun Lin, Yu-Hsun Wang, Ying-Ru Chen, and Shun-Fa Yang. 2025. "Impact of Dietitian-Guided Individualized Nutrition (DGIN) on ICU Outcomes in Critically Ill Patients: A Retrospective Cohort Study in Taiwan" Nutrients 17, no. 18: 2995. https://doi.org/10.3390/nu17182995
APA StyleLo, S.-C., Lin, H.-C., Wang, Y.-H., Chen, Y.-R., & Yang, S.-F. (2025). Impact of Dietitian-Guided Individualized Nutrition (DGIN) on ICU Outcomes in Critically Ill Patients: A Retrospective Cohort Study in Taiwan. Nutrients, 17(18), 2995. https://doi.org/10.3390/nu17182995









