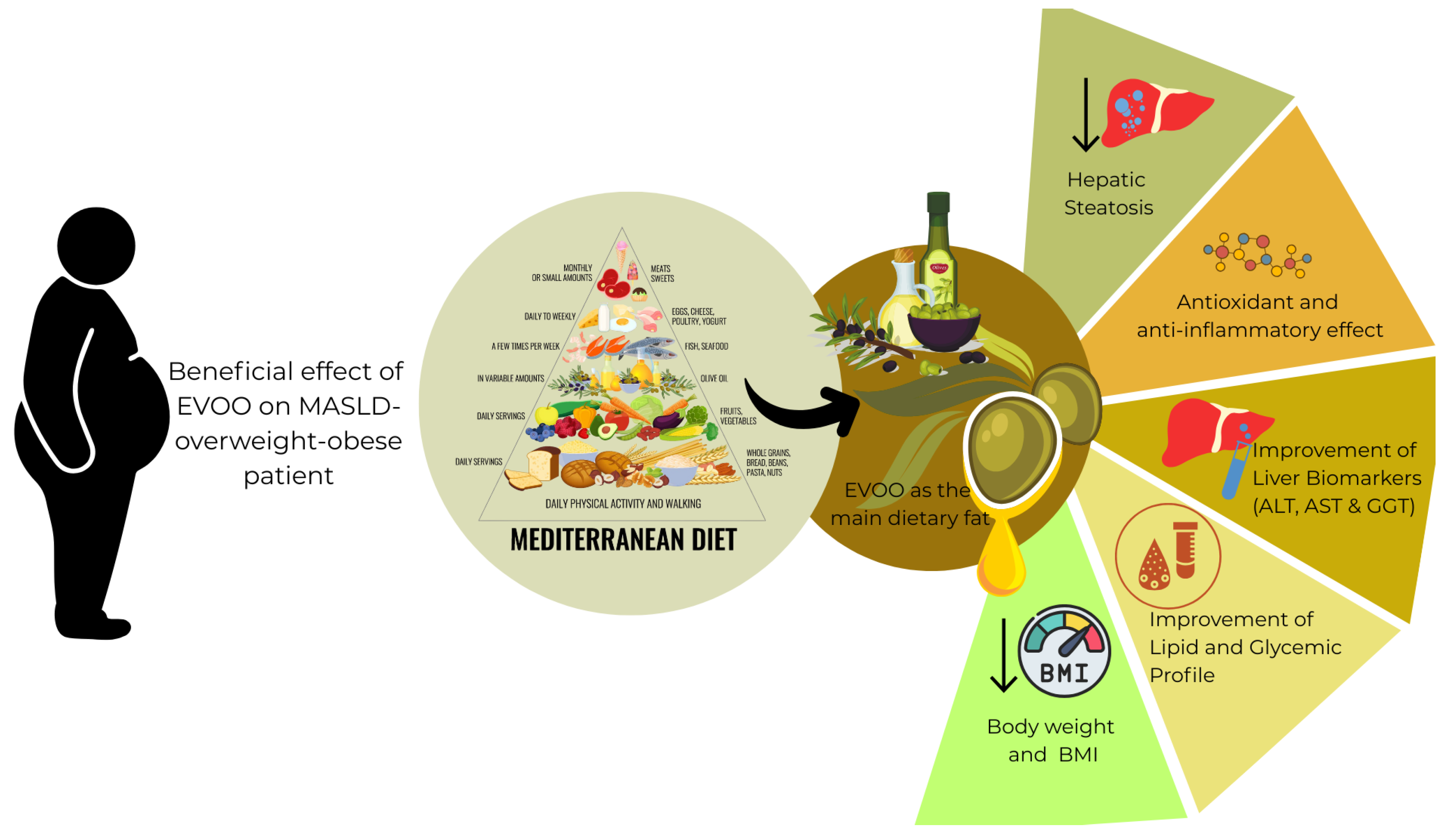
Exploring the Role of Extra Virgin Olive Oil (EVOO) in MASLD: Evidence from Human Consumption
Abstract
1. Introduction
2. Materials and Methods
Literature Search Strategy and Data Synthesis
3. Results
3.1. Olive and MASLD
3.1.1. Effects of Olive Oil on Hepatic Steatosis, Inflammation, and Oxidative Stress
3.1.2. Effects of Olive Oil on Liver Enzymes
3.1.3. Effects on Blood Lipid and Glucose Profiles
3.1.4. Effect of Olive on Anthropometric and Clinical Outcomes
3.1.5. Dose and Type of Olive Oil
3.1.6. Dietary Context and Study Duration
3.2. Olive Oil as a Core Component of the Mediterranean Diet and Its Clinical Relevance for MASLD Dietary Management
3.2.1. Hepatic Steatosis and Liver Function Markers
3.2.2. Inflammation, Oxidative Stress, and Fibrosis
3.2.3. Glycemic Control, Insulin Resistance and Lipid Profile
| Study (Author Year and Country) | Type and Duration of Study | Participants Characteristics | Intervention/Grouping and Comparator/Control | Incorporation of Olive Oil | Effects of Mediterranean Diet | |||
|---|---|---|---|---|---|---|---|---|
| Hepatic Steatosis, Inflammation and Oxidative Stress | Liver Parameters | Blood Lipid and Blood Sugar Profile | Anthropometric and Clinical Measurements | |||||
| Quetglas-Llabrés et al., 2024; Spain [33] | Prospective Randomized Trial; 2 years intervention | 40 adult patients diagnose with MASLD and Metabolic syndrome; 40–60 years old; average BMI of 32 kg/m2 Magnetic Resonance Imaging; Intrahepatic Fat Content | Low Adherence to Mediterranean Diet vs. High Adherence to Mediterranean Diet | Adherence to Mediterranean Diet 17 item questionnaire; [50] Use only EVOO for cooking, salad dressings, and spreads. | ↓ Intra Hepatic Fat Content (IFC) on High Adherence Group On High Adherence Group ↑ CAT ↑ SOD ↑ GPx ↑ GRd ↑ GSH level in erythrocytes ↓ MDA ↓ oxLDL ↑ TLR4 expression | High Adherence Group: ↓ AST ↓ ALT ↓ GGT ↓ Cytokeratin-18 plasma levels | Both group ↓ LDL ↓ TC ↓ TG ↑ HDL High Adherence Group: ↓ Glycemia ↓ HbA1C | Both group ↓ BMI High Adherence Group: ↓ Body Fat % ↓ Visceral Fat |
| George et al., 2019; Australia [48] | Multicenter Randomized Controlled Trial: 12 week intervention | 18 adult patients with MASLD; male/female; average age of 52 years old; average BMI of 31.6 kg/m2 Ultrasound or liver biopsy; Intrahepatic Lipids Liver stiffness measurement (Fibroscan) | Mediterranean Diet vs. Low fat Diet | Adherence to Mediterranean Diet 14 item questionnaire [51] Extra virgin olive oil > 4 tablespoon Mediterranean Diet Group were given supply of EVOO and nuts | ↔ Intrahepatic Lipids Non-significant but Mediterranean Diet group showed 8% reduction ↔ Liver Stiffness Measurement ↔ hs-CRP levels | ↔ AST ↔ ALT ↔ GGT | ↔ TG ↔ TC ↔ LDL ↔ HDL ↔ HOMA-IR | ↔ BMI ↔ Weight ↔ WC ↓ Visceral Fat |
| Ristic-Medic et al., 2021; Serbia [35] | Randomized Controlled Trial: 3 months | 12 adult MASLD patients; all male; 27–42 years old; BMI of 25 to 35 kg/m2; Liver ultrasound and Fatty Liver Index | Calorie Restricted Mediterranean Diet vs. Low fat Diet | EVOO as the principal fat, ensuring total fat intake comprised up to −35% of total energy | ↓ Hepatic Steatosis Index ↓Fatty Liver Index ↓ lipid accumulation index ↓ hs-CRP levels | ↓ AST ↓ ALT ↓ GGT | ↓ TG ↓ TC ↓ LDL ↓ TG-HDL ratio ↑ HDL ↓ Fasting Glucose ↓ Insulin ↓ HOMA IR | ↓ BMI ↓ WC ↓ Body Fat % ↓ Visceral Fat |
| Marin-Alejandre et al., 2019; Spain [36] | Randomized Controlled Trial: Fatty Liver in Obesity (FLiO); 6 months intervention | 39 adult MASLD patients; male/female; age 40–80 years old BMI 27.5 kg/m2 to <40 kg/m2; abdominal ultrasonography | Personalized Dietary Strategies; Fatty Liver in Obesity (FLiO) characterized by high adherence to the Mediterranean Diet (MedDiet) vs. Control diet based on American Heart Association (AHA) guidelines | FLiO Diet emphasize the used of extra virgin olive oil as a primary fat source Adherence to Mediterranean Diet 17 item questionnaire; [50] Use only EVOO for cooking, salad dressings, and spreads. | ↓ hepatic volume and hepatic fat content ↔ Liver Stiffness Measurement ↓ hs-CRP levels ↑ adiponectin levels ↑ Total Antioxidant Capacity (TAC) of the Diet | ↓ AST ↓ ALT ↓ GGT | ↓ TG ↓ TC ↓ LDL ↓ TG-HDL ratio ↑ HDL ↓ Fasting Glucose ↓ Insulin ↓ HOMA IR | ↓ BMI ↓ WC |
| Kaliora et al., 2019; Greece [37] | Prospective Non Randomized Intervention Trial | 44 adult patients with MASLD with nonsignificant fibrosis; male/female; 18 years of age and above BMI > 25 kg/m2; Abdominal ultrasound (US) and elastography ultrasound stiffness | Mediterranean diet | Emphasis was given to use EVOO as the main fat in diet. Adherence to Mediterranean diet [52] | ↓ liver fibrosis score/hepatic steatosis ↓ C-reactive protein (CRP), ↓ oxLDL ↔ IL-6 ↔ TNF-a ↔ leptin | ↔ AST ↔ ALT | ↓ fasting glucose, ↓ HbA1c ↓ visfatin, | ↓ BMI ↓ WC ↓ Body Fat ↓ Weight ↓ blood pressure, |
| Montemayor et al., 2022; Spain [39] | Multi-center prospective randomized trial; 6 months intervention | 138 adult patients with MASLD with Metabolic Syndrome; aged 40 to 60 years old; BMI 27–40 kg/m2; Magnetic Resonance Imaging Intrahepatic Fat Content | Mediterranean Diet adherence changes after 6-month: No changes in adherence, Moderate Changes in Adherence and High Changes in Adherence | Adherence to Mediterranean Diet 17 item questionnaire: [53] How much olive oil do you consume per day >4 tablespoon | Increase Adherence to Mediterranean Diet showed in ↓ IFC | Increase Adherence to Mediterranean leads to: ↓ AST ↓ ALT ↓ GGT | ↓ TG in moderate adherence group Increase Adherence to Mediterranean leads to: ↓ HOMA-IR | Increase Adherence to Mediterranean Diet showed larger reduction in BMI, WC and Blood Pressure |
| Properzi et al. 2018; Australia [40] | Prospective Randomized Trial; 12 weeks trial; | 24 adult patients with MASLD; male/female; average age of 51 years old; average BMI of 31.5 kg/m2; MRS/proton density fat fraction (MRS-PDFF) | Ad libitum isocaloric diets Mediterranean vs Low fat | 750 mL of olive oil for the MD group supply every visit | ↓ Hepatic Steatosis/Hepatic triglyceride content ↔ Liver Stiffness Measurement | ↓ ALT ↓ GGT | ↓ TG ↓ TC ↓ HbA1c | ↔ BMI ↔ Weight |
| Katsagoni et al., 2018; Greece [41] | Randomized Controlled Trial: 6 months | 21 patients in the Mediterranean lifestyle group (MLG) with MASLD; and 21 patients in the Mediterranean diet group (MDG) adult with MASLD males/females; median ages 44 and 48 years old; median BMI 31.67 and 32.44 Liver ultrasound | Control group (CG), (B) Mediterranean diet group (MDG) or (C) Mediterranean lifestyle group (MLG). | Emphasis was given to use EVOO as the main fat in diet. Adherence to Mediterranean diet [52] | All groups: ↔ MASLD fibrosis score There is an improvement in liver function tests and liver stiffness measurement in the MLG | ↓ ALT in MLG ↓ GGT in MLG | ↔ TG ↔ TC ↔ LDL ↔ HDL Fasting glucose and insulin resistance (HOMA-IR) significantly improved in the Mediterranean lifestyle group. | ↓ BMI ↓ Weight for MDG and MLG |
| Abbate et al., 2021 Spain [43] | Randomized Controlled Trial: 6 months | 43 adult patients with MASLD and Mets (MD-HMF) 43 adult patients with MASLD and Mets (MD-PA) male/female; aged 40 to 60 years; BMI between 27 and 40 kg/m2; Liver ultrasound | Conventional Diet (CD) group, which followed the American Association for the Study of Liver Disease (AASLD) recommendations Mediterranean Diet–high meal frequency (MD-HMF) Mediterranean Diet–physical activity (MD-PA) | Adherence to Mediterranean Diet 17 item questionnaire: [53] How much olive oil do you consume per day >4 tablespoon Consumption of at least 30 g per day of Olive Oil | ↓ intrahepatic fat content/hepatic steatosis across all Mediterranean diet groups ↔ Liver Stiffness Measurement in all group | ↓ ALT ↓ GGT | ↓ TG ↔ TC ↔ LDL ↑ HDL ↓ HOMA-IR | ↓ BMI ↔ WC |
| Study (Author Year and Country) | Type and Duration of Study | Participants Characteristics | Intervention/Grouping and Comparator/Control | Incorporation of Olive Oil | Effects of Mediterranean Diet | |||
|---|---|---|---|---|---|---|---|---|
| Hepatic Steatosis, Inflammation and Oxidative Stress | Liver Parameters | Blood Lipid and Blood Sugar Profile | Anthropometric and Clinical Measurements | |||||
| Barrea et al., 2023; Italy [38] | Cross Sectional Observational Study | 336 adult patients with 46% prevalence of MASLD; male/female; average age of 35 years old; average BMI 31.18 kg/m2; Fatty Liver Index | Degree of Adherence: Low, Medium, and High Adherence to Mediterranean Diet | Adherence to Mediterranean Diet 14 item questionnaire [51] EVOO > 4 tablespoons | FLI was significantly higher in subjects with low adherence to MD than subjects with average and high adherence to MD. | Low adherence to MD had significantly ↑ AST, ALT, and GGT than subjects with average and high adherence to MD. | Low adherence to MD had significantly ↑ fasting plasma glucose, fasting plasma insulin, LDL cholesterol, and TG, than subjects with average and high adherence to MD | Visceral adipose index (VAI) was significantly higher in subjects with low adherence to MD than subjects with average and high adherence to MD |
| Gelli et al., 2017; Italy [42] | Observational study; 6 months | 46 adult patients with MASLD; male/female; 26–71 years old; BMI range of 18.9–45.3 kg/m2; Liver Ultrasound | Mediterranean Diet Before vs. after the intervention | Emphasis was given to use EVOO as the main fat in diet. Adherence to Mediterranean diet [52] | ↓ liver fat severity/hepatic steatosis | ↓ AST ↓ ALT ↓ GGT | ↓ TG ↑ HDL ↓ Serum Glucose ↓ HOMA-IR Improvement in total-Chol/HDL, LDL/HDL, TG/HDL ratios and Atherogenic Index of Plasma (AIP) | ↓ BMI ↓ WC ↓ Waist to Hip Ratio |
| Aller et al., 2015 Spain [44] | Observational-Association Study | 82 patients with MASLD; male/female; average age 44.2 years old; average BMI of 32.9 kg/m2; Percutaneous liver biopsy | Adherence to Mediterranean diet | Adherence to Mediterranean Diet 14 item questionnaire [51] EVOO > 4 tablespoons | Higher adherence to Mediterranean diet leads to higher odds of protect from high grade of steatosis; Patients without liver inflammation and fibrosis tend to exhibit higher adherence to the Mediterranean diet. Higher levels of adiponectin were observed in patients with lower-grade steatosis. | Higher adherence to Mediterranean diet ↓ AST ↓ ALT | Higher adherence to Mediterranean diet ↓ LDL ↑ HDL ↓ degree of insulin resistance | Higher adherence to Mediterranean ↓ BMI ↓ Weight |
| Baratta et al., 2017 Italy [45] | Observational-Association Study | 584 patients presenting with one or more cardiovascular risk factor; male/female; average age 56 years old; average BMI of 30 kg/m2; 82.7% of patients of the patients have steatosis; Liver ultrasound | Adherence to Mediterranean diet: Low vs. Intermediate vs. High | Adherence to Mediterranean Diet 14 item questionnaire [51] EVOO > 4 tablespoons | High adherence Mediterranean diet is associated with a lower prevalence of MASLD | Higher adherence to Mediterranean diet ↓ AST ↓ GGT | Higher adherence to Mediterranean diet ↓ TG ↑ HDL ↓ HOMA IR (showing direct association with olive oil intake) | Higher adherence to Mediterranean ↓ BMI |
3.2.4. Anthropometric and Clinical Outcomes
3.2.5. EVOO Dosage and Duration of the Study
3.2.6. Type of Studies
4. Discussion
4.1. EVOO as Essential Part of Mediterranean Diet in the Context of MASLD
4.2. The Role of Polyphenols in EVOO in the Context of MASLD
4.3. Limitations and Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine Aminotransferase |
| ANDQCC | Academy of Nutrition and Dietetics Quality Criteria Checklist |
| AST | Aspartate Aminotransferase |
| BMI | Body Mass Index |
| CAT | Catalase |
| CVD | Cardiovascular Disease |
| DHA | Docosahexaenoic Acid |
| EPA | Eicosapentaenoic Acid |
| EVOO | Extra Virgin Olive Oil |
| GGT | Gamma-Glutamyl Transferase |
| (GLP-1) | Glucagon-Like Peptide-1 |
| GPx | Glutathione Peroxidase |
| GrR | Glutathione Reductase |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| hs-CRP | High-Sensitivity C-Reactive Protein |
| IFC | Intrahepatic Fat Content |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-1β | Interleukin-1 Beta |
| IL-7A | Interleukin-7 Alpha |
| LDL | Low-Density Lipoprotein |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| MASL | Metabolic Dysfunction-Associated Steatotic Liver |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MDA | Malondialdehyde |
| MICOL Study | Multicenter Italian Study on Cholelithiasis |
| MUFA | Monounsaturated Fatty Acids |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NUTRIHEP Study | Nutrition and Hepatic Health Study |
| oxLDL | Oxidized Low-Density Lipoprotein |
| PUFA | Polyunsaturated Fatty Acids |
| SOD | Superoxide Dismutase |
| SREBP | Sterol Regulatory Element-Binding Protein |
| TBARS | Thiobarbituric Acid Reactive Substances |
| TC | Total Cholesterol |
| TG | Triglycerides |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor Alpha |
| WC | Waist Circumference |
| WELCOM Study | Western-Eastern Liver Cardio-Metabolic Study (confirm if referring to specific dataset) |
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin. Mol. Hepatol. 2024, 31, S32–S50. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rydqvist, P.; Ramezani, T.; Haas, J.S.; Bantel, H.; Buggisch, P.; Geier, A.; Hofmann, W.-P.; Mauss, S.; Roeb, E.; et al. Metabolic Dysfunction–Associated Steatohepatitis Diagnosis and Management in Germany: Insights from an Expert Consensus Panel. Liver Int. 2025, 45, e70225. [Google Scholar] [CrossRef]
- Zeng, X.-F.; Varady, K.A.; Wang, X.-D.; Targher, G.; Byrne, C.D.; Tayyem, R.; Latella, G.; Bergheim, I.; Valenzuela, R.; George, J.; et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: An international multidisciplinary expert consensus. Metabolism 2024, 161, 156028. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Reyes-Goya, C.; Santana-Garrido, Á.; Espinosa-Martín, P.; Vázquez, C.M.; Mate, A. Wild and cultivated olive trees: Nutraceutical insights of extra virgin olive oils in cardiovascular and ocular diseases. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2024, 1870, 166904. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Assy, N.; Nassar, F.; Nasser, G.; Grosovski, M. Olive oil consumption and non-alcoholic fatty liver disease. World J. Gastroenterol. 2009, 15, 1809–1815. [Google Scholar] [CrossRef]
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef]
- Handu, D.; Moloney, L.; Wolfram, T.; Ziegler, P.; Acosta, A.; Steiber, A. Academy of Nutrition and Dietetics Methodology for Conducting Systematic Reviews for the Evidence Analysis Library. J. Acad. Nutr. Diet. 2016, 116, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Myers, E.; Parrott, J.; Cummins, D.; Splett, P. Funding Source and Research Report Quality in Nutrition Practice-Related Research. PLoS ONE 2011, 6, e28437. [Google Scholar] [CrossRef] [PubMed]
- Mondon, C.; Tan, P.Y.; Chan, C.L.; Tran, T.N.; Gong, Y.Y. Prevalence, determinants, intervention strategies and current gaps in addressing childhood malnutrition in Vietnam: A systematic review. BMC Public Health 2024, 24, 960. [Google Scholar] [CrossRef]
- Kenneally, S.; Sier, J.H.; Moore, J.B. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: A systematic review. BMJ Open Gastroenterol. 2017, 4, e000139. [Google Scholar] [CrossRef]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction–associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212. [Google Scholar] [CrossRef]
- Hsu, C.L.; Loomba, R. From NAFLD to MASLD: Implications of the new nomenclature for preclinical and clinical research. Nat. Metab. 2024, 6, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161. [Google Scholar] [CrossRef]
- Sofi, F.; Giangrandi, I.; Cesari, F.; Corsani, I.; Abbate, R.; Gensini, G.F.; Casini, A. Effects of a 1-year dietary intervention with n-3 polyunsaturated fatty acid-enriched olive oil on non-alcoholic fatty liver disease patients: A preliminary study. Int. J. Food Sci. Nutr. 2010, 61, 792–802. [Google Scholar] [CrossRef]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G.; et al. Daily Use of Extra Virgin Olive Oil with High Oleocanthal Concentration Reduced Body Weight, Waist Circumference, Alanine Transaminase, Inflammatory Cytokines and Hepatic Steatosis in Subjects with the Metabolic Syndrome: A 2-Month Intervention Study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Nigam, P.; Bhatt, S.; Misra, A.; Chadha, D.S.; Vaidya, M.; Dasgupta, J.; Pasha, Q.M.A. Effect of a 6-Month Intervention with Cooking Oils Containing a High Concentration of Monounsaturated Fatty Acids (Olive and Canola Oils) Compared with Control Oil in Male Asian Indians with Nonalcoholic Fatty Liver Disease. Diabetes Technol. Ther. 2014, 16, 255–261. [Google Scholar] [CrossRef]
- Kruse, M.; Kemper, M.; Gancheva, S.; Osterhoff, M.; Dannenberger, D.; Markgraf, D.; Machann, J.; Hierholzer, J.; Roden, M.; Pfeiffer, A.F.H. Dietary Rapeseed Oil Supplementation Reduces Hepatic Steatosis in Obese Men—A Randomized Controlled Trial. Mol. Nutr. Food Res. 2020, 64, 2000419. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.; Brevik-Andersen, M.; Qin, Y.; Innes, J.K.; Calder, P.C. Evaluation of a High Concentrate Omega-3 for Correcting the Omega-3 Fatty Acid Nutritional Deficiency in Non-Alcoholic Fatty Liver Disease (CONDIN). Nutrients 2018, 10, 1126. [Google Scholar] [CrossRef]
- Shidfar, F.; Bahrololumi, S.S.; Doaei, S.; Mohammadzadeh, A.; Gholamalizadeh, M.; Mohammadimanesh, A. The Effects of Extra Virgin Olive Oil on Alanine Aminotransferase, Aspartate Aminotransferase, and Ultrasonographic Indices of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients Undergoing Low Calorie Diet. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1053710. [Google Scholar] [CrossRef]
- Cueto-Galán, R.; Barón, F.J.; Valdivielso, P.; Pintó, X.; Corbella, E.; Gómez-Gracia, E.; Wärnberg, J. Changes in fatty liver index after consuming a Mediterranean diet: 6-Year follow-up of the PREDIMED-Malaga trial. Med. Clín. Engl. Ed. 2017, 148, 435–443. [Google Scholar] [CrossRef]
- Yahay, M.; Heidari, Z.; Allameh, Z.; Amani, R. The effects of canola and olive oils consumption compared to sunflower oil, on lipid profile and hepatic steatosis in women with polycystic ovarian syndrome: A randomized controlled trial. Lipids Health Dis. 2021, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Keshk, W.; Ziada, D.; Soliman, S.; EL-Kalla, F. The Effect of a Hypocaloric Diet Containing Olive Oil on Hepatic Steatosis Grading Using Tissue Elastography: A Randomized Controlled Trial. Afro-Egypt. J. Infect. Endem. Dis. 2022, 12, 57–65. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; McCormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D.; Sheron, N.; et al. Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the WELCOME* study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; García, S.; Argelich, E.; Casares, M.; Ugarriza, L.; Llompart, I.; Tur, J.A.; Sureda, A. Impact of Adherence to the Mediterranean Diet on Antioxidant Status and Metabolic Parameters in NAFLD Patients: A 24-Month Lifestyle Intervention Study. Antioxidants 2024, 13, 480. [Google Scholar] [CrossRef]
- George, E.S.; Reddy, A.; Nicoll, A.J.; Ryan, M.C.; Itsiopoulos, C.; Abbott, G.; Johnson, N.A.; Sood, S.; Roberts, S.K.; Tierney, A.C. Impact of a Mediterranean diet on hepatic and metabolic outcomes in non-alcoholic fatty liver disease: The MEDINA randomised controlled trial. Liver Int. 2022, 42, 1308–1322. [Google Scholar] [CrossRef]
- Ristic-Medic, D.; Kovacic, M.; Takic, M.; Arsic, A.; Petrovic, S.; Paunovic, M.; Jovicic, M.; Vucic, V. Calorie-Restricted Mediterranean and Low-Fat Diets Affect Fatty Acid Status in Individuals with Nonalcoholic Fatty Liver Disease. Nutrients 2021, 13, 15. [Google Scholar] [CrossRef]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. The Metabolic and Hepatic Impact of Two Personalized Dietary Strategies in Subjects with Obesity and Nonalcoholic Fatty Liver Disease: The Fatty Liver in Obesity (FLiO) Randomized Controlled Trial. Nutrients 2019, 11, 2543. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Gioxari, A.; Kalafati, I.P.; Diolintzi, A.; Kokkinos, A.; Dedoussis, G.V. The Effectiveness of Mediterranean Diet in Nonalcoholic Fatty Liver Disease Clinical Course: An Intervention Study. J. Med. Food 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Savastano, S.; Colao, A.; Muscogiuri, G. Adherence to Mediterranean Diet: Any Association with NAFLD? Antioxidants 2023, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Mascaró, C.M.; Ugarriza, L.; Casares, M.; Llompart, I.; Abete, I.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A.; Bouzas, C. Adherence to Mediterranean Diet and NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 3186. [Google Scholar] [CrossRef] [PubMed]
- Properzi, C.; O’Sullivan, T.A.; Sherriff, J.L.; Ching, H.L.; Jeffrey, G.P.; Buckley, R.F.; Tibballs, J.; MacQuillan, G.C.; Garas, G.; Adams, L.A. Ad Libitum Mediterranean and Low-Fat Diets Both Significantly Reduce Hepatic Steatosis: A Randomized Controlled Trial. Hepatology 2018, 68, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Katsagoni, C.N.; Papatheodoridis, G.V.; Ioannidou, P.; Deutsch, M.; Alexopoulou, A.; Papadopoulos, N.; Papageorgiou, M.-V.; Fragopoulou, E.; Kontogianni, M.D. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: A randomised controlled clinical trial. Br. J. Nutr. 2018, 120, 164–175. [Google Scholar] [CrossRef]
- Gelli, C.; Tarocchi, M.; Abenavoli, L.; Di Renzo, L.; Galli, A.; De Lorenzo, A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3150. [Google Scholar] [CrossRef]
- Abbate, M.; Mascaró, C.M.; Montemayor, S.; Barbería-Latasa, M.; Casares, M.; Gómez, C.; Angullo-Martinez, E.; Tejada, S.; Abete, I.; Zulet, M.A.; et al. Energy Expenditure Improved Risk Factors Associated with Renal Function Loss in NAFLD and MetS Patients. Nutrients 2021, 13, 629. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; de la Fuente, B.; De Luis Román, D.A. Mediterranean Diet Is Associated With Liver Histology In Patients With Non Alcoholic Fatty Liver Disease. Nutr. Hosp. 2015, 32, 2518–2524. [Google Scholar] [CrossRef] [PubMed]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease: Synopsis of the Evidence Available from Systematic Reviews and Meta-Analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. EFSA Health Claims-Based Virgin Olive Oil Shelf-Life. Antioxidants 2023, 12, 1563. [Google Scholar] [CrossRef]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.-C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef]
- Shabalala, S.C.; Dludla, P.V.; Mabasa, L.; Kappo, A.P.; Basson, A.K.; Pheiffer, C.; Johnson, R. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed. Pharmacother. 2020, 131, 110785. [Google Scholar] [CrossRef]
- Bouzas, C.; Bibiloni, M.M.; Julibert, A.; Ruiz-Canela, M.; Salas-Salvadó, J.; Corella, D.; Zomeño, M.D.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Adherence to the Mediterranean Lifestyle and Desired Body Weight Loss in a Mediterranean Adult Population with Overweight: A PREDIMED-Plus Study. Nutrients 2020, 12, 2114. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Arvaniti, F.; Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev. Med. 2007, 44, 335–340. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: A comprehensive overview. J. Intern. Med. 2021, 290, 549–566. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Blanco Mejía, S.; Viguiliouk, E.; Khan, T.; Kendall, C.W.C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 1207–1227. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Long-term impact of mediterranean diet on cardiovascular disease prevention: A systematic review and meta-analysis of randomized controlled trials. Curr. Probl. Cardiol. 2024, 49, 102509. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Huang, Q.; Zhang, Q.; Li, M.; Wu, Y. The effectiveness of lifestyle interventions for diabetes remission on patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Worldviews Evid. Based Nurs. 2023, 20, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Kastorini, C.-M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean diet and metabolic syndrome: An updated systematic review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef]
- Hansrivijit, P.; Oli, S.; Khanal, R.; Ghahramani, N.; Thongprayoon, C.; Cheungpasitporn, W. Mediterranean diet and the risk of chronic kidney disease: A systematic review and meta-analysis. Nephrol. Carlton Vic 2020, 25, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Charkviani, M.; Thongprayoon, C.; Tangpanithandee, S.; Krisanapan, P.; Miao, J.; Mao, M.A.; Cheungpasitporn, W. Effects of Mediterranean Diet, DASH Diet, and Plant-Based Diet on Outcomes among End Stage Kidney Disease Patients: A Systematic Review and Meta-Analysis. Clin. Pract. 2022, 13, 41–51. [Google Scholar] [CrossRef]
- Soltani, S.; Jayedi, A. Adherence to healthy dietary pattern and risk of kidney disease: A systematic review and meta-analysis of observational studies. Int. J. Vitam. Nutr. Res. Int. Z. Vitam.-Ernahrungsforschung J. Int. Vitaminol. Nutr. 2022, 92, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Sommariva, A.; Degoni, L.M.; Gallo, G.; Mancarella, M.; Natarelli, F.; Savoia, A.; Catalini, A.; Ferranti, R.; Pregliasco, F.E.; et al. Association between Mediterranean diet and dementia and Alzheimer disease: A systematic review with meta-analysis. Aging Clin. Exp. Res. 2024, 36, 77. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J. Neurol. Sci. 2022, 434, 120166. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- González-Palacios Torres, C.; Barrios-Rodríguez, R.; Muñoz-Bravo, C.; Toledo, E.; Dierssen, T.; Jiménez-Moleón, J.J. Mediterranean diet and risk of breast cancer: An umbrella review. Clin. Nutr. Edinb. Scotl. 2023, 42, 600–608. [Google Scholar] [CrossRef]
- Zupo, R.; Castellana, F.; Piscitelli, P.; Crupi, P.; Desantis, A.; Greco, E.; Severino, F.P.; Pulimeno, M.; Guazzini, A.; Kyriakides, T.C.; et al. Scientific evidence supporting the newly developed one-health labeling tool “Med-Index”: An umbrella systematic review on health benefits of mediterranean diet principles and adherence in a planeterranean perspective. J. Transl. Med. 2023, 21, 755. [Google Scholar] [CrossRef]
- Rajewski, P.; Cieściński, J.; Rajewski, P.; Suwała, S.; Rajewska, A.; Potasz, M. Dietary Interventions and Physical Activity as Crucial Factors in the Prevention and Treatment of Metabolic Dysfunction-Associated Steatotic Liver Disease. Biomedicines 2025, 13, 217. [Google Scholar] [CrossRef]
- WHO Updates Guidelines on Fats and Carbohydrates. Available online: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (accessed on 15 July 2025).
- Green, C.J.; Hodson, L. The Influence of Dietary Fat on Liver Fat Accumulation. Nutrients 2014, 6, 5018–5033. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, C.C.; Bonfiglio, C.; Notarnicola, M.; Rendina, M.; Castellaneta, A.; Di Leo, A.; Giannelli, G.; Fontana, L. High Extra Virgin Olive Oil Consumption Is Linked to a Lower Prevalence of NAFLD with a Prominent Effect in Obese Subjects: Results from the MICOL Study. Nutrients 2023, 15, 4673. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, C.; Cuccaro, F.; Campanella, A.; Rosso, N.; Tatoli, R.; Giannelli, G.; Donghia, R. Effect of Intake of Extra Virgin Olive Oil on Mortality in a South Italian Cohort with and without NAFLD. Nutrients 2023, 15, 4593. [Google Scholar] [CrossRef]
- Donghia, R.; Tatoli, R.; Campanella, A.; Losurdo, G.; Di Leo, A.; De Pergola, G.; Bonfiglio, C.; Giannelli, G. Extra Virgin Olive Oil Reduces the Risk of Non-Alcoholic Fatty Liver Disease in Females but Not in Males: Results from the NUTRIHEP Cohort. Nutrients 2024, 16, 3234. [Google Scholar] [CrossRef]
- Flynn, M.; Tierney, A.; Itsiopoulos, C. Is Extra Virgin Olive Oil the Critical Ingredient Driving the Health Benefits of a Mediterranean Diet? A Narrative Review. Nutrients 2023, 15, 2916. [Google Scholar] [CrossRef] [PubMed]
- Seidita, A.; Soresi, M.; Giannitrapani, L.; Di Stefano, V.; Citarrella, R.; Mirarchi, L.; Cusimano, A.; Augello, G.; Carroccio, A.; Iovanna, J.L.; et al. The clinical impact of an extra virgin olive oil enriched mediterranean diet on metabolic syndrome: Lights and shadows of a nutraceutical approach. Front. Nutr. 2022, 9, 980429. [Google Scholar] [CrossRef] [PubMed]
- Lucas, L.; Russell, A.; Keast, R. Molecular mechanisms of inflammation. Anti-inflammatory benefits of virgin olive oil and the phenolic compound oleocanthal. Curr. Pharm. Des. 2011, 17, 754–768. [Google Scholar] [CrossRef]
- Sato, K.; Arai, H.; Mizuno, A.; Fukaya, M.; Sato, T.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Doi, T.; et al. Dietary palatinose and oleic acid ameliorate disorders of glucose and lipid metabolism in Zucker fatty rats. J. Nutr. 2007, 137, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, J.A.; Gallego de la Sacristana, A.; Romero, I.; Vidal-Puig, A.; Latre, J.M.; Sanchez, E.; Perez-Martinez, P.; Lopez-Miranda, J.; Perez-Jimenez, F. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes Care 2007, 30, 1717–1723. [Google Scholar] [CrossRef]
- Njei, B.; Ameyaw, P.; Al-Ajlouni, Y.; Njei, L.-P.; Boateng, S. Diagnosis and Management of Lean Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A Systematic Review. Cureus 2024, 16, e71451. [Google Scholar] [CrossRef]
- Sato-Espinoza, K.; Chotiprasidhi, P.; Huaman, M.R.; Díaz-Ferrer, J. Update in lean metabolic dysfunction-associated steatotic liver disease. World J. Hepatol. 2024, 16, 452–464. [Google Scholar] [CrossRef]
| Author and Year of Publication | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rezaei et al. (2019) [21] | + | + | + | − | − | + | + | + | + | + | Positive |
| Sofi et al. (2010) [22] | + | − | + | − | − | + | + | + | + | + | Positive |
| Patti et al. (2020) [23] | + | − | − | − | − | + | + | + | + | + | Positive |
| Pintó et al. (2019) [24] | + | + | + | + | − | + | + | + | + | + | Positive |
| Nigam et al. (2014) [25] | + | + | + | + | − | + | + | + | + | + | Positive |
| Kruse et al. (2020) [26] | + | + | + | + | − | + | + | + | + | + | Positive |
| Tobin et al. (2018) [27] | + | + | + | + | + | + | + | + | + | + | Positive |
| Shidfar et al. (2018) [28] | + | + | + | + | − | + | + | + | + | + | Positive |
| Cueto-Galán et al. (2017) [29] | + | + | + | − | − | + | + | + | + | + | Positive |
| Yahay et al. (2021) [30] | + | + | + | − | − | + | + | + | + | + | Positive |
| Keshk et al. (2022) [31] | + | + | + | − | − | + | + | + | + | + | Positive |
| Scorletti et al. (2014) [32] | + | + | + | + | + | + | + | + | + | + | Positive |
| Quetglas-Llabrés et al. (2024) [33] | + | + | + | + | − | + | + | + | + | + | Positive |
| George et al. (2022) [34] | + | + | + | + | − | + | + | + | + | + | Positive |
| Ristic-Medic et al. (2021) [35] | + | + | + | − | − | + | + | + | + | + | Positive |
| Marin-Alejandre et al. (2019) [36] | + | + | + | − | − | + | + | + | + | + | Positive |
| Kaliora et al. (2019) [37] | + | + | + | − | − | + | + | + | + | + | Positive |
| Barrea et al. (2023) [38] | + | − | § | § | − | + | + | + | + | + | Positive |
| Montemayor et al. (2022) [39] | + | + | + | + | − | + | + | + | + | + | Positive |
| Properzi et al. (2018) [40] | + | + | + | + | − | + | + | + | + | + | Positive |
| Katsagoni et al. (2018) [41] | + | + | + | + | − | + | + | + | + | + | Positive |
| Gelli et al. (2017) [42] | + | − | § | − | − | + | + | + | + | + | Positive |
| Abbate et al. (2021) [43] | + | + | + | + | − | + | + | + | + | + | Positive |
| Aller (2015) [44] | + | − | § | § | − | + | + | + | + | + | Positive |
| Baratta (2017) [45] | + | − | § | § | − | + | + | + | + | + | Positive |
| Study (Author Year and Country) | Type and Duration of Study | Participants Characteristics for Olive Oil Arm and MASLD Diagnosis | Type and Dose of Olive Oil | Diet During the Study | Effects of Olive Oil | |||
|---|---|---|---|---|---|---|---|---|
| Hepatic Steatosis, Inflammation and Oxidative Stress | Liver Parameters | Blood Lipid and Blood Sugar Profile | Anthropometric and Clinical Measurements | |||||
| Rezaei et al. (2019) Iran [21] | Randomized Clinical Trial; Olive Oil vs. Sunflower Oil; 12 weeks intervention | 26 adult MASLD patients; male/female; ≥18 years old; BMI of: ≥25 kg/m2; Liver Ultrasound | 20 mL of standard Olive Oil per day; Composition: Oleic acid (64.3%) Linoleic (15.4%) Palmitic (15.2%) Omega 3 fatty acid (1%) | Hypocaloric diet with 500 kcal deficit (10–15% from protein, 30–35% from fat and 50–55% from the carbohydrates) | ↓ Hepatic Steatosis (2x reduction vs. sunflower oil) ↔ IL-6 ↔ Serum malondialdehyde ↔ Total antioxidant capacity n | ↓ AST ↓ ALT | ↓ Serum TG ↔ FBG | ↓ Weight ↓ BMI ↓ WC ↓ Blood Pressure |
| Sofi et al. (2010) Italy [22] | Randomized Clinical Trial (Pilot study); Olive Oil enriched with PUFA vs. Olive Oil alone; 12 months intervention | 6 adult patients with MASLD; male/female; ≥18 years old; average BMI of ≥29.3 kg/m2; Liver Ultrasound | 6.5 mL of olive oil enriched with n-3 PUFA (EPA/DHA) | The usual diet;not standardized or modified by the researchers | ↓ Hepatic Steatosis ↑ Adiponectin levels ↔ TBARS ↔ dROMS ↔ BAP ↔ SHp | ↓ AST ↓ ALT ↓ GGT | ↔ TC ↓ LDL ↑ HDL ↔ Blood Glucose ↔ HOMA | ↔ BMI |
| Patti et al. (2020) Italy [23] | Intervention study; before vs. after treatment of High Oleocanthal concentration EVOO; 2 months | 23 adult patients with Metabolic Syndrome with Hepatic steatosis; male/female ≥18 years old; average BMI of ≥31 kg/m2; Ultrasound Examination | 32 mL of EVOO Mono cultivar EVOO with High Oleocanthal Concentration | Mediterranean Diet | ↓ Hepatic Steatosis ↓ IL6, ↓ IL 17 A, ↓ TNF-a, ↓ IL-1B ↑IL-10 | ↔ AST ↓ ALT ↔ GGT | ↔ TC ↔ TG ↔ LDL ↔ HDL ↔ Glycemia ↔ Hb1AC ↔ HOMA | ↓ Weight ↓ BMI ↓ WC |
| Pintó et al., 2019 Spain [24] | Randomized Control Trial; Bellvitge PREDIMED trial; EVOO + MD vs. Nuts+ MD; Control group: Low Fat Diet; 3 years intervention | 34 adult participants high risk of CVD; male/female; older adults with average age of 64 years old; average BMI of ≥28.7 kg/m2; NMR imaging | 60 mL/day of EVOO (contains high amount of phenolic compound, antioxidants) | Energy Unrestricted Mediterranean Diet | ↓ Hepatic Steatosis prevalence ↓ 12-HETE levels ↔ hs-CRP | ↔ AST ↔ ALT | ↔ TC ↔ TG ↔ LDL ↔ HDL ↔ Fasting glucose | ↔ BMI ↔ Weight ↔ WC |
| Nigam et al., 2014 India [25] | Randomized Control Trial; Olive Oil vs. Canola oil and soybean/safflower; 6 months intervention | 30 adult patients with MASLD; all male; average age of 37 years old; average BMI of 27.2 kg/m2; Liver Ultrasound | 20 mL of Standard Olive Oil per day | Therapeutic Lifestyle Change (TLD diet) with daily energy intake of 15–21% protein, 55–70% carbohydrates, 20% from fats | ↓ Hepatic Steatosis ↓ hs-CRP | ↔ AST ↔ ALT | ↓ TG ↑ HDL ↓ Fasting Insulin ↓ HOMA-IR ↓ HOMA-BCF ↓ Disposition Index | ↓ Weight ↓ BMI ↔ WC |
| Kruse et al., 2020 (Germany) [26] | Parallel-group randomized controlled trial; 8 weeks of daily oil supplementation | 11 adult with MASLD; all male; aged 18–65 old; BMI of 30–35 kg/m2; H-MRS | Refined olive oil 50 g/day (~3.5 tablespoons) an | Isocaloric diet | ↑ Intrahepatic lipid (IHL) ↔ IL-6 ↔ IL-8 ↔ CK 18 | ↔ AST ↔ ALT | ↔ TG ↔ TC ↔ LDL ↔ HDL, ↔ apolipoprotein ↔ B serum ↔ glucose, ↔ insulin, ↔ HOMA IR | ↔ Weight ↔ BMI ↔ WC ↔ total body fat ↔ Waist-to-hip ratio (WHR) of |
| Tobin et al., 2018 Norway [27] | Randomized Control Trial; Omega-3 concentrate vs. olive oil (as placebo) 24 weeks intervention | 86 adult patients with MASLD; male/female; ≥18 years old; average BMI of 32.4 kg/m2; MRI | Olive oil (gel capsules containing 1 g of olive oil)—3 caps daily. | Calorie restricted diet | ↓ Hepatic Steatosis | ↓ AST ↓ ALT ↓ GGT | ↓ TG | ↔ BMI ↔ Weight ↔ WC |
| Shidfar et al., 2018 Iran [28] | Randomized Clinical Trial Olive Oil vs. Normal Cooking Oil; 12 weeks intervention | 25 adult patients with MASLD; male/female; average age of 45 years old; average BMI of 29.7 kg/m2; Liver Ultrasound | 20% of the total energy intake virgin olive oil | Hypocaloric diet with an aim of 5% weight reduction within 3 months with 50% carbohydrates, 20% protein and 30% fats | ↔ Hepatic Steatosis (but more improvement on olive oil arm vs. the control) | ↓ AST ↓ ALT | ↓ Weight ↓ BMI ↔WC | |
| Cueto-Galán et al., 2017 Spain [29] | Randomized Clinical Trial; Part of the PREDIMED Malaga Trial; EVOO + MD, EVOO+ Dried Fruits and Nuts, Control Diet: Low Fat Diet 6 years follow up | 117 adult participant where 57% of the total sample have MASLD; male/female; average age of 67 years old; average BMI of 29.55 kg/m2; Fatty Liver Index | 1 L per week (estimation of 143 mL/day consumption) | Mediterranean Diet | ↓ Fatty Liver Index vs. the control group indicating reduced steatosis | Fatty Liver Index Calculation includes triglycerides, specific HDL/LDL changes were not separately reported | Minimal change in BMI ↔ WC | |
| Yahay et al., 2021 Iran [30] | Randomized Clinical Trial; Olive Oil vs. Canola and control: sunflower oil, 10 weeks intervention | 24 adult female with PCOS; 18–45 years old; average BMI of 28.84 kg/m2; Liver Ultrasound | 25 mL per day of Olive oil; High MUFA 69.22%), n-6 PUFA (~11.20%), n-3 PUFA (~0.63%) | Balanced Diet with 45–60% from Carbohydrates, 30–35% from fats and 15–18% from protein | ↓ Hepatic Steatosis (Fatty Liver severity measure of the extent of fat deposition in the liver) | ↔ SHGB | ↔ TC ↔ TG ↔ LDL ↔ HDL ↓ HOMA-IR | ↔ BMI ↔ Weight |
| Keshk et al., 2022 Egypt [31] | Randomized Clinical Trial Hypocaloric Diet with Olive Oil vs. Hypocaloric Diet without Olive Oil; 6 months duration | 30 adult patients with MASLD; male/female; ≥18 years old; BMI of 30–40 kg/m2 Transient Elastography Fibroscan | 20% of the total energy intake from refined olive oil blend | Hypocaloric diet (-500 kcal/day) but following Mediterranean dietary pattern (50% energy from carbs and 20% from protein) | ↓ Controlled Attenuation Parameter (but much lower in Hypocaloric Diet without Oilve Oil) | ↓ AST ↓ ALT | ↓ TC ↓ TG ↔ LDL ↔ HDL | ↓ Weight ↓ BMI ↓ WC |
| Scorletti et al., 2014 United Kingdom [32] | Randomized Clinical Trial, WELCOME study Omacor (DHA/EPA) vs. olive oil (as placebo) 72 weeks intervention | 45 adult patients with MASLD; male/female; average age of 54 years old; average BMI of 32 kg/m2 magnetic resonance spectroscopy | 4 g per day of olive oil; 1 g of olive oil contains 600 mg of oleic acid plus lesser amounts of linoleic, palmitic, stearic, and a-linolenic acids | General dietary advice | ↔ Hepatic Steatosis | ↓ AST ↓ ALT | ↔ TC ↔ TG ↔ LDL ↔ HDL ↔ Hb1AC ↔Fasting glucose ↔ Fasting Insulin | ↔ BMI ↔ Weight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardino, M.; Tiribelli, C.; Rosso, N. Exploring the Role of Extra Virgin Olive Oil (EVOO) in MASLD: Evidence from Human Consumption. Nutrients 2025, 17, 2932. https://doi.org/10.3390/nu17182932
Bernardino M, Tiribelli C, Rosso N. Exploring the Role of Extra Virgin Olive Oil (EVOO) in MASLD: Evidence from Human Consumption. Nutrients. 2025; 17(18):2932. https://doi.org/10.3390/nu17182932
Chicago/Turabian StyleBernardino, Melvin, Claudio Tiribelli, and Natalia Rosso. 2025. "Exploring the Role of Extra Virgin Olive Oil (EVOO) in MASLD: Evidence from Human Consumption" Nutrients 17, no. 18: 2932. https://doi.org/10.3390/nu17182932
APA StyleBernardino, M., Tiribelli, C., & Rosso, N. (2025). Exploring the Role of Extra Virgin Olive Oil (EVOO) in MASLD: Evidence from Human Consumption. Nutrients, 17(18), 2932. https://doi.org/10.3390/nu17182932







