Effects of Diet and Exercise on Mitochondrial Health in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Role of Ceramides
Abstract
1. Introduction
2. The Relationship Between Mitochondria and Ceramides
2.1. Ceramide Synthesis, Degradation, and Species
2.2. Normal Mitochondrial Function
2.3. Localization of Ceramides in the Mitochondria
2.4. The Role of Ceramides in Mitochondria
3. The Role of Ceramides and Mitochondria in MASLD
3.1. Pathogenesis of MASLD
3.2. FAO and ROS in MASLD
3.3. Biogenesis, Mitophagy, and Dynamics in MASLD
3.3.1. Ceramide Concentrations in MASLD
3.3.2. Ceramides and Insulin Resistance in MASLD
3.3.3. Ceramides That Increase Fatty Acid Availability
3.3.4. Ceramides and MASH
4. Diet Interventions in MASLD
4.1. Clinical Benefits of Dietary Treatment in MASLD
4.2. Energy Restriction and Macronutrient Composition
4.3. Mediterranean Diet
4.4. Unique Dietary Components and Strategies
5. Exercise
5.1. Clinical Benefits of Exercise in MASLD
5.2. Impacts of Exercise on Mitochondrial Function
5.2.1. FAO and ROS
5.2.2. Biogenesis and Mitophagy
5.3. Ceramides During Physical Activity
5.4. The Synergistic Roles of Diet and Exercise in the Clinical Treatment of MASLD
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and Nonalcoholic Fatty Liver Disease: Biochemical, Metabolic, and Clinical Implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Valvano, C.M.; De Nardo, W.; Watt, M.J. Integrative Metabolism in MASLD and MASH: Pathophysiology and Emerging Mechanisms. J. Hepatol. 2025, 83, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Fazel, Y.; Koenig, A.B.; Sayiner, M.; Goodman, Z.D.; Younossi, Z.M. Epidemiology and Natural History of Non-Alcoholic Fatty Liver Disease. Metabolism 2016, 65, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Ramanathan, R.; Ali, A.H.; Ibdah, J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 7280. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.; Lee, J.Y.; Kim, J.; Oh, C.-M. Mitochondrial Quality Control: Its Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Obes. Metab. Syndr. 2023, 32, 289–302. [Google Scholar] [CrossRef]
- Bionda, C.; Portoukalian, J.; Schmitt, D.; Rodriguez-Lafrasse, C.; Ardail, D. Subcellular Compartmentalization of Ceramide Metabolism: MAM (Mitochondria-Associated Membrane) and/or Mitochondria? Biochem. J. 2004, 382, 527–533. [Google Scholar] [CrossRef]
- Shimeno, H.; Soeda, S.; Sakamoto, M.; Kouchi, T.; Kowakame, T.; Kihara, T. Partial Purification and Characterization of Sphingosine N-Acyltransferase (Ceramide Synthase) from Bovine Liver Mitochondrion-Rich Fraction. Lipids 1998, 33, 601–605. [Google Scholar] [CrossRef]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and Other Sphingolipids as Drivers of Cardiovascular Disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Wolf, D. Skeletal Muscle Lipid Accumulation in Obesity, Insulin Resistance, and Type 2 Diabetes. Pediatr. Diabetes 2004, 5, 219–226. [Google Scholar] [CrossRef]
- Jiang, M.; Li, C.; Liu, Q.; Wang, A.; Lei, M. Inhibiting Ceramide Synthesis Attenuates Hepatic Steatosis and Fibrosis in Rats With Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2019, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Deevska, G.M.; Rozenova, K.A.; Giltiay, N.V.; Chambers, M.A.; White, J.; Boyanovsky, B.B.; Wei, J.; Daugherty, A.; Smart, E.J.; Reid, M.B.; et al. Acid Sphingomyelinase Deficiency Prevents Diet-Induced Hepatic Triacylglycerol Accumulation and Hyperglycemia in Mice. J. Biol. Chem. 2009, 284, 8359–8368. [Google Scholar] [CrossRef] [PubMed]
- Kasumov, T.; Li, L.; Li, M.; Gulshan, K.; Kirwan, J.P.; Liu, X.; Previs, S.; Willard, B.; Smith, J.D.; McCullough, A. Ceramide as a Mediator of Non-Alcoholic Fatty Liver Disease and Associated Atherosclerosis. PLoS ONE 2015, 10, e0126910. [Google Scholar] [CrossRef]
- Longato, L.; Tong, M.; Wands, J.R.; de la Monte, S.M. High Fat Diet Induced Hepatic Steatosis and Insulin Resistance: Role of Dysregulated Ceramide Metabolism. Hepatol. Res. 2012, 42, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S.; Brunner, B.; Bielohuby, M.; Hansen, G.; Pfenninger, A.; Wang, B.; Bruning, J.C.; Larsen, P.J.; Tennagels, N. The Role of C16:0 Ceramide in the Development of Obesity and Type 2 Diabetes: CerS6 Inhibition as a Novel Therapeutic Approach. Mol. Metab. 2019, 21, 36–50. [Google Scholar] [CrossRef]
- Yetukuri, L.; Katajamaa, M.; Medina-Gomez, G.; Seppänen-Laakso, T.; Vidal-Puig, A.; Orešič, M. Bioinformatics Strategies for Lipidomics Analysis: Characterization of Obesity Related Hepatic Steatosis. BMC Syst. Biol. 2007, 1, 12. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A Comprehensive Classification System for Lipids. Eur. J. Lipid Sci. Technol. 2005, 107, 337–364. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef]
- Mandon, E.C.; Ehses, I.; Rother, J.; van Echten, G.; Sandhoff, K. Subcellular Localization and Membrane Topology of Serine Palmitoyltransferase, 3-Dehydrosphinganine Reductase, and Sphinganine N-Acyltransferase in Mouse Liver. J. Biol. Chem. 1992, 267, 11144–11148. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive Sphingolipids: Metabolism and Function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Zelnik, I.D.; Rozman, B.; Rosenfeld-Gur, E.; Ben-Dor, S.; Futerman, A.H. A Stroll Down the CerS Lane. Adv. Exp. Med. Biol. 2019, 1159, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.M.; Li, Y.; Chaurasia, B.; Kaddai, V.A.; Summers, S.A. Dihydroceramides: From Bit Players to Lead Actors. J. Biol. Chem. 2015, 290, 15371–15379. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Kumagai, K.; Tomishige, N.; Kawano, M. CERT and Intracellular Trafficking of Ceramide. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2007, 1771, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Park, W.-J.; Song, J.-H.; Kim, G.-T.; Park, T.-S. Ceramide and Sphingosine 1-Phosphate in Liver Diseases. Mol. Cells 2020, 43, 419–430. [Google Scholar] [CrossRef]
- Hammerschmidt, P.; Brüning, J.C. Contribution of Specific Ceramides to Obesity-Associated Metabolic Diseases. Cell Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-Induced CerS6-Dependent C16:0 Ceramide Production Promotes Weight Gain and Glucose Intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a Ceramide Double Bond Improves Insulin Resistance and Hepatic Steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef]
- Zigdon, H.; Kogot-Levin, A.; Park, J.-W.; Goldschmidt, R.; Kelly, S.; Merrill, A.H.; Scherz, A.; Pewzner-Jung, Y.; Saada, A.; Futerman, A.H. Ablation of Ceramide Synthase 2 Causes Chronic Oxidative Stress Due to Disruption of the Mitochondrial Respiratory Chain. J. Biol. Chem. 2013, 288, 4947–4956. [Google Scholar] [CrossRef]
- Jensen, P.N.; Fretts, A.M.; Hoofnagle, A.N.; McKnight, B.; Howard, B.V.; Umans, J.G.; Sitlani, C.M.; Siscovick, D.S.; King, I.B.; Sotoodehnia, N.; et al. Circulating Ceramides and Sphingomyelins and the Risk of Incident Cardiovascular Disease among People with Diabetes: The Strong Heart Study. Cardiovasc. Diabetol. 2022, 21, 167. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Yu, C.; Hoofnagle, A.; Hari, N.; Jensen, P.N.; Fretts, A.M.; Umans, J.G.; Howard, B.V.; Sitlani, C.M.; Siscovick, D.S.; et al. Circulating Sphingolipids, Insulin, HOMA-IR, and HOMA-B: The Strong Heart Family Study. Diabetes 2018, 67, 1663–1672. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, W.-J.; Futerman, A.H. Ceramide Synthases as Potential Targets for Therapeutic Intervention in Human Diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Brown, S.H.J.; Lim, X.Y.; Fiveash, C.E.; Osborne, B.; Bentley, N.L.; Braude, J.P.; Mitchell, T.W.; Coster, A.C.F.; Don, A.S.; et al. Regulation of Glucose Homeostasis and Insulin Action by Ceramide Acyl-Chain Length: A Beneficial Role for Very Long-Chain Sphingolipid Species. Biochim. Biophys. Acta 2016, 1861, 1828–1839. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Aslanidis, C. Role of Lipids in Pathophysiology, Diagnosis and Therapy of Hepatocellular Carcinoma. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158658. [Google Scholar] [CrossRef] [PubMed]
- Kurz, J.; Parnham, M.J.; Geisslinger, G.; Schiffmann, S. Ceramides as Novel Disease Biomarkers. Trends Mol. Med. 2019, 25, 20–32. [Google Scholar] [CrossRef]
- Hilvo, M.; Vasile, V.C.; Donato, L.J.; Hurme, R.; Laaksonen, R. Ceramides and Ceramide Scores: Clinical Applications for Cardiometabolic Risk Stratification. Front. Endocrinol. 2020, 11, 570628. [Google Scholar] [CrossRef]
- Gaggini, M.; Pingitore, A.; Vassalle, C. Plasma Ceramides Pathophysiology, Measurements, Challenges, and Opportunities. Metabolites 2021, 11, 719. [Google Scholar] [CrossRef]
- Kasumov, T.; Solomon, T.P.J.; Hwang, C.; Huang, H.; Haus, J.M.; Zhang, R.; Kirwan, J.P. Improved Insulin Sensitivity after Exercise Training Is Linked to Reduced Plasma C14:0 Ceramide in Obesity and Type 2 Diabetes. Obesity 2015, 23, 1414–1421. [Google Scholar] [CrossRef]
- Scott, I.; Youle, R.J. Mitochondrial Fission and Fusion. Essays Biochem. 2010, 47, 85–98. [Google Scholar] [CrossRef]
- Ding, M.; Liu, C.; Shi, R.; Yu, M.; Zeng, K.; Kang, J.; Fu, F.; Mi, M. Mitochondrial Fusion Promoter Restores Mitochondrial Dynamics Balance and Ameliorates Diabetic Cardiomyopathy in an Optic Atrophy 1-Dependent Way. Acta Physiol. 2020, 229, e13428. [Google Scholar] [CrossRef]
- Jang, S.; Javadov, S. OPA1 Regulates Respiratory Supercomplexes Assembly: The Role of Mitochondrial Swelling. Mitochondrion 2020, 51, 30–39. [Google Scholar] [CrossRef]
- Cerveny, K.L.; Tamura, Y.; Zhang, Z.; Jensen, R.E.; Sesaki, H. Regulation of Mitochondrial Fusion and Division. Trends Cell Biol. 2007, 17, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Sumneang, N.; Siri-Angkul, N.; Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. The Effects of Iron Overload on Mitochondrial Function, Mitochondrial Dynamics, and Ferroptosis in Cardiomyocytes. Arch. Biochem. Biophys. 2020, 680, 108241. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ning, J.; Feng, N.; Li, Z.; Liu, Z.; Wang, Y.; Wang, Y.; Li, X.; Huo, C.; Jia, X.; et al. Dynamin-Related Protein 1-Mediated Mitochondrial Fission Contributes to Post-Traumatic Cardiac Dysfunction in Rats and the Protective Effect of Melatonin. J. Pineal Res. 2018, 64, e12447. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; van der Bliek, A.M. Dynamin-Related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Mol. Biol. Cell 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Elgass, K.; Pakay, J.; Ryan, M.T.; Palmer, C.S. Recent Advances into the Understanding of Mitochondrial Fission. Biochim. Biophys. Acta 2013, 1833, 150–161. [Google Scholar] [CrossRef]
- Horbay, R.; Bilyy, R. Mitochondrial Dynamics during Cell Cycling. Apoptosis 2016, 21, 1327–1335. [Google Scholar] [CrossRef]
- Feng, S.-T.; Wang, Z.-Z.; Yuan, Y.-H.; Wang, X.-L.; Sun, H.-M.; Chen, N.-H.; Zhang, Y. Dynamin-Related Protein 1: A Protein Critical for Mitochondrial Fission, Mitophagy, and Neuronal Death in Parkinson’s Disease. Pharmacol. Res. 2020, 151, 104553. [Google Scholar] [CrossRef]
- Ding, W.-X.; Yin, X.-M. Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 2012, 393, 547–564. [Google Scholar] [CrossRef]
- Willems, P.H.G.M.; Rossignol, R.; Dieteren, C.E.J.; Murphy, M.P.; Koopman, W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin Is Recruited Selectively to Impaired Mitochondria and Promotes Their Autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, K.Z.Q.; Chu, C.T. After the Banquet: Mitochondrial Biogenesis, Mitophagy, and Cell Survival. Autophagy 2013, 9, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Di, W.; Lv, J.; Jiang, S.; Lu, C.; Yang, Z.; Ma, Z.; Hu, W.; Yang, Y.; Xu, B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr. Issues Mol. Biol. 2018, 28, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Edgett, B.A.; Gurd, B.J. Coordination of Mitochondrial Biogenesis by PGC-1α in Human Skeletal Muscle: A Re-Evaluation. Metabolism 2018, 79, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of Mitochondrial Biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef]
- Barshad, G.; Marom, S.; Cohen, T.; Mishmar, D. Mitochondrial DNA Transcription and Its Regulation: An Evolutionary Perspective. Trends Genet. 2018, 34, 682–692. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Huss, J.M.; Torra, I.P.; Staels, B.; Giguère, V.; Kelly, D.P. Estrogen-Related Receptor Alpha Directs Peroxisome Proliferator-Activated Receptor Alpha Signaling in the Transcriptional Control of Energy Metabolism in Cardiac and Skeletal Muscle. Mol. Cell Biol. 2004, 24, 9079–9091. [Google Scholar] [CrossRef]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a New Target of PGC-1alpha, Plays an Important Role in the Suppression of ROS and Mitochondrial Biogenesis. PLoS ONE 2010, 5, e11707. [Google Scholar] [CrossRef]
- Hirschey, M.D.; Shimazu, T.; Goetzman, E.; Jing, E.; Schwer, B.; Lombard, D.B.; Grueter, C.A.; Harris, C.; Biddinger, S.; Ilkayeva, O.R.; et al. SIRT3 Regulates Mitochondrial Fatty-Acid Oxidation by Reversible Enzyme Deacetylation. Nature 2010, 464, 121–125. [Google Scholar] [CrossRef]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A Role for the Mitochondrial Deacetylase Sirt3 in Regulating Energy Homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef]
- Someya, S.; Yu, W.; Hallows, W.C.; Xu, J.; Vann, J.M.; Leeuwenburgh, C.; Tanokura, M.; Denu, J.M.; Prolla, T.A. Sirt3 Mediates Reduction of Oxidative Damage and Prevention of Age-Related Hearing Loss under Caloric Restriction. Cell 2010, 143, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Birkenfeld, A.L.; Lee, H.-Y.; Guebre-Egziabher, F.; Alves, T.C.; Jurczak, M.J.; Jornayvaz, F.R.; Zhang, D.; Hsiao, J.J.; Martin-Montalvo, A.; Fischer-Rosinsky, A.; et al. Deletion of the Mammalian INDY Homolog Mimics Aspects of Dietary Restriction and Protects against Adiposity and Insulin Resistance in Mice. Cell Metab. 2011, 14, 184–195. [Google Scholar] [CrossRef] [PubMed]
- El Bawab, S.; Roddy, P.; Qian, T.; Bielawska, A.; Lemasters, J.J.; Hannun, Y.A. Molecular Cloning and Characterization of a Human Mitochondrial Ceramidase *. J. Biol. Chem. 2000, 275, 21508–21513. [Google Scholar] [CrossRef] [PubMed]
- Ardail, D.; Popa, I.; Alcantara, K.; Pons, A.; Zanetta, J.P.; Louisot, P.; Thomas, L.; Portoukalian, J. Occurrence of Ceramides and Neutral Glycolipids with Unusual Long-Chain Base Composition in Purified Rat Liver Mitochondria. FEBS Lett. 2001, 488, 160–164. [Google Scholar] [CrossRef]
- Babiychuk, E.B.; Atanassoff, A.P.; Monastyrskaya, K.; Brandenberger, C.; Studer, D.; Allemann, C.; Draeger, A. The Targeting of Plasmalemmal Ceramide to Mitochondria during Apoptosis. PLoS ONE 2011, 6, e23706. [Google Scholar] [CrossRef]
- Crompton, M. The Mitochondrial Permeability Transition Pore and Its Role in Cell Death. Biochem. J. 1999, 341 Pt 2, 233–249. [Google Scholar] [CrossRef]
- Susin, S.A.; Zamzami, N.; Kroemer, G. Mitochondria as Regulators of Apoptosis: Doubt No More. Biochim. Biophys. Acta 1998, 1366, 151–165. [Google Scholar] [CrossRef]
- Vance, J.E. Phospholipid Synthesis in a Membrane Fraction Associated with Mitochondria. J. Biol. Chem. 1990, 265, 7248–7256. [Google Scholar] [CrossRef]
- Merrill, A.H. De Novo Sphingolipid Biosynthesis: A Necessary, but Dangerous, Pathway. J. Biol. Chem. 2002, 277, 25843–25846. [Google Scholar] [CrossRef]
- Marsh, B.J.; Mastronarde, D.N.; Buttle, K.F.; Howell, K.E.; McIntosh, J.R. Organellar Relationships in the Golgi Region of the Pancreatic Beta Cell Line, HIT-T15, Visualized by High Resolution Electron Tomography. Proc. Natl. Acad. Sci. USA 2001, 98, 2399–2406. [Google Scholar] [CrossRef]
- Ardail, D.; Gasnier, F.; Lermé, F.; Simonot, C.; Louisot, P.; Gateau-Roesch, O. Involvement of Mitochondrial Contact Sites in the Subcellular Compartmentalization of Phospholipid Biosynthetic Enzymes. J. Biol. Chem. 1993, 268, 25985–25992. [Google Scholar] [CrossRef]
- El Bawab, S.; Birbes, H.; Roddy, P.; Szulc, Z.M.; Bielawska, A.; Hannun, Y.A. Biochemical Characterization of the Reverse Activity of Rat Brain Ceramidase. A CoA-Independent and Fumonisin B1-Insensitive Ceramide Synthase. J. Biol. Chem. 2001, 276, 16758–16766. [Google Scholar] [CrossRef]
- Novgorodov, S.A.; Wu, B.X.; Gudz, T.I.; Bielawski, J.; Ovchinnikova, T.V.; Hannun, Y.A.; Obeid, L.M. Novel Pathway of Ceramide Production in Mitochondria: Thioesterase and Neutral Ceramidase Produce Ceramide from Sphingosine and Acyl-CoA. J. Biol. Chem. 2011, 286, 25352–25362. [Google Scholar] [CrossRef]
- Birbes, H.; Luberto, C.; Hsu, Y.-T.; El Bawab, S.; Hannun, Y.A.; Obeid, L.M. A Mitochondrial Pool of Sphingomyelin Is Involved in TNFα-Induced Bax Translocation to Mitochondria. Biochem. J. 2005, 386, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Gudz, T.I.; Tserng, K.Y.; Hoppel, C.L. Direct Inhibition of Mitochondrial Respiratory Chain Complex III by Cell-Permeable Ceramide. J. Biol. Chem. 1997, 272, 24154–24158. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, M.; Cocco, T.; Lorusso, M. Ceramide Interaction with the Respiratory Chain of Heart Mitochondria. Biochemistry 2000, 39, 6660–6668. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, C.; Colell, A.; Marí, M.; Morales, A.; Fernández-Checa, J.C. Direct Effect of Ceramide on the Mitochondrial Electron Transport Chain Leads to Generation of Reactive Oxygen Species. Role of Mitochondrial Glutathione. J. Biol. Chem. 1997, 272, 11369–11377. [Google Scholar] [CrossRef]
- Mullen, T.D.; Obeid, L.M. Ceramide and Apoptosis: Exploring the Enigmatic Connections between Sphingolipid Metabolism and Programmed Cell Death. Anti-Cancer Agents Med. Chem. 2012, 12, 340–363. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yu, J.Y.; Yin, D.; Patwardhan, G.A.; Gupta, V.; Hirabayashi, Y.; Holleran, W.M.; Giuliano, A.E.; Jazwinski, S.M.; Gouaze-Andersson, V.; et al. A Role for Ceramide in Driving Cancer Cell Resistance to Doxorubicin. FASEB J. 2008, 22, 2541–2551. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Pilátová, M.B.; Solárová, Z.; Mezencev, R.; Solár, P. Ceramides and Their Roles in Programmed Cell Death. Adv. Med. Sci. 2023, 68, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Draeger, A. Fluorescent Annexin A1 Reveals Dynamics of Ceramide Platforms in Living Cells. Traffic 2008, 9, 1757–1775. [Google Scholar] [CrossRef] [PubMed]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed Cell Death Induced by Ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as Multifaceted Regulators of Cell Death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Ganesan, V.; Perera, M.N.; Colombini, D.; Datskovskiy, D.; Chadha, K.; Colombini, M. Ceramide and Activated Bax Act Synergistically to Permeabilize the Mitochondrial Outer Membrane. Apoptosis 2010, 15, 553–562. [Google Scholar] [CrossRef]
- von Haefen, C.; Wieder, T.; Gillissen, B.; Stärck, L.; Graupner, V.; Dörken, B.; Daniel, P.T. Ceramide Induces Mitochondrial Activation and Apoptosis via a Bax-Dependent Pathway in Human Carcinoma Cells. Oncogene 2002, 21, 4009–4019. [Google Scholar] [CrossRef]
- Lee, H.; Rotolo, J.A.; Mesicek, J.; Penate-Medina, T.; Rimner, A.; Liao, W.-C.; Yin, X.; Ragupathi, G.; Ehleiter, D.; Gulbins, E.; et al. Mitochondrial Ceramide-Rich Macrodomains Functionalize Bax upon Irradiation. PLoS ONE 2011, 6, e19783. [Google Scholar] [CrossRef]
- Hou, Q.; Jin, J.; Zhou, H.; Novgorodov, S.A.; Bielawska, A.; Szulc, Z.M.; Hannun, Y.A.; Obeid, L.M.; Hsu, Y.-T. Mitochondrially Targeted Ceramides Preferentially Promote Autophagy, Retard Cell Growth, and Induce Apoptosis. J. Lipid Res. 2011, 52, 278–288. [Google Scholar] [CrossRef]
- Dadsena, S.; Bockelmann, S.; Mina, J.G.M.; Hassan, D.G.; Korneev, S.; Razzera, G.; Jahn, H.; Niekamp, P.; Müller, D.; Schneider, M.; et al. Ceramides Bind VDAC2 to Trigger Mitochondrial Apoptosis. Nat. Commun. 2019, 10, 1832. [Google Scholar] [CrossRef]
- Greggio, C.; Jha, P.; Kulkarni, S.S.; Lagarrigue, S.; Broskey, N.T.; Boutant, M.; Wang, X.; Conde Alonso, S.; Ofori, E.; Auwerx, J.; et al. Enhanced Respiratory Chain Supercomplex Formation in Response to Exercise in Human Skeletal Muscle. Cell Metab. 2017, 25, 301–311. [Google Scholar] [CrossRef]
- Ojuka, E.O.; Jones, T.E.; Han, D.-H.; Chen, M.; Holloszy, J.O. Raising Ca2+ in L6 Myotubes Mimics Effects of Exercise on Mitochondrial Biogenesis in Muscle. FASEB J. 2003, 17, 675–681. [Google Scholar] [CrossRef]
- Roberts, F.L.; Markby, G.R. New Insights into Molecular Mechanisms Mediating Adaptation to Exercise; A Review Focusing on Mitochondrial Biogenesis, Mitochondrial Function, Mitophagy and Autophagy. Cells 2021, 10, 2639. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Li, G.; Fu, T.; Zhang, T.; Lu, X.; Li, N.; Geng, Q. Ceramides and Mitochondrial Homeostasis. Cell. Signal. 2024, 117, 111099. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Dulovic-Mahlow, M.; Mandik, F.; Frese, L.; Kanana, Y.; Haissatou Diaw, S.; Depperschmidt, J.; Böhm, C.; Rohr, J.; Lohnau, T.; et al. Ceramide Accumulation Induces Mitophagy and Impairs β-Oxidation in PINK1 Deficiency. Proc. Natl. Acad. Sci. USA 2021, 118, e2025347118. [Google Scholar] [CrossRef]
- Gaudioso, A.; Garcia-Rozas, P.; Casarejos, M.J.; Pastor, O.; Rodriguez-Navarro, J.A. Lipidomic Alterations in the Mitochondria of Aged Parkin Null Mice Relevant to Autophagy. Front. Neurosci. 2019, 13, 329. [Google Scholar] [CrossRef]
- Sentelle, R.D.; Senkal, C.E.; Jiang, W.; Ponnusamy, S.; Gencer, S.; Selvam, S.P.; Ramshesh, V.K.; Peterson, Y.K.; Lemasters, J.J.; Szulc, Z.M.; et al. Ceramide Targets Autophagosomes to Mitochondria and Induces Lethal Mitophagy. Nat. Chem. Biol. 2012, 8, 831–838. [Google Scholar] [CrossRef]
- Twig, G.; Elorza, A.; Molina, A.J.A.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and Selective Fusion Govern Mitochondrial Segregation and Elimination by Autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef]
- Mattenberger, Y.; James, D.I.; Martinou, J.C. Fusion of Mitochondria in Mammalian Cells Is Dependent on the Mitochondrial Inner Membrane Potential and Independent of Microtubules or Actin. FEBS Lett. 2003, 538, 53–59. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Tabassum, G.; Dohare, R.; Syed, M.A. miR-495–3p Regulates Sphingolipid Metabolic Reprogramming to Induce Sphk1/Ceramide Mediated Mitophagy and Apoptosis in NSCLC. Free Radic. Biol. Med. 2022, 189, 71–84. [Google Scholar] [CrossRef]
- Mendham, A.E.; Goedecke, J.H.; Zeng, Y.; Larsen, S.; George, C.; Hauksson, J.; Fortuin-de Smidt, M.C.; Chibalin, A.V.; Olsson, T.; Chorell, E. Exercise Training Improves Mitochondrial Respiration and Is Associated with an Altered Intramuscular Phospholipid Signature in Women with Obesity. Diabetologia 2021, 64, 1642–1659. [Google Scholar] [CrossRef]
- Jamil, M.; Cowart, L.A. Sphingolipids in Mitochondria—From Function to Disease. Front. Cell Dev. Biol. 2023, 11, 1302472. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Villamor, E.; Perez-Vizcaino, F.; Moreno, L. Ceramide and Regulation of Vascular Tone. Int. J. Mol. Sci. 2019, 20, 411. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Lu, Y.; Xu, M. Acid Sphingomyelinase Downregulation Alleviates Vascular Endothelial Leptin Resistance in Rats. Acta Pharmacol. Sin. 2020, 41, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ye, C.; Cheng, Q.; Zhang, X.; Yao, L.; Li, Q.; Huang, J.; Liu, Y.; Zou, Z.; Wang, H.; et al. Macrophage Raptor Deficiency-Induced Lysosome Dysfunction Exacerbates Nonalcoholic Steatohepatitis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 211–231. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in Nonalcoholic Fatty Liver Disease: Revisited After a Decade. Hepatology 2021, 73, 833–842. [Google Scholar] [CrossRef]
- Eccleston, H.B.; Andringa, K.K.; Betancourt, A.M.; King, A.L.; Mantena, S.K.; Swain, T.M.; Tinsley, H.N.; Nolte, R.N.; Nagy, T.R.; Abrams, G.A.; et al. Chronic Exposure to a High-Fat Diet Induces Hepatic Steatosis, Impairs Nitric Oxide Bioavailability, and Modifies the Mitochondrial Proteome in Mice. Antioxid. Redox Signal 2011, 15, 447–459. [Google Scholar] [CrossRef]
- Ooi, G.J.; Meikle, P.J.; Huynh, K.; Earnest, A.; Roberts, S.K.; Kemp, W.; Parker, B.L.; Brown, W.; Burton, P.; Watt, M.J. Hepatic Lipidomic Remodeling in Severe Obesity Manifests with Steatosis and Does Not Evolve with Non-Alcoholic Steatohepatitis. J. Hepatol. 2021, 75, 524–535. [Google Scholar] [CrossRef]
- Mayo, R.; Crespo, J.; Martínez-Arranz, I.; Banales, J.M.; Arias, M.; Mincholé, I.; de la Fuente, R.A.; Jimenez-Agüero, R.; Alonso, C.; de Luis, D.A.; et al. Metabolomic-Based Noninvasive Serum Test to Diagnose Nonalcoholic Steatohepatitis: Results from Discovery and Validation Cohorts. Hepatol. Commun. 2018, 2, 807–820. [Google Scholar] [CrossRef]
- Puri, P.; Baillie, R.A.; Wiest, M.M.; Mirshahi, F.; Choudhury, J.; Cheung, O.; Sargeant, C.; Contos, M.J.; Sanyal, A.J. A Lipidomic Analysis of Nonalcoholic Fatty Liver Disease. Hepatology 2007, 46, 1081–1090. [Google Scholar] [CrossRef]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.-K.; et al. The Presence and Severity of Nonalcoholic Steatohepatitis Is Associated with Specific Changes in Circulating Bile Acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of Fatty Acids Stored in Liver and Secreted via Lipoproteins in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.W. Lipid Metabolism and Liver Inflammation. I. Hepatic Fatty Acid Uptake: Possible Role in Steatosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G194–G198. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, K.M.; Kahn, S.E. Review: The Role of Insulin Resistance in Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2006, 91, 4753–4761. [Google Scholar] [CrossRef]
- Hebbard, L.; George, J. Animal Models of Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 35–44. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Cusi, K. Role of Insulin Resistance and Lipotoxicity in Non-Alcoholic Steatohepatitis. Clin. Liver Dis. 2009, 13, 545–563. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Oxidative Stress, Cardiolipin and Mitochondrial Dysfunction in Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 14205–14218. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Pistolese, M.; Ruggiero, F.M. Reactive Oxygen Species Affect Mitochondrial Electron Transport Complex I Activity through Oxidative Cardiolipin Damage. Gene 2002, 286, 135–141. [Google Scholar] [CrossRef]
- Namachivayam, A.; Valsala Gopalakrishnan, A. A Review on Molecular Mechanism of Alcoholic Liver Disease. Life Sci. 2021, 274, 119328. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Thematic Review Series: Adipocyte Biology. Adipocyte Stress: The Endoplasmic Reticulum and Metabolic Disease. J. Lipid Res. 2007, 48, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Marsano, L.S.; McClain, C.J. Gut-Liver Axis, Nutrition, and Non-Alcoholic Fatty Liver Disease. Clin. Biochem. 2015, 48, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Namjou, B.; Lingren, T.; Huang, Y.; Parameswaran, S.; Cobb, B.L.; Stanaway, I.B.; Connolly, J.J.; Mentch, F.D.; Benoit, B.; Niu, X.; et al. GWAS and Enrichment Analyses of Non-Alcoholic Fatty Liver Disease Identify New Trait-Associated Genes and Pathways across eMERGE Network. BMC Med. 2019, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Schwimmer, J.B.; Celedon, M.A.; Lavine, J.E.; Salem, R.; Campbell, N.; Schork, N.J.; Shiehmorteza, M.; Yokoo, T.; Chavez, A.; Middleton, M.S.; et al. Heritability of Nonalcoholic Fatty Liver Disease. Gastroenterology 2009, 136, 1585–1592. [Google Scholar] [CrossRef]
- Cherubini, A.; Casirati, E.; Tomasi, M.; Valenti, L. PNPLA3 as a Therapeutic Target for Fatty Liver Disease: The Evidence to Date. Expert. Opin. Ther. Targets 2021, 25, 1033–1043. [Google Scholar] [CrossRef]
- Morio, B.; Panthu, B.; Bassot, A.; Rieusset, J. Role of Mitochondria in Liver Metabolic Health and Diseases. Cell Calcium 2021, 94, 102336. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased De Novo Lipogenesis Is a Distinct Characteristic of Individuals With Nonalcoholic Fatty Liver Disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and Lipogenesis in the Liver: An Update1. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef]
- Sanders, F.W.B.; Acharjee, A.; Walker, C.; Marney, L.; Roberts, L.D.; Imamura, F.; Jenkins, B.; Case, J.; Ray, S.; Virtue, S.; et al. Hepatic Steatosis Risk Is Partly Driven by Increased de Novo Lipogenesis Following Carbohydrate Consumption. Genome Biol. 2018, 19, 79. [Google Scholar] [CrossRef]
- Rohrbach, T.; Maceyka, M.; Spiegel, S. Sphingosine Kinase and Sphingosine-1-Phosphate in Liver Pathobiology. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 543–553. [Google Scholar] [CrossRef]
- Rector, R.S.; Thyfault, J.P.; Uptergrove, G.M.; Morris, E.M.; Naples, S.P.; Borengasser, S.J.; Mikus, C.R.; Laye, M.J.; Laughlin, M.H.; Booth, F.W.; et al. Mitochondrial Dysfunction Precedes Insulin Resistance and Hepatic Steatosis and Contributes to the Natural History of Non-Alcoholic Fatty Liver Disease in an Obese Rodent Model. J. Hepatol. 2010, 52, 727–736. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Rector, R.S.; Uptergrove, G.M.; Borengasser, S.J.; Morris, E.M.; Wei, Y.; Laye, M.J.; Burant, C.F.; Qi, N.R.; Ridenhour, S.E.; et al. Rats Selectively Bred for Low Aerobic Capacity Have Reduced Hepatic Mitochondrial Oxidative Capacity and Susceptibility to Hepatic Steatosis and Injury. J. Physiol. 2009, 587, 1805–1816. [Google Scholar] [CrossRef]
- Koliaki, C.; Szendroedi, J.; Kaul, K.; Jelenik, T.; Nowotny, P.; Jankowiak, F.; Herder, C.; Carstensen, M.; Krausch, M.; Knoefel, W.T.; et al. Adaptation of Hepatic Mitochondrial Function in Humans with Non-Alcoholic Fatty Liver Is Lost in Steatohepatitis. Cell Metab. 2015, 21, 739–746. [Google Scholar] [CrossRef]
- Ibdah, J.A.; Perlegas, P.; Zhao, Y.; Angdisen, J.; Borgerink, H.; Shadoan, M.K.; Wagner, J.D.; Matern, D.; Rinaldo, P.; Cline, J.M. Mice Heterozygous for a Defect in Mitochondrial Trifunctional Protein Develop Hepatic Steatosis and Insulin Resistance. Gastroenterology 2005, 128, 1381–1390. [Google Scholar] [CrossRef]
- Rector, R.S.; Morris, E.M.; Ridenhour, S.; Meers, G.M.; Hsu, F.-F.; Turk, J.; Ibdah, J.A. Selective Hepatic Insulin Resistance in a Murine Model Heterozygous for a Mitochondrial Trifunctional Protein Defect. Hepatology 2013, 57, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.P.; Cunningham, R.P.; Meers, G.M.; Johnson, S.A.; Wheeler, A.A.; Ganga, R.R.; Spencer, N.M.; Pitt, J.B.; Diaz-Arias, A.; Swi, A.I.A.; et al. Compromised Hepatic Mitochondrial Fatty Acid Oxidation and Reduced Markers of Mitochondrial Turnover in Human NAFLD. Hepatology 2022, 76, 1452–1465. [Google Scholar] [CrossRef]
- Dornas, W.; Schuppan, D. Mitochondrial Oxidative Injury: A Key Player in Nonalcoholic Fatty Liver Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G400–G411. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Abdelmegeed, M.A.; Banerjee, A.; Yoo, S.-H.; Jang, S.; Gonzalez, F.J.; Song, B.-J. Critical Role of Cytochrome P450 2E1 (CYP2E1) in the Development of High Fat-Induced Non-Alcoholic Steatohepatitis. J. Hepatol. 2012, 57, 860–866. [Google Scholar] [CrossRef]
- Pérez-Carreras, M.; Del Hoyo, P.; Martín, M.A.; Rubio, J.C.; Martín, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective Hepatic Mitochondrial Respiratory Chain in Patients with Nonalcoholic Steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Campbell-Sargent, C.; Mirshahi, F.; Rizzo, W.B.; Contos, M.J.; Sterling, R.K.; Luketic, V.A.; Shiffman, M.L.; Clore, J.N. Nonalcoholic Steatohepatitis: Association of Insulin Resistance and Mitochondrial Abnormalities. Gastroenterology 2001, 120, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Shokolenko, I.; Venediktova, N.; Bochkareva, A.; Wilson, G.L.; Alexeyev, M.F. Oxidative Stress Induces Degradation of Mitochondrial DNA. Nucleic Acids Res. 2009, 37, 2539–2548. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.; Alhasawi, A.; Contavadoo, M.; Appanna, V.D. Dysfunctional Mitochondrial Bioenergetics and the Pathogenesis of Hepatic Disorders. Front. Cell Dev. Biol. 2015, 3, 40. [Google Scholar] [CrossRef]
- Malik, A.N.; Simões, I.C.M.; Rosa, H.S.; Khan, S.; Karkucinska-Wieckowska, A.; Wieckowski, M.R. A Diet Induced Maladaptive Increase in Hepatic Mitochondrial DNA Precedes OXPHOS Defects and May Contribute to Non-Alcoholic Fatty Liver Disease. Cells 2019, 8, 1222. [Google Scholar] [CrossRef]
- Pessayre, D. Role of Mitochondria in Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. 1), S20–S27. [Google Scholar] [CrossRef]
- Pessayre, D.; Mansouri, A.; Fromenty, B. Nonalcoholic Steatosis and Steatohepatitis. V. Mitochondrial Dysfunction in Steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G193–G199. [Google Scholar] [CrossRef]
- Feldstein, A.E.; Werneburg, N.W.; Canbay, A.; Guicciardi, M.E.; Bronk, S.F.; Rydzewski, R.; Burgart, L.J.; Gores, G.J. Free Fatty Acids Promote Hepatic Lipotoxicity by Stimulating TNF-Alpha Expression via a Lysosomal Pathway. Hepatology 2004, 40, 185–194. [Google Scholar] [CrossRef]
- Feldstein, A.E. Novel Insights into the Pathophysiology of Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2010, 30, 391–401. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Doll, D.N.; Rellick, S.L.; Barr, T.L.; Ren, X.; Simpkins, J.W. Rapid Mitochondrial Dysfunction Mediates TNF-Alpha-Induced Neurotoxicity. J. Neurochem. 2015, 132, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Mihm, S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int. J. Mol. Sci. 2018, 19, 3104. [Google Scholar] [CrossRef] [PubMed]
- Handa, P.; Vemulakonda, A.; Kowdley, K.V.; Uribe, M.; Méndez-Sánchez, N. Mitochondrial DNA from Hepatocytes as a Ligand for TLR9: Drivers of Nonalcoholic Steatohepatitis? World J. Gastroenterol. 2016, 22, 6965–6971. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte Mitochondrial DNA Drives Nonalcoholic Steatohepatitis by Activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of Nonalcoholic Fatty Liver Disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Taghizadeh, M.; Khalese-Ranjbar, B.; Hamedi-Shahraki, S.; Asghari, S. The Clinical Value of Serum Sirtuin-1 Concentration in the Diagnosis of Metabolic Dysfunction-Associated Steatotic Liver Disease. BMC Gastroenterol. 2025, 25, 27. [Google Scholar] [CrossRef]
- Nassir, F.; Ibdah, J.A. Sirtuins and Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2016, 22, 10084–10092. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid. Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef]
- Nassir, F.; Arndt, J.J.; Johnson, S.A.; Ibdah, J.A. Regulation of Mitochondrial Trifunctional Protein Modulates Nonalcoholic Fatty Liver Disease in Mice. J. Lipid Res. 2018, 59, 967–973. [Google Scholar] [CrossRef]
- Krishnasamy, Y.; Gooz, M.; Li, L.; Lemasters, J.J.; Zhong, Z. Role of Mitochondrial Depolarization and Disrupted Mitochondrial Homeostasis in Non-Alcoholic Steatohepatitis and Fibrosis in Mice. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 190–204. [Google Scholar]
- Hernández-Alvarez, M.I.; Sebastián, D.; Vives, S.; Ivanova, S.; Bartoccioni, P.; Kakimoto, P.; Plana, N.; Veiga, S.R.; Hernández, V.; Vasconcelos, N.; et al. Deficient Endoplasmic Reticulum-Mitochondrial Phosphatidylserine Transfer Causes Liver Disease. Cell 2019, 177, 881–895.e17. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Zhang, W.; Beaton, M.; Marsboom, G.; Gruber, M.; Simon, M.C.; Hart, J.; Dorn, G.W.; Brady, M.J.; Macleod, K.F. BNip3 Regulates Mitochondrial Function and Lipid Metabolism in the Liver. Mol. Cell Biol. 2012, 32, 2570–2584. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.O.; Passos, E.; Diogo, C.V.; Rocha-Rodrigues, S.; Santos-Alves, E.; Oliveira, P.J.; Ascensão, A.; Magalhães, J. Exercise Mitigates Mitochondrial Permeability Transition Pore and Quality Control Mechanisms Alterations in Nonalcoholic Steatohepatitis. Appl. Physiol. Nutr. Metab. 2016, 41, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Su, X.; Bradley, D.; Fabbrini, E.; Conte, C.; Eagon, J.C.; Varela, J.E.; Brunt, E.M.; Patterson, B.W.; Klein, S. Intrahepatic Diacylglycerol Content Is Associated with Hepatic Insulin Resistance in Obese Subjects. Gastroenterology 2012, 142, 1444–1446.e2. [Google Scholar] [CrossRef]
- Kotronen, A.; Seppänen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepää, A.-L.; Oresic, M.; Yki-Järvinen, H. Hepatic Stearoyl-CoA Desaturase (SCD)-1 Activity and Diacylglycerol but Not Ceramide Concentrations Are Increased in the Nonalcoholic Human Fatty Liver. Diabetes 2009, 58, 203–208. [Google Scholar] [CrossRef]
- Kumashiro, N.; Erion, D.M.; Zhang, D.; Kahn, M.; Beddow, S.A.; Chu, X.; Still, C.D.; Gerhard, G.S.; Han, X.; Dziura, J.; et al. Cellular Mechanism of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2011, 108, 16381–16385. [Google Scholar] [CrossRef]
- Watt, M.J.; Barnett, A.C.; Bruce, C.R.; Schenk, S.; Horowitz, J.F.; Hoy, A.J. Regulation of Plasma Ceramide Levels with Fatty Acid Oversupply: Evidence That the Liver Detects and Secretes de Novo Synthesised Ceramide. Diabetologia 2012, 55, 2741–2746. [Google Scholar] [CrossRef]
- Senkal, C.E.; Salama, M.F.; Snider, A.J.; Allopenna, J.J.; Rana, N.A.; Koller, A.; Hannun, Y.A.; Obeid, L.M. Ceramide Is Metabolized to Acylceramide and Stored in Lipid Droplets. Cell Metab. 2017, 25, 686–697. [Google Scholar] [CrossRef]
- Hajduch, E.; Lachkar, F.; Ferré, P.; Foufelle, F. Roles of Ceramides in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 792. [Google Scholar] [CrossRef]
- Lyn-Cook, L.E.; Lawton, M.; Tong, M.; Silbermann, E.; Longato, L.; Jiao, P.; Mark, P.; Wands, J.R.; Xu, H.; de la Monte, S.M. Hepatic Ceramide May Mediate Brain Insulin Resistance and Neurodegeneration in Type 2 Diabetes and Non-Alcoholic Steatohepatitis. J. Alzheimers Dis. 2009, 16, 715–729. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Mato, J.M.; Vance, D.; Kaplowitz, N.; Fernández-Checa, J.C. Acid Sphingomyelinase-Ceramide System in Steatohepatitis: A Novel Target Regulating Multiple Pathways. J. Hepatol. 2015, 62, 219–233. [Google Scholar] [CrossRef]
- Gorden, D.L.; Myers, D.S.; Ivanova, P.T.; Fahy, E.; Maurya, M.R.; Gupta, S.; Min, J.; Spann, N.J.; McDonald, J.G.; Kelly, S.L.; et al. Biomarkers of NAFLD Progression: A Lipidomics Approach to an Epidemic. J. Lipid Res. 2015, 56, 722–736. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Gordillo, R.; Koliaki, C.; Gancheva, S.; Jelenik, T.; De Filippo, E.; Herder, C.; Markgraf, D.; Jankowiak, F.; Esposito, I.; et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care 2018, 41, 1235–1243. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Zhou, Y.; Sädevirta, S.; Leivonen, M.; Arola, J.; Orešič, M.; Hyötyläinen, T.; Yki-Järvinen, H. Hepatic Ceramides Dissociate Steatosis and Insulin Resistance in Patients with Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Carlier, A.; Phan, F.; Szpigel, A.; Hajduch, E.; Salem, J.-E.; Gautheron, J.; Le Goff, W.; Guérin, M.; Lachkar, F.; Ratziu, V.; et al. Dihydroceramides in Triglyceride-Enriched VLDL Are Associated with Nonalcoholic Fatty Liver Disease Severity in Type 2 Diabetes. Cell Rep. Med. 2020, 1, 100154. [Google Scholar] [CrossRef]
- Bandet, C.L.; Tan-Chen, S.; Bourron, O.; Le Stunff, H.; Hajduch, E. Sphingolipid Metabolism: New Insight into Ceramide-Induced Lipotoxicity in Muscle Cells. Int. J. Mol. Sci. 2019, 20, 479. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Talbot, C.L.; Chaurasia, B. Ceramides in Adipose Tissue. Front. Endocrinol. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Turban, S.; Hajduch, E. Protein Kinase C Isoforms: Mediators of Reactive Lipid Metabolites in the Development of Insulin Resistance. FEBS Lett. 2011, 585, 269–274. [Google Scholar] [CrossRef]
- Blouin, C.M.; Prado, C.; Takane, K.K.; Lasnier, F.; Garcia-Ocana, A.; Ferré, P.; Dugail, I.; Hajduch, E. Plasma Membrane Subdomain Compartmentalization Contributes to Distinct Mechanisms of Ceramide Action on Insulin Signaling. Diabetes 2010, 59, 600–610. [Google Scholar] [CrossRef]
- Powell, D.J.; Hajduch, E.; Kular, G.; Hundal, H.S. Ceramide Disables 3-Phosphoinositide Binding to the Pleckstrin Homology Domain of Protein Kinase B (PKB)/Akt by a PKCzeta-Dependent Mechanism. Mol. Cell Biol. 2003, 23, 7794–7808. [Google Scholar] [CrossRef]
- Fox, T.E.; Houck, K.L.; O’Neill, S.M.; Nagarajan, M.; Stover, T.C.; Pomianowski, P.T.; Unal, O.; Yun, J.K.; Naides, S.J.; Kester, M. Ceramide Recruits and Activates Protein Kinase C Zeta (PKC Zeta) within Structured Membrane Microdomains. J. Biol. Chem. 2007, 282, 12450–12457. [Google Scholar] [CrossRef] [PubMed]
- Cinar, R.; Godlewski, G.; Liu, J.; Tam, J.; Jourdan, T.; Mukhopadhyay, B.; Harvey-White, J.; Kunos, G. Hepatic Cannabinoid-1 Receptors Mediate Diet-Induced Insulin Resistance by Increasingde Novosynthesis of Long-Chain Ceramides. Hepatology 2014, 59, 143. [Google Scholar] [CrossRef] [PubMed]
- Cazzolli, R.; Carpenter, L.; Biden, T.J.; Schmitz-Peiffer, C. A Role for Protein Phosphatase 2A-like Activity, but Not Atypical Protein Kinase Czeta, in the Inhibition of Protein Kinase B/Akt and Glycogen Synthesis by Palmitate. Diabetes 2001, 50, 2210–2218. [Google Scholar] [CrossRef]
- Raichur, S.; Wang, S.T.; Chan, P.W.; Li, Y.; Ching, J.; Chaurasia, B.; Dogra, S.; Öhman, M.K.; Takeda, K.; Sugii, S.; et al. CerS2 Haploinsufficiency Inhibits β-Oxidation and Confers Susceptibility to Diet-Induced Steatohepatitis and Insulin Resistance. Cell Metab. 2014, 20, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Park, W.-J.; Kuperman, Y.; Boura-Halfon, S.; Pewzner-Jung, Y.; Futerman, A.H. Ablation of Very Long Acyl Chain Sphingolipids Causes Hepatic Insulin Resistance in Mice Due to Altered Detergent-Resistant Membranes. Hepatology 2013, 57, 525–532. [Google Scholar] [CrossRef]
- Pewzner-Jung, Y.; Brenner, O.; Braun, S.; Laviad, E.L.; Ben-Dor, S.; Feldmesser, E.; Horn-Saban, S.; Amann-Zalcenstein, D.; Raanan, C.; Berkutzki, T.; et al. A Critical Role for Ceramide Synthase 2 in Liver Homeostasis: II. Insights into Molecular Changes Leading to Hepatopathy. J. Biol. Chem. 2010, 285, 10911–10923. [Google Scholar] [CrossRef]
- de Almeida, I.T.; Cortez-Pinto, H.; Fidalgo, G.; Rodrigues, D.; Camilo, M.E. Plasma Total and Free Fatty Acids Composition in Human Non-Alcoholic Steatohepatitis. Clin. Nutr. 2002, 21, 219–223. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of Hepatic Triglyceride Accumulation in Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef]
- Xia, J.Y.; Holland, W.L.; Kusminski, C.M.; Sun, K.; Sharma, A.X.; Pearson, M.J.; Sifuentes, A.J.; McDonald, J.G.; Gordillo, R.; Scherer, P.E. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015, 22, 266–278. [Google Scholar] [CrossRef]
- Shimomura, I.; Matsuda, M.; Hammer, R.E.; Bashmakov, Y.; Brown, M.S.; Goldstein, J.L. Decreased IRS-2 and Increased SREBP-1c Lead to Mixed Insulin Resistance and Sensitivity in Livers of Lipodystrophic and Ob/Ob Mice. Mol. Cell 2000, 6, 77–86. [Google Scholar] [CrossRef]
- Kammoun, H.L.; Chabanon, H.; Hainault, I.; Luquet, S.; Magnan, C.; Koike, T.; Ferré, P.; Foufelle, F. GRP78 Expression Inhibits Insulin and ER Stress-Induced SREBP-1c Activation and Reduces Hepatic Steatosis in Mice. J. Clin. Investig. 2009, 119, 1201–1215. [Google Scholar] [CrossRef]
- Yang, Z.-X.; Shen, W.; Sun, H. Effects of Nuclear Receptor FXR on the Regulation of Liver Lipid Metabolism in Patients with Non-Alcoholic Fatty Liver Disease. Hepatol. Int. 2010, 4, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Pettinelli, P.; Videla, L.A. Up-Regulation of PPAR-Gamma mRNA Expression in the Liver of Obese Patients: An Additional Reinforcing Lipogenic Mechanism to SREBP-1c Induction. J. Clin. Endocrinol. Metab. 2011, 96, 1424–1430. [Google Scholar] [CrossRef]
- Auguet, T.; Berlanga, A.; Guiu-Jurado, E.; Martinez, S.; Porras, J.A.; Aragonès, G.; Sabench, F.; Hernandez, M.; Aguilar, C.; Sirvent, J.J.; et al. Altered Fatty Acid Metabolism-Related Gene Expression in Liver from Morbidly Obese Women with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 22173–22187. [Google Scholar] [CrossRef] [PubMed]
- Worgall, T.S.; Juliano, R.A.; Seo, T.; Deckelbaum, R.J. Ceramide Synthesis Correlates with the Posttranscriptional Regulation of the Sterol-Regulatory Element-Binding Protein. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Kondo, T.; Sajan, M.; Luo, J.; Bronson, R.; Asano, T.; Farese, R.; Cantley, L.C.; Kahn, C.R. Divergent Regulation of Hepatic Glucose and Lipid Metabolism by Phosphoinositide 3-Kinase via Akt and PKClambda/Zeta. Cell Metab. 2006, 3, 343–353. [Google Scholar] [CrossRef]
- Sokolowska, E.; Blachnio-Zabielska, A. The Role of Ceramides in Insulin Resistance. Front. Endocrinol. 2019, 10, 577. [Google Scholar] [CrossRef]
- Chen, J.Y.; Newcomb, B.; Zhou, C.; Pondick, J.V.; Ghoshal, S.; York, S.R.; Motola, D.L.; Coant, N.; Yi, J.K.; Mao, C.; et al. Tricyclic Antidepressants Promote Ceramide Accumulation to Regulate Collagen Production in Human Hepatic Stellate Cells. Sci. Rep. 2017, 7, 44867. [Google Scholar] [CrossRef]
- Moles, A.; Tarrats, N.; Fernández-Checa, J.C.; Marí, M. Cathepsins B and D Drive Hepatic Stellate Cell Proliferation and Promote Their Fibrogenic Potential. Hepatology 2009, 49, 1297–1307. [Google Scholar] [CrossRef]
- Alsamman, S.; Christenson, S.A.; Yu, A.; Ayad, N.M.E.; Mooring, M.S.; Segal, J.M.; Hu, J.K.-H.; Schaub, J.R.; Ho, S.S.; Rao, V.; et al. Targeting Acid Ceramidase Inhibits YAP/TAZ Signaling to Reduce Fibrosis in Mice. Sci. Transl. Med. 2020, 12, eaay8798. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Suzuki, A.; Guy, C.; Unalp-Arida, A.; Colvin, R.; Johnson, R.J.; Diehl, A.M. Network, for the N.S.C.R. Increased Fructose Consumption Is Associated with Fibrosis Severity in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2010, 51, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.-H.; et al. Fructose and Sugar: A Major Mediator of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Dubé, J.J.; Amati, F.; Toledo, F.G.S.; Stefanovic-Racic, M.; Rossi, A.; Coen, P.; Goodpaster, B.H. Effects of Weight Loss and Exercise on Insulin Resistance, and Intramyocellular Triacylglycerol, Diacylglycerol and Ceramide. Diabetologia 2011, 54, 1147–1156. [Google Scholar] [CrossRef]
- Johnson, M.L.; Distelmaier, K.; Lanza, I.R.; Irving, B.A.; Robinson, M.M.; Konopka, A.R.; Shulman, G.I.; Nair, K.S. Mechanism by Which Caloric Restriction Improves Insulin Sensitivity in Sedentary Obese Adults. Diabetes 2016, 65, 74–84. [Google Scholar] [CrossRef]
- Pilvi, T.-K.; Seppanen-Laakso, T.; Simolin, H.; Finckenberg, P.; Huotari, A.; Herzig, K.-H.; Korpela, R.; Oresic, M.; Mervaala, E.-M. Metabolomic Changes in Fatty Liver Can Be Modified by Dietary Protein and Calcium during Energy Restriction. World J. Gastroenterol. 2008, 14, 4462–4472. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Watts, V.L.; Niu, X.; Halushka, M.; Miller, K.L.; Vandegaer, K.; Bedja, D.; Fox-Talbot, K.; Bielawska, A.; Gabrielson, K.L.; et al. Beneficial Cardiac Effects of Caloric Restriction Are Lost with Age in a Murine Model of Obesity. J. Cardiovasc. Transl. Res. 2013, 6, 436–445. [Google Scholar] [CrossRef]
- David, C.E.B.; Lucas, A.M.B.; Cunha, P.L.O.; Viana, Y.I.P.; Yoshinaga, M.Y.; Miyamoto, S.; Filho, A.B.C.; Varela, A.L.N.; Kowaltowski, A.J.; Facundo, H.T. Calorie Restriction Changes Lipidomic Profiles and Maintains Mitochondrial Function and Redox Balance during Isoproterenol-Induced Cardiac Hypertrophy. J. Physiol. Biochem. 2022, 78, 283–294. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E. CALERIE Pennington Team Calorie Restriction Increases Muscle Mitochondrial Biogenesis in Healthy Humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef]
- Lee, C.K.; Klopp, R.G.; Weindruch, R.; Prolla, T.A. Gene Expression Profile of Aging and Its Retardation by Caloric Restriction. Science 1999, 285, 1390–1393. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Dowla, S.; Pendergrass, M.; Bolding, M.; Gower, B.; Fontaine, K.; Ashraf, A.; Soleymani, T.; Morrison, S.; Goss, A. Effectiveness of a Carbohydrate Restricted Diet to Treat Non-Alcoholic Fatty Liver Disease in Adolescents with Obesity: Trial Design and Methodology. Contemp. Clin. Trials 2018, 68, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Iwasa, M.; Iwata, K.; Kaito, M.; Sugimoto, R.; Urawa, N.; Mifuji, R.; Konishi, M.; Kobayashi, Y.; Adachi, Y. Restriction of Dietary Calories, Fat and Iron Improves Non-Alcoholic Fatty Liver Disease. J. Gastroenterol. Hepatol. 2007, 22, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Varkaneh, H.K.; Poursoleiman, F.; Al Masri, M.K.; Alras, K.A.; Shayah, Y.; Masmoum, M.D.; Alangari, F.A.; Alras, A.A.; Rinaldi, G.; Day, A.S.; et al. Low Fat Diet versus Low Carbohydrate Diet for Management of Non-Alcohol Fatty Liver Disease: A Systematic Review. Front. Nutr. 2022, 9, 987921. [Google Scholar] [CrossRef]
- Haufe, S.; Haas, V.; Utz, W.; Birkenfeld, A.L.; Jeran, S.; Böhnke, J.; Mähler, A.; Luft, F.C.; Schulz-Menger, J.; Boschmann, M.; et al. Long-Lasting Improvements in Liver Fat and Metabolism Despite Body Weight Regain After Dietary Weight Loss. Diabetes Care 2013, 36, 3786–3792. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Romero-Gomez, M. The Role of Nutrition in Non-Alcoholic Fatty Liver Disease: Pathophysiology and Management. Liver Int. 2020, 40 (Suppl. 1), 102–108. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the Clinical Assessment and Management of Nonalcoholic Fatty Liver Disease. Hepatology 2023, 77, 1797. [Google Scholar] [CrossRef]
- Grattagliano, I.; Di Ciaula, A.; Baj, J.; Molina-Molina, E.; Shanmugam, H.; Garruti, G.; Wang, D.Q.-H.; Portincasa, P. Protocols for Mitochondria as the Target of Pharmacological Therapy in the Context of Nonalcoholic Fatty Liver Disease (NAFLD). Methods Mol. Biol. 2021, 2310, 201–246. [Google Scholar] [CrossRef]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Anthony Sinha, R. Current Treatment Paradigms and Emerging Therapies for NAFLD/NASH. Front. Biosci. (Landmark Ed) 2021, 26, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Pafili, K.; Roden, M. Nonalcoholic Fatty Liver Disease (NAFLD) from Pathogenesis to Treatment Concepts in Humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.C.E.; Cassanye, A.; Martín-Gari, M.; Granado-Serrano, A.B.; Portero-Otín, M. Effect of Dietary Bioactive Compounds on Mitochondrial and Metabolic Flexibility. Diseases 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, W.; Hu, X.; Liu, Z.; Wang, F.; Wang, J. The Role of Polyphenols in Modulating Mitophagy: Implications for Therapeutic Interventions. Pharmacol. Res. 2024, 207, 107324. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol Prevents Diet-Induced Metabolic Syndrome and Attenuates Mitochondrial Abnormalities in Obese Mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.-J.; Böhm, M.; de Sotomayor, M.A.; Laufs, U. Ferulic Acid, a Bioactive Component of Rice Bran, Improves Oxidative Stress and Mitochondrial Biogenesis and Dynamics in Mice and in Human Mononuclear Cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención Con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef]
- Daidone, M.; Casuccio, A.; Puleo, M.G.; Cuore, A.D.; Pacinella, G.; Chiara, T.D.; Raimondo, D.D.; Immordino, P.; Tuttolomondo, A. Mediterranean Diet Effects on Vascular Health and Serum Levels of Adipokines and Ceramides. PLoS ONE 2024, 19, e0300844. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. Characterizing the Effects of Saturated Fatty Acids on Insulin Signaling and Ceramide and Diacylglycerol Accumulation in 3T3-L1 Adipocytes and C2C12 Myotubes. Arch. Biochem. Biophys. 2003, 419, 101–109. [Google Scholar] [CrossRef]
- Schmitz-Peiffer, C.; Craig, D.L.; Biden, T.J. Ceramide Generation Is Sufficient to Account for the Inhibition of the Insulin-Stimulated PKB Pathway in C2C12 Skeletal Muscle Cells Pretreated with Palmitate. J. Biol. Chem. 1999, 274, 24202–24210. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Mu, Y.-M.; Wang, B.-A.; Li, X.-L.; Lu, J.-M.; Li, J.-Y.; Pan, C.-Y.; Yanase, T.; Nawata, H. Saturated Free Fatty Acids, Palmitic Acid and Stearic Acid, Induce Apoptosis by Stimulation of Ceramide Generation in Rat Testicular Leydig Cell. Biochem. Biophys. Res. Commun. 2003, 303, 1002–1007. [Google Scholar] [CrossRef]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Klapa, M.I.; Candela, M.; Rampelli, S.; Lehoux, S.; Lázaro, I.; Sala-Vila, A.; et al. Mechanisms Underlying the Cardiometabolic Protective Effect of Walnut Consumption in Obese People: A Cross-over, Randomized, Double-Blind, Controlled Inpatient Physiology Study. Diabetes Obes. Metab. 2019, 21, 2086–2095. [Google Scholar] [CrossRef]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between Adherence to the Mediterranean Diet and Oxidative Stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Skoumas, J.; Pitsavos, C.; Masoura, C.; Siasos, G.; Galiatsatos, N.; Psaltopoulou, T.; Mylonakis, C.; Margazas, A.; Kyvelou, S.; et al. Long-Term Adherence to the Mediterranean Diet Reduces the Prevalence of Hyperuricaemia in Elderly Individuals, without Known Cardiovascular Disease: The Ikaria Study. Maturitas 2011, 70, 58–64. [Google Scholar] [CrossRef]
- Kastorini, C.-M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The Effect of Mediterranean Diet on Metabolic Syndrome and Its Components: A Meta-Analysis of 50 Studies and 534,906 Individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.-P.; Sacanella, E.; Vazquez-Agell, M.; Morales, M.; Fitó, M.; Escoda, R.; Serrano-Martínez, M.; Salas-Salvadó, J.; Benages, N.; Casas, R.; et al. Inhibition of Circulating Immune Cell Activation: A Molecular Antiinflammatory Effect of the Mediterranean Diet. Am. J. Clin. Nutr. 2009, 89, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Salas-Salvadó, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The Mediterranean Diet Pattern and Its Main Components Are Associated with Lower Plasma Concentrations of Tumor Necrosis Factor Receptor 60 in Patients at High Risk for Cardiovascular Disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean Diet Is Associated with the Severity of Non-Alcoholic Fatty Liver Disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.-V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in Clinical Characteristics of Patients with Non-Alcoholic Fatty Liver Disease, after an Intervention Based on the Mediterranean Lifestyle: A Randomised Controlled Clinical Trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a Counseling-Supported Treatment with the Mediterranean Diet and Physical Activity on the Severity of the Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2017, 23, 3150–3162. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The Beneficial Effects of Mediterranean Diet over Low-Fat Diet May Be Mediated by Decreasing Hepatic Fat Content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Marchiò, M.; Timofeeva, E.; Biagini, G. Neuroactive Peptides as Putative Mediators of Antiepileptic Ketogenic Diets. Front. Neurol. 2014, 5, 63. [Google Scholar] [CrossRef]
- Berilgen, M.S.; Mungen, B.; Ustundag, B.; Demir, C. Serum Ghrelin Levels Are Enhanced in Patients with Epilepsy. Seizure 2006, 15, 106–111. [Google Scholar] [CrossRef]
- Holmer, M.; Lindqvist, C.; Petersson, S.; Moshtaghi-Svensson, J.; Tillander, V.; Brismar, T.B.; Hagström, H.; Stål, P. Treatment of NAFLD with Intermittent Calorie Restriction or Low-Carb High-Fat Diet—A Randomised Controlled Trial. JHEP Rep. 2021, 3, 100256. [Google Scholar] [CrossRef]
- Cunha, G.M.; Guzman, G.; De Mello, L.L.C.; Trein, B.; Spina, L.; Bussade, I.; Marques Prata, J.; Sajoux, I.; Countinho, W. Efficacy of a 2-Month Very Low-Calorie Ketogenic Diet (VLCKD) Compared to a Standard Low-Calorie Diet in Reducing Visceral and Liver Fat Accumulation in Patients With Obesity. Front. Endocrinol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Hall, K.D.; Guo, J.; Ravussin, E.; Mayer, L.S.; Reitman, M.L.; Smith, S.R.; Walsh, B.T.; Leibel, R.L. Glucose and Lipid Homeostasis and Inflammation in Humans Following an Isocaloric Ketogenic Diet. Obesity 2019, 27, 971–981. [Google Scholar] [CrossRef]
- Chirapongsathorn, S.; Rintaravitoon, W.; Tangjaturonrasme, B.; Chotsriluecha, S.; Pumsutas, Y.; Kanchanapradith, A.; Treeprasertsuk, S. Effect of a Ketogenic Diet on Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Progression: A Randomized Controlled Trial. JGH Open 2025, 9, e70099. [Google Scholar] [CrossRef] [PubMed]
- Jani, S.; Da Eira, D.; Stefanovic, M.; Ceddia, R.B. The Ketogenic Diet Prevents Steatosis and Insulin Resistance by Reducing Lipogenesis, Diacylglycerol Accumulation and Protein Kinase C Activity in Male Rat Liver. J. Physiol. 2022, 600, 4137–4151. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, P.K.; Dufour, S.; Lyu, K.; Zhang, X.-M.; Hakkarainen, A.; Lehtimäki, T.E.; Cline, G.W.; Petersen, K.F.; Shulman, G.I.; Yki-Järvinen, H. Effect of a Ketogenic Diet on Hepatic Steatosis and Hepatic Mitochondrial Metabolism in Nonalcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7347–7354. [Google Scholar] [CrossRef] [PubMed]
- Hasan-Olive, M.M.; Lauritzen, K.H.; Ali, M.; Rasmussen, L.J.; Storm-Mathisen, J.; Bergersen, L.H. A Ketogenic Diet Improves Mitochondrial Biogenesis and Bioenergetics via the PGC1α-SIRT3-UCP2 Axis. Neurochem. Res. 2019, 44, 22–37. [Google Scholar] [CrossRef]
- Dupuis, N.; Curatolo, N.; Benoist, J.-F.; Auvin, S. Ketogenic Diet Exhibits Anti-Inflammatory Properties. Epilepsia 2015, 56, e95–e98. [Google Scholar] [CrossRef]
- Shang, F.; Li, X.; Jiang, X. Coffee Consumption and Risk of the Metabolic Syndrome: A Meta-Analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef]
- Bambha, K.; Wilson, L.A.; Unalp, A.; Loomba, R.; Neuschwander-Tetri, B.A.; Brunt, E.M.; Bass, N.M. Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) Coffee Consumption in NAFLD Patients with Lower Insulin Resistance Is Associated with Lower Risk of Severe Fibrosis. Liver Int. 2014, 34, 1250–1258. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.; Ahn, S.B. Different Associations of Coffee Consumption with the Risk of Incident Metabolic Dysfunction-Associated Steatotic Liver Disease and Advanced Liver Fibrosis. Nutrients 2023, 16, 140. [Google Scholar] [CrossRef]
- Takahashi, K.; Yanai, S.; Shimokado, K.; Ishigami, A. Coffee Consumption in Aged Mice Increases Energy Production and Decreases Hepatic mTOR Levels. Nutrition 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Seow, W.J.; Low, D.Y.; Pan, W.-C.; Gunther, S.H.; Sim, X.; Torta, F.; Herr, D.R.; Kovalik, J.-P.; Ching, J.; Khoo, C.M.; et al. Coffee, Black Tea, and Green Tea Consumption in Relation to Plasma Metabolites in an Asian Population. Mol. Nutr. Food Res. 2020, 64, e2000527. [Google Scholar] [CrossRef] [PubMed]
- Wittenbecher, C.; Cuadrat, R.; Johnston, L.; Eichelmann, F.; Jäger, S.; Kuxhaus, O.; Prada, M.; Del Greco, M.F.; Hicks, A.A.; Hoffman, P.; et al. Dihydroceramide- and Ceramide-Profiling Provides Insights into Human Cardiometabolic Disease Etiology. Nat. Commun. 2022, 13, 936. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic Acid Improves High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Galvano, F.; Li Volti, G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients 2017, 9, 85. [Google Scholar] [CrossRef]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee Polyphenols Suppress Diet-Induced Body Fat Accumulation by Downregulating SREBP-1c and Related Molecules in C57BL/6J Mice. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-Diabetic and Anti-Lipidemic Effects of Chlorogenic Acid Are Mediated by Ampk Activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Karimi, S.; Sanginabadi, M.; Poustchi, H.; Enayati, S.; Asgarbeik, S.; Nasrollahzadeh, J.; Hekmatdoost, A. Short Term Effects of Coffee Components Consumption on Gut Microbiota in Patients with Non-Alcoholic Fatty Liver and Diabetes: A Pilot Randomized Placebo-Controlled, Clinical Trial. EXCLI J. 2020, 19, 241–250. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Dang, X.; Liu, Y.; Jiang, J.; Wang, Y.; Lu, X.; Guo, X. Effect of Chlorogenic Acid on LPS-Induced Proinflammatory Signaling in Hepatic Stellate Cells. Inflamm. Res. 2013, 62, 581–587. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef]
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of Beneficial Effects of Exercise Training on Non-Alcoholic Fatty Liver Disease (NAFLD): Roles of Oxidative Stress and Inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003. [Google Scholar] [CrossRef]
- Stevanović, J.; Beleza, J.; Coxito, P.; Ascensão, A.; Magalhães, J. Physical Exercise and Liver “Fitness”: Role of Mitochondrial Function and Epigenetics-Related Mechanisms in Non-Alcoholic Fatty Liver Disease. Mol. Metab. 2019, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-del-Olmo, M.Á.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef]
- Wei, H.; Qu, H.; Wang, H.; Deng, H. Associations between Sitting Time and Non-Alcoholic Fatty Liver Diseases in Chinese Male Workers: A Cross-Sectional Study. BMJ Open 2016, 6, e011939. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, K.; Thoma, C.; Moore, S.; Ploetz, T.; Anstee, Q.M.; Taylor, R.; Day, C.P.; Trenell, M.I. Non-Alcoholic Fatty Liver Disease Is Associated with Higher Levels of Objectively Measured Sedentary Behaviour and Lower Levels of Physical Activity than Matched Healthy Controls. Frontline Gastroenterol. 2015, 6, 44–51. [Google Scholar] [CrossRef]
- Gerber, L.; Otgonsuren, M.; Mishra, A.; Escheik, C.; Birerdinc, A.; Stepanova, M.; Younossi, Z.M. Non-Alcoholic Fatty Liver Disease (NAFLD) Is Associated with Low Level of Physical Activity: A Population-Based Study. Aliment. Pharmacol. Ther. 2012, 36, 772–781. [Google Scholar] [CrossRef]
- Kwak, M.-S.; Kim, D.; Chung, G.E.; Kim, W.; Kim, Y.J.; Yoon, J.-H. Role of Physical Activity in Nonalcoholic Fatty Liver Disease in Terms of Visceral Obesity and Insulin Resistance. Liver Int. 2015, 35, 944–952. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Zvibel, I.; Goldiner, I.; Blendis, L.; Halpern, Z.; Oren, R. Role of Leisure-Time Physical Activity in Nonalcoholic Fatty Liver Disease: A Population-Based Study. Hepatology 2008, 48, 1791–1798. [Google Scholar] [CrossRef]
- Stine, J.G.; DiJoseph, K.; Pattison, Z.; Harrington, A.; Chinchilli, V.M.; Schmitz, K.H.; Loomba, R. Exercise Training Is Associated With Treatment Response in Liver Fat Content by Magnetic Resonance Imaging Independent of Clinically Significant Body Weight Loss in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2023, 118, 1204. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Shojaee-Moradie, F.; Sprung, V.S.; Jones, H.; Pugh, C.J.A.; Richardson, P.; Kemp, G.J.; Barrett, M.; Jackson, N.C.; Thomas, E.L.; et al. Dissociation between Exercise-Induced Reduction in Liver Fat and Changes in Hepatic and Peripheral Glucose Homoeostasis in Obese Patients with Non-Alcoholic Fatty Liver Disease. Clin. Sci. 2015, 130, 93–104. [Google Scholar] [CrossRef]
- Sullivan, S.; Kirk, E.P.; Mittendorfer, B.; Patterson, B.W.; Klein, S. Randomized Trial of Exercise Effect on Intrahepatic Triglyceride Content and Lipid Kinetics in Nonalcoholic Fatty Liver Disease. Hepatology 2012, 55, 1738–1745. [Google Scholar] [CrossRef]
- van der Heijden, G.-J.; Wang, Z.J.; Chu, Z.D.; Sauer, P.J.J.; Haymond, M.W.; Rodriguez, L.M.; Sunehag, A.L. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity 2010, 18, 384–390. [Google Scholar] [CrossRef]
- Hallsworth, K.; Fattakhova, G.; Hollingsworth, K.G.; Thoma, C.; Moore, S.; Taylor, R.; Day, C.P.; Trenell, M.I. Resistance Exercise Reduces Liver Fat and Its Mediators in Non-Alcoholic Fatty Liver Disease Independent of Weight Loss. Gut 2011, 60, 1278–1283. [Google Scholar] [CrossRef]
- Winn, N.C.; Liu, Y.; Rector, R.S.; Parks, E.J.; Ibdah, J.A.; Kanaley, J.A. Energy-Matched Moderate and High Intensity Exercise Training Improves Nonalcoholic Fatty Liver Disease Risk Independent of Changes in Body Mass or Abdominal Adiposity—A Randomized Trial. Metabolism 2018, 78, 128–140. [Google Scholar] [CrossRef]
- Keating, S.E.; Croci, I.; Wallen, M.P.; Cox, E.R.; Thuzar, M.; Pham, U.; Mielke, G.I.; Coombes, J.S.; Macdonald, G.A.; Hickman, I.J. High-Intensity Interval Training Is Safe, Feasible and Efficacious in Nonalcoholic Steatohepatitis: A Randomized Controlled Trial. Dig. Dis. Sci. 2023, 68, 2123–2139. [Google Scholar] [CrossRef]
- Sabag, A.; Barr, L.; Armour, M.; Armstrong, A.; Baker, C.J.; Twigg, S.M.; Chang, D.; Hackett, D.A.; Keating, S.E.; George, J.; et al. The Effect of High-Intensity Interval Training vs Moderate-Intensity Continuous Training on Liver Fat: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2022, 107, 862–881. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Tantawy, S.A.; Kamel, D.M.; Alqahtani, B.A.; Soliman, G.S. A Randomized Controlled Trial on the Effectiveness of 8-Week High-Intensity Interval Exercise on Intrahepatic Triglycerides, Visceral Lipids, and Health-Related Quality of Life in Diabetic Obese Patients with Nonalcoholic Fatty Liver Disease. Medicine 2019, 98, e14918. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Hackett, D.A.; Parker, H.M.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Baker, M.K.; Chuter, V.H.; Caterson, I.D.; George, J.; et al. Effect of Aerobic Exercise Training Dose on Liver Fat and Visceral Adiposity. J. Hepatol. 2015, 63, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Ascensão, A.; Gonçalves, I.O.; Lumini-Oliveira, J.; Marques-Aleixo, I.; Dos Passos, E.; Rocha-Rodrigues, S.; Machado, N.G.; Moreira, A.C.; Oliveira, P.J.; Torrella, J.R.; et al. Endurance Training and Chronic Intermittent Hypoxia Modulate in Vitro Salicylate-Induced Hepatic Mitochondrial Dysfunction. Mitochondrion 2012, 12, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.A.; Meers, G.M.; Linden, M.A.; Kearney, M.L.; Morris, E.M.; Thyfault, J.P.; Rector, R.S. Impact of Various Exercise Modalities on Hepatic Mitochondrial Function. Med. Sci. Sports Exerc. 2014, 46, 1089–1097. [Google Scholar] [CrossRef]
- Sun, L.; Shen, W.; Liu, Z.; Guan, S.; Liu, J.; Ding, S. Endurance Exercise Causes Mitochondrial and Oxidative Stress in Rat Liver: Effects of a Combination of Mitochondrial Targeting Nutrients. Life Sci. 2010, 86, 39–44. [Google Scholar] [CrossRef]
- Lima, F.D.; Stamm, D.N.; Della-Pace, I.D.; Dobrachinski, F.; de Carvalho, N.R.; Royes, L.F.F.; Soares, F.A.; Rocha, J.B.; González-Gallego, J.; Bresciani, G. Swimming Training Induces Liver Mitochondrial Adaptations to Oxidative Stress in Rats Submitted to Repeated Exhaustive Swimming Bouts. PLoS ONE 2013, 8, e55668. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.A.; Fletcher, J.A.; Morris, E.M.; Meers, G.M.; Kearney, M.L.; Crissey, J.M.; Laughlin, M.H.; Booth, F.W.; Sowers, J.R.; Ibdah, J.A.; et al. Combining Metformin and Aerobic Exercise Training in the Treatment of Type 2 Diabetes and NAFLD in OLETF Rats. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E300–E310. [Google Scholar] [CrossRef] [PubMed]
- Pilegaard, H.; Saltin, B.; Neufer, P.D. Exercise Induces Transient Transcriptional Activation of the PGC-1α Gene in Human Skeletal Muscle. J. Physiol. 2003, 546, 851–858. [Google Scholar] [CrossRef]
- Fuller, S.E.; Huang, T.-Y.; Simon, J.; Batdorf, H.M.; Essajee, N.M.; Scott, M.C.; Waskom, C.M.; Brown, J.M.; Burke, S.J.; Collier, J.J.; et al. Low-Intensity Exercise Induces Acute Shifts in Liver and Skeletal Muscle Substrate Metabolism but Not Chronic Adaptations in Tissue Oxidative Capacity. J. Appl. Physiol. 2019, 127, 143–156. [Google Scholar] [CrossRef]
- Aharoni-Simon, M.; Hann-Obercyger, M.; Pen, S.; Madar, Z.; Tirosh, O. Fatty Liver Is Associated with Impaired Activity of PPARγ-Coactivator 1α (PGC1α) and Mitochondrial Biogenesis in Mice. Lab. Investig. 2011, 91, 1018–1028. [Google Scholar] [CrossRef]
- Laye, M.J.; Rector, R.S.; Borengasser, S.J.; Naples, S.P.; Uptergrove, G.M.; Ibdah, J.A.; Booth, F.W.; Thyfault, J.P. Cessation of Daily Wheel Running Differentially Alters Fat Oxidation Capacity in Liver, Muscle, and Adipose Tissue. J. Appl. Physiol. 2009, 106, 161–168. [Google Scholar] [CrossRef]
- Haase, T.N.; Ringholm, S.; Leick, L.; Biensø, R.S.; Kiilerich, K.; Johansen, S.; Nielsen, M.M.; Wojtaszewski, J.F.; Hidalgo, J.; Pedersen, P.A.; et al. Role of PGC-1α in Exercise and Fasting-Induced Adaptations in Mouse Liver. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1501–R1509. [Google Scholar] [CrossRef]
- Lei, X.; Rodriguez, S.; Petersen, P.S.; Seldin, M.M.; Bowman, C.E.; Wolfgang, M.J.; Wong, G.W. Loss of CTRP5 Improves Insulin Action and Hepatic Steatosis. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E1036–E1052. [Google Scholar] [CrossRef]
- Ghareghani, P.; Shanaki, M.; Ahmadi, S.; Khoshdel, A.R.; Rezvan, N.; Meshkani, R.; Delfan, M.; Gorgani-Firuzjaee, S. Aerobic Endurance Training Improves Nonalcoholic Fatty Liver Disease (NAFLD) Features via miR-33 Dependent Autophagy Induction in High Fat Diet Fed Mice. Obes. Res. Clin. Pract. 2018, 12, 80–89. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-P.; Stephens, T.J.; Murthy, S.; Canny, B.J.; Hargreaves, M.; Witters, L.A.; Kemp, B.E.; McConell, G.K. Effect of Exercise Intensity on Skeletal Muscle AMPK Signaling in Humans. Diabetes 2003, 52, 2205–2212. [Google Scholar] [CrossRef]
- Brooks, G.A. Importance of the “crossover” Concept in Exercise Metabolism. Clin. Exp. Pharmacol. Physiol. 1997, 24, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Gunadi, J.W.; Tarawan, V.M.; Daniel Ray, H.R.; Wahyudianingsih, R.; Lucretia, T.; Tanuwijaya, F.; Lesmana, R.; Supratman, U.; Setiawan, I. Different Training Intensities Induced Autophagy and Histopathology Appearances Potentially Associated with Lipid Metabolism in Wistar Rat Liver. Heliyon 2020, 6, e03874. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.C.; Martens, C.R.; Santos-Parker, J.R.; Bassett, C.J.; Strahler, T.R.; Cruickshank-Quinn, C.; Reisdorph, N.; McQueen, M.B.; Seals, D.R. Amino Acid and Lipid Associated Plasma Metabolomic Patterns Are Related to Healthspan Indicators with Ageing. Clin. Sci. 2018, 132, 1765–1777. [Google Scholar] [CrossRef]
- Bergman, B.C.; Brozinick, J.T.; Strauss, A.; Bacon, S.; Kerege, A.; Bui, H.H.; Sanders, P.; Siddall, P.; Kuo, M.S.; Perreault, L. Serum Sphingolipids: Relationships to Insulin Sensitivity and Changes with Exercise in Humans. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E398–E408. [Google Scholar] [CrossRef]
- Bruce, C.R.; Thrush, A.B.; Mertz, V.A.; Bezaire, V.; Chabowski, A.; Heigenhauser, G.J.F.; Dyck, D.J. Endurance Training in Obese Humans Improves Glucose Tolerance and Mitochondrial Fatty Acid Oxidation and Alters Muscle Lipid Content. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E99–E107. [Google Scholar] [CrossRef]
- Dobrzyń, A.; Zendzian-Piotrowska, M.; Górski, J. Effect of Endurance Training on the Sphingomyelin-Signalling Pathway Activity in the Skeletal Muscles of the Rat. J. Physiol. Pharmacol. 2004, 55, 305–313. [Google Scholar]
- Reidy, P.T.; Mahmassani, Z.S.; McKenzie, A.I.; Petrocelli, J.J.; Summers, S.A.; Drummond, M.J. Influence of Exercise Training on Skeletal Muscle Insulin Resistance in Aging: Spotlight on Muscle Ceramides. Int. J. Mol. Sci. 2020, 21, 1514. [Google Scholar] [CrossRef]
- Gaggini, M.; Vassalle, C.; Carli, F.; Maltinti, M.; Sabatino, L.; Buzzigoli, E.; Mastorci, F.; Sbrana, F.; Gastaldelli, A.; Pingitore, A. Changes in Plasma Bioactive Lipids and Inflammatory Markers during a Half-Marathon in Trained Athletes. Appl. Sci. 2021, 11, 4622. [Google Scholar] [CrossRef]
- Mardare, C.; Krüger, K.; Liebisch, G.; Seimetz, M.; Couturier, A.; Ringseis, R.; Wilhelm, J.; Weissmann, N.; Eder, K.; Mooren, F.-C. Endurance and Resistance Training Affect High Fat Diet-Induced Increase of Ceramides, Inflammasome Expression, and Systemic Inflammation in Mice. J. Diabetes Res. 2016, 2016, 4536470. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and Their Metabolism in Physiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- American Gastroenterological Association. American Gastroenterological Association Medical Position Statement: Nonalcoholic Fatty Liver Disease. Gastroenterology 2002, 123, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Rector, R.S.; Uptergrove, G.M.; Morris, E.M.; Borengasser, S.J.; Laughlin, M.H.; Booth, F.W.; Thyfault, J.P.; Ibdah, J.A. Daily Exercise vs. Caloric Restriction for Prevention of Nonalcoholic Fatty Liver Disease in the OLETF Rat Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G874–G883. [Google Scholar] [CrossRef]
- Wang, S.-T.; Zheng, J.; Peng, H.-W.; Cai, X.-L.; Pan, X.-T.; Li, H.-Q.; Hong, Q.-Z.; Peng, X.-E. Physical Activity Intervention for Non-Diabetic Patients with Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. BMC Gastroenterol. 2020, 20, 66. [Google Scholar] [CrossRef]
- Mucinski, J.M.; Salvador, A.F.; Moore, M.P.; Fordham, T.M.; Anderson, J.M.; Shryack, G.; Cunningham, R.P.; Lastra, G.; Gaballah, A.H.; Diaz-Arias, A.; et al. Histological Improvements Following Energy Restriction and Exercise: The Role of Insulin Resistance in Resolution of MASH. J. Hepatol. 2024, 81, 781–793. [Google Scholar] [CrossRef]
- Wang, E.; Norred, W.P.; Bacon, C.W.; Riley, R.T.; Merrill, A.H. Inhibition of Sphingolipid Biosynthesis by Fumonisins. Implications for Diseases Associated with Fusarium Moniliforme. J. Biol. Chem. 1991, 266, 14486–14490. [Google Scholar] [CrossRef]
- Gelderblom, W.C.; Abel, S.; Smuts, C.M.; Marnewick, J.; Marasas, W.F.; Lemmer, E.R.; Ramljak, D. Fumonisin-Induced Hepatocarcinogenesis: Mechanisms Related to Cancer Initiation and Promotion. Environ. Health Perspect. 2001, 109 (Suppl. 2), 291–300. [Google Scholar] [CrossRef][Green Version]
- Pelletier, D.; Hafler, D.A. Fingolimod for Multiple Sclerosis. N. Engl. J. Med. 2012, 366, 339–347. [Google Scholar] [CrossRef]
- Preitner, F.; Mody, N.; Graham, T.E.; Peroni, O.D. Long-Term Fenretinide Treatment Prevents High-Fat Diet-Induced Obesity, Insulin Resistance, and Hepatic Steatosis. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1420–E1429. [Google Scholar] [CrossRef]
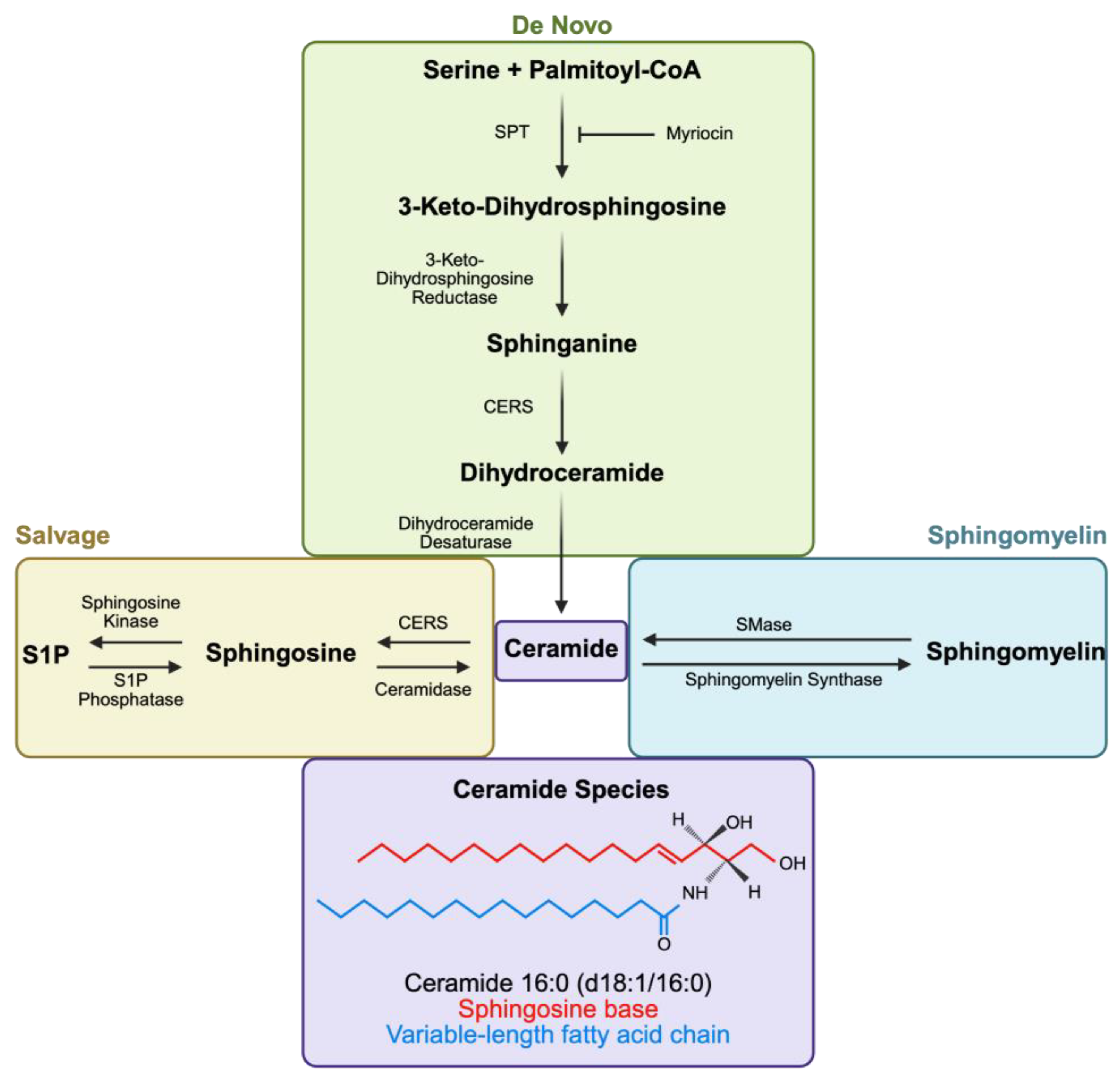
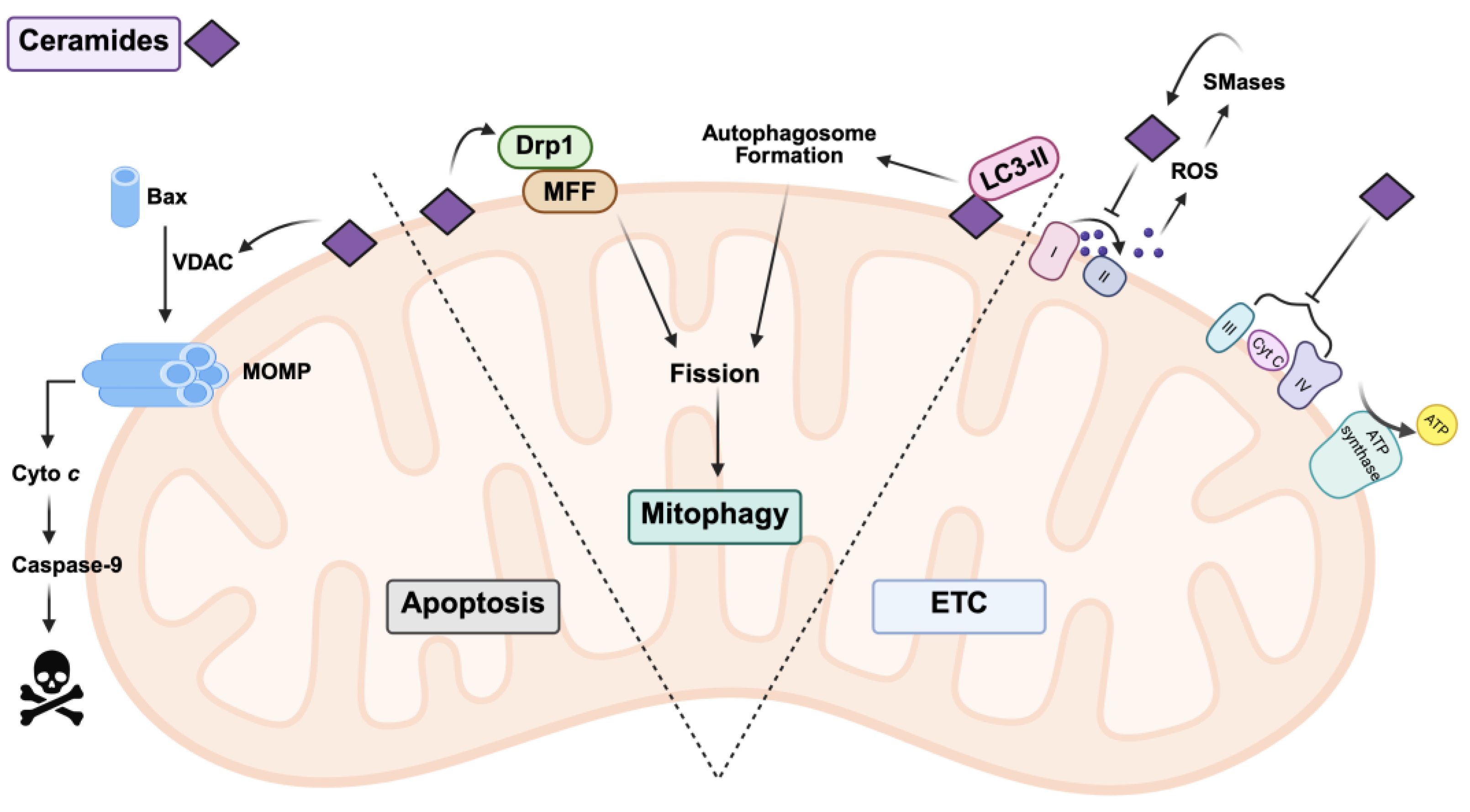
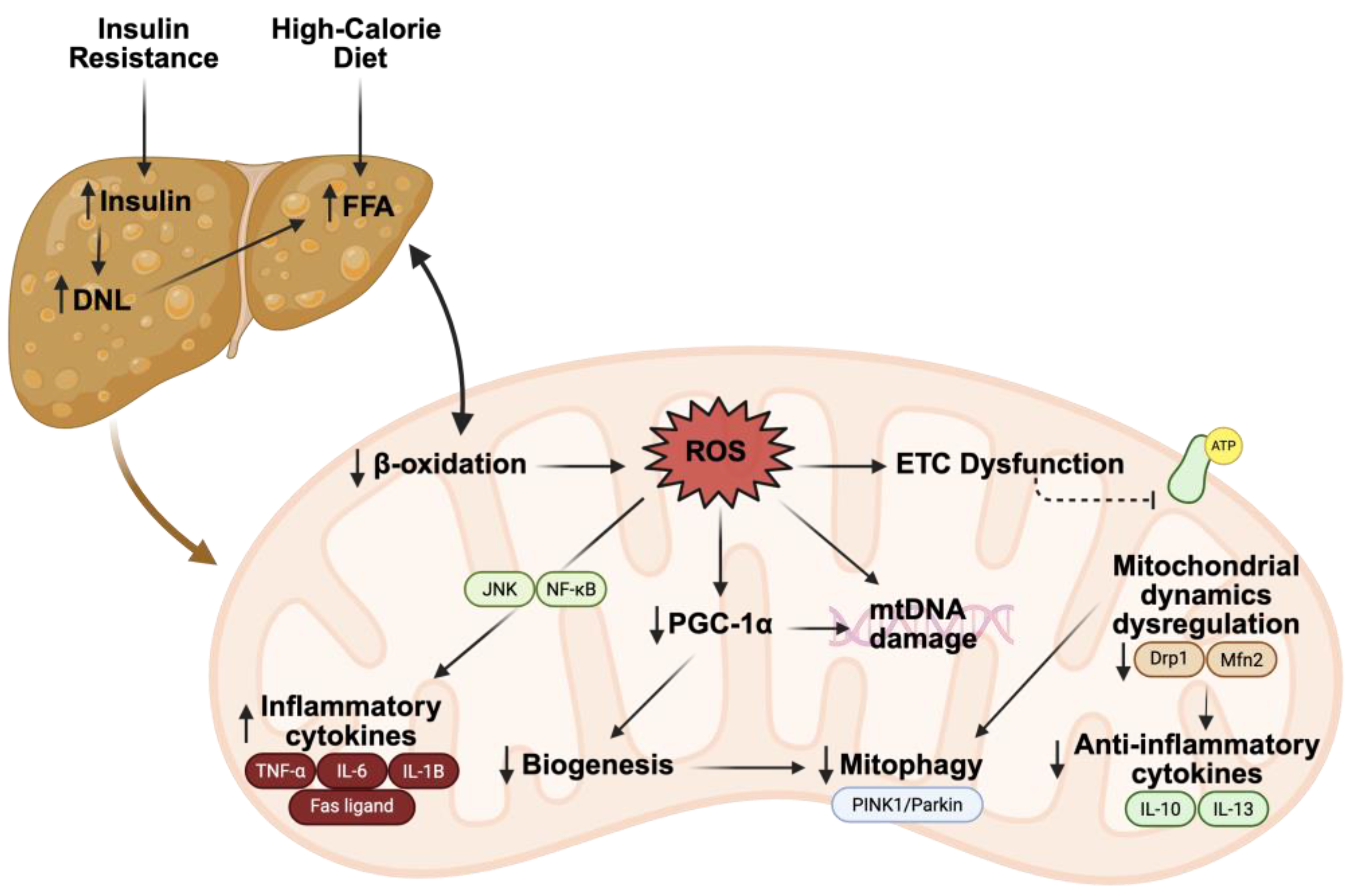

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCaffrey, J.M.; Ibdah, J.A. Effects of Diet and Exercise on Mitochondrial Health in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Role of Ceramides. Nutrients 2025, 17, 2972. https://doi.org/10.3390/nu17182972
McCaffrey JM, Ibdah JA. Effects of Diet and Exercise on Mitochondrial Health in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Role of Ceramides. Nutrients. 2025; 17(18):2972. https://doi.org/10.3390/nu17182972
Chicago/Turabian StyleMcCaffrey, Jonas M., and Jamal A. Ibdah. 2025. "Effects of Diet and Exercise on Mitochondrial Health in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Role of Ceramides" Nutrients 17, no. 18: 2972. https://doi.org/10.3390/nu17182972
APA StyleMcCaffrey, J. M., & Ibdah, J. A. (2025). Effects of Diet and Exercise on Mitochondrial Health in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): Role of Ceramides. Nutrients, 17(18), 2972. https://doi.org/10.3390/nu17182972






