α-Lack-SPI Alleviates MASLD in Rats via Regulating Hepatic Lipid Accumulation and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
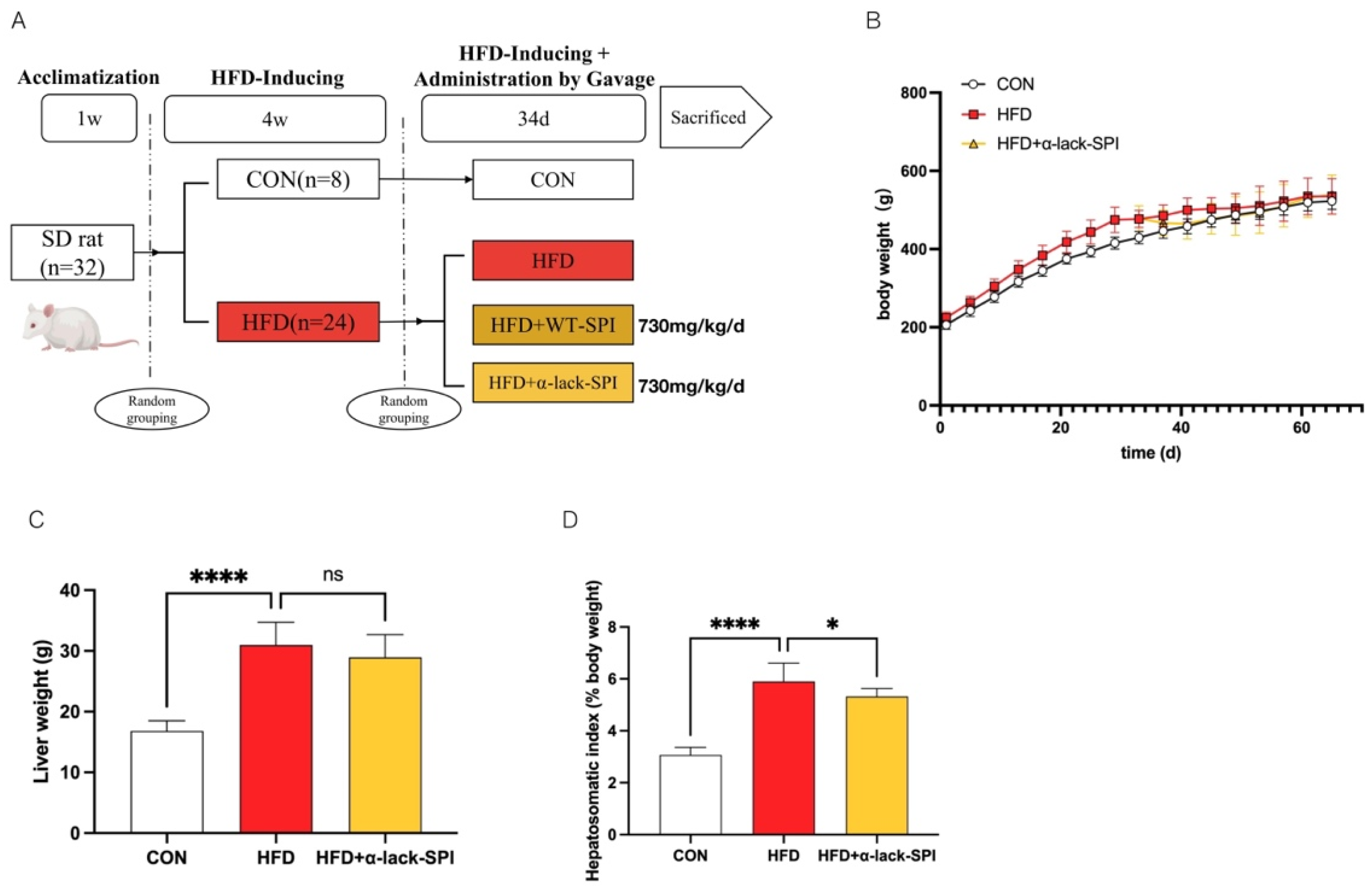
2.2. Animal Experiments
2.3. Measurements of Serum and Liver Biochemical Markers
2.4. Histopathological Analysis
2.5. Western Blot Analysis
2.6. Real-Time Quantitative Polymerase Chain Reaction Analysis
2.7. Statistical Analysis
3. Results
3.1. α-Lack-SPI Has No Effect on Either Body Weight or Liver Weight in Rats Fed with HFD
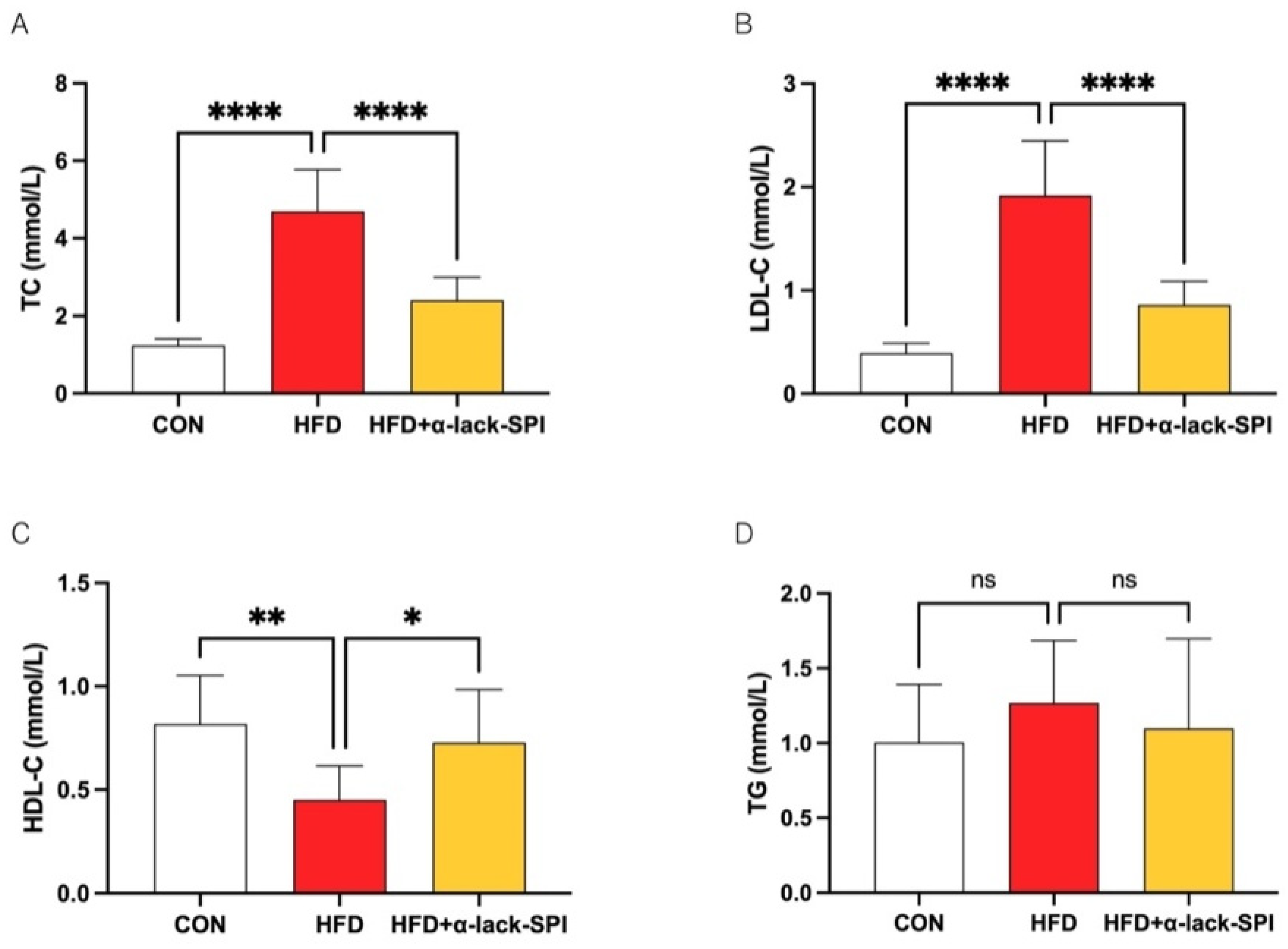
3.2. α-Lack-SPI Has Positive Effects on Lipid Profiles of Rats with HFD-Induced MASLD
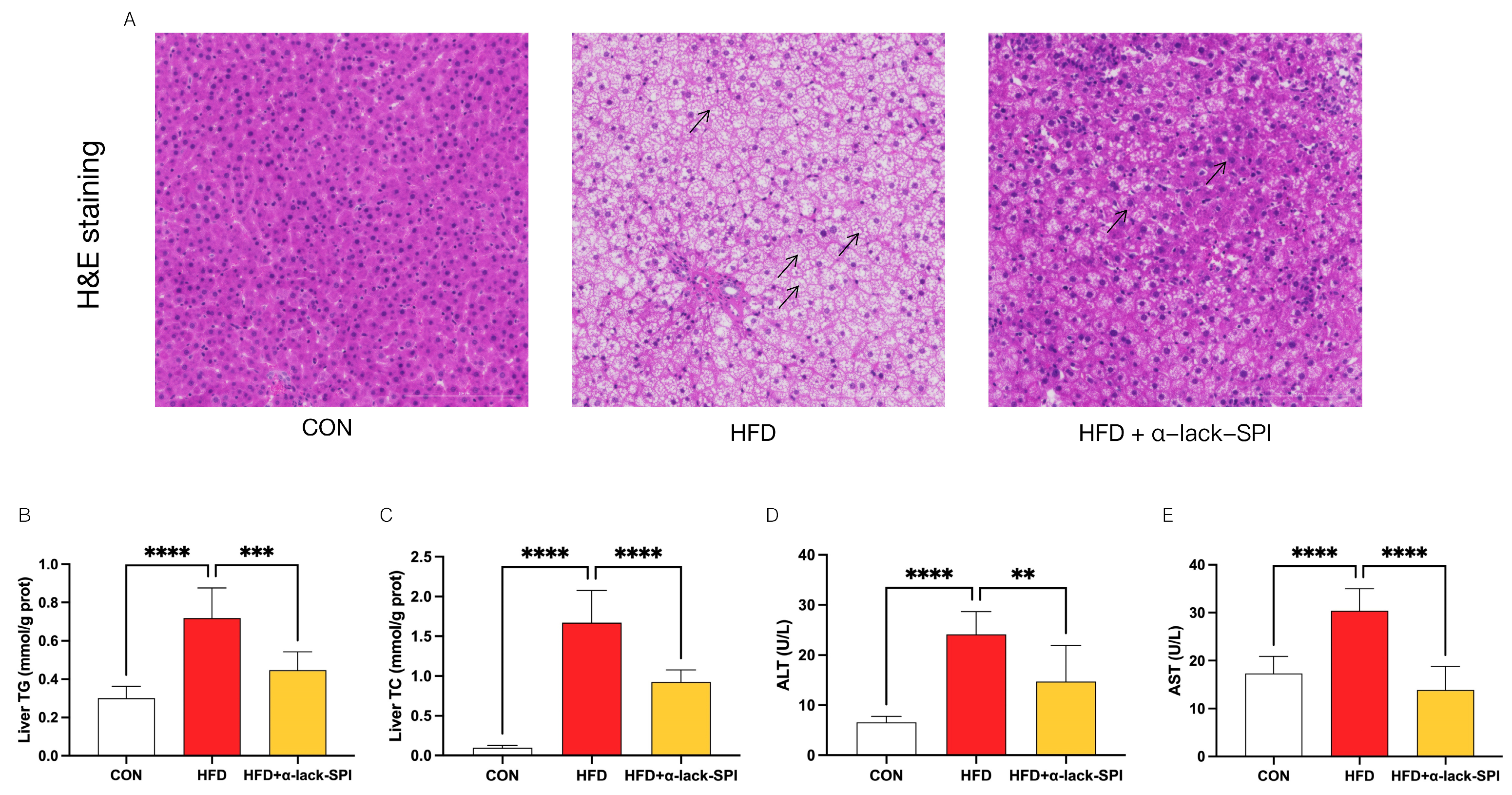
3.3. α-Lack-SPI Ameliorates Hepatic Steatosis in Rats with HFD-Induced MASLD
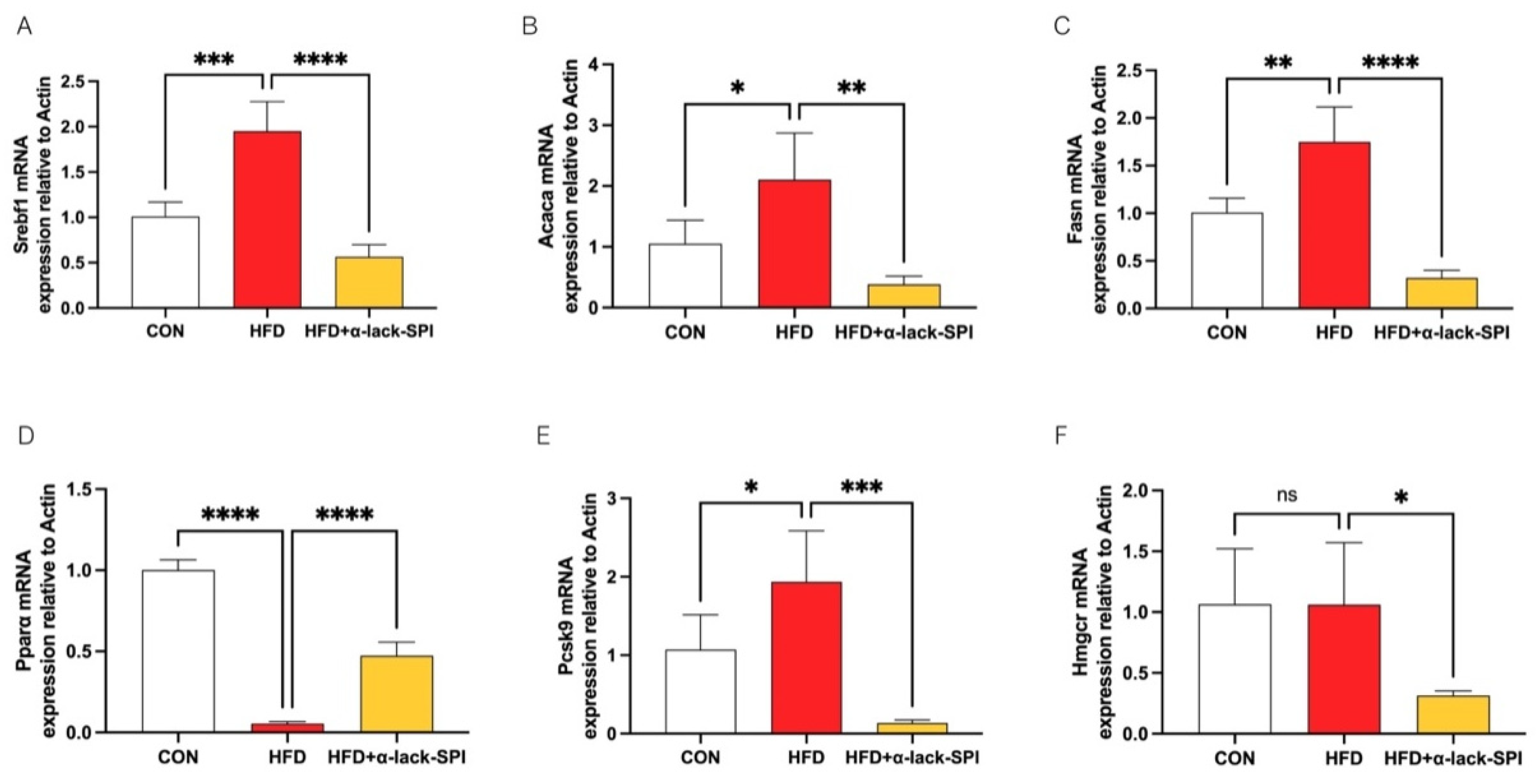
3.4. α-Lack-SPI Regulates Hepatic Lipid Synthesis and Disposal in Rats with HFD-Induced MASLD
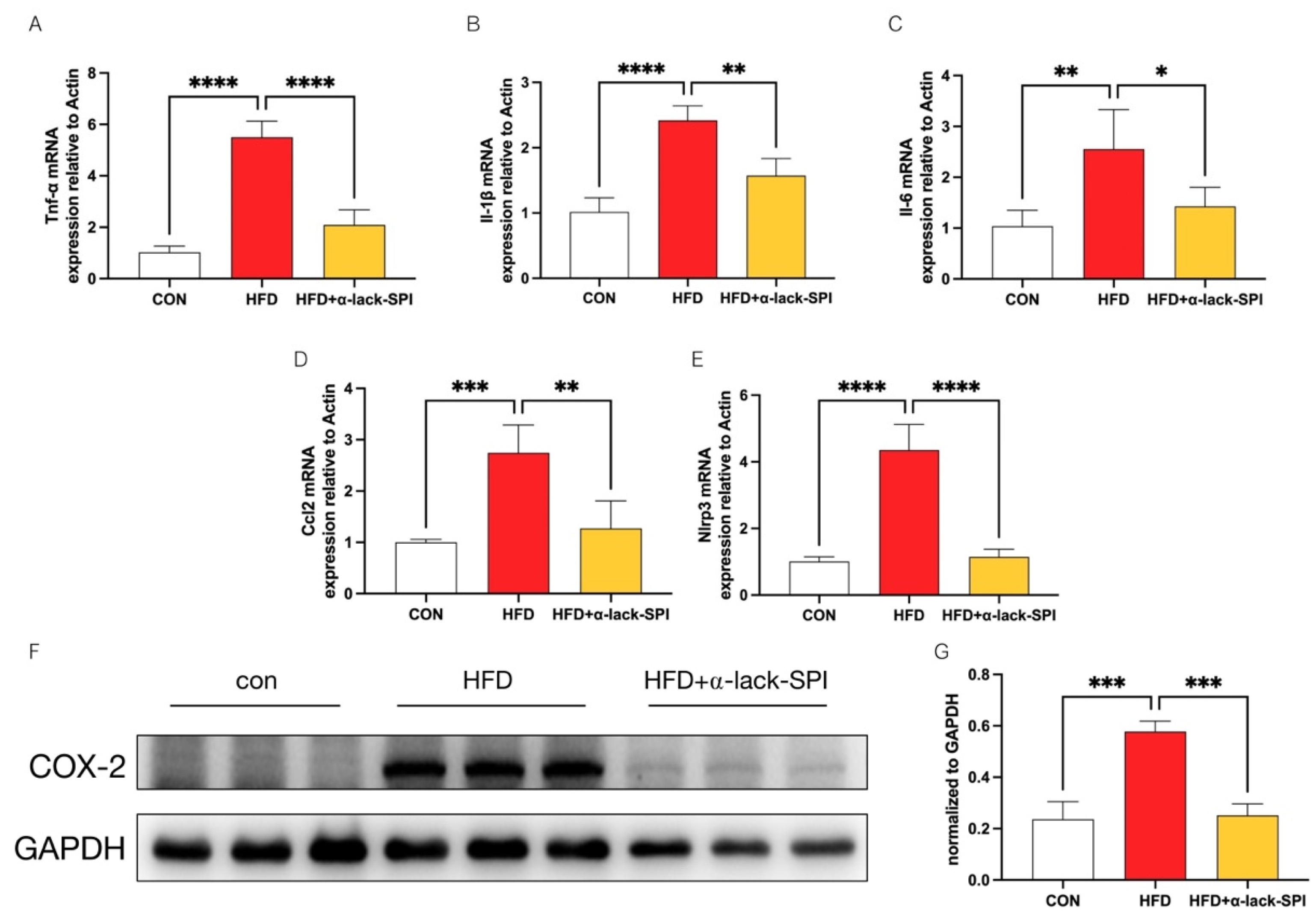
3.5. α-Lack-SPI Inhibits Hepatic Inflammation in Rats with HFD-Induced MASLD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| MASH | Metabolic Dysfunction-Associated Steatohepatitis |
| α-lack-SPI | α-subunit-deficient soy protein isolate |
| WT-SPI | Wild type soy protein isolate |
| HFD | High fat high cholesterol diet |
| TC | Total cholesterol |
| TG | Triglycerides |
| LDL-C | Low density lipoprotein cholesterol |
| HDL-C | High density lipoprotein cholesterol |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| COX-2 | Cyclooxygenase-2 |
| Srebf1 | Sterol regulatory element-binding transcription factor 1 |
| Acaca | Acetyl-CoA carboxylase |
| Fasn | Fatty acid synthase |
| Pcsk9 | Proprotein convertase subtilisin/kexin type 9 |
| Pparα | Peroxisome proliferator-activated receptor alpha |
| Hmgcr | 3-Hydroxy-3-methylglutaryl-CoA reductase |
| Nlrp3 | Nucleotide-binding domain, leucine-rich repeat-containing family, pyrin domain-containing 3 |
| Tnf-α | Tumor necrosis factor alpha |
| Il-1β | Interleukin-1 beta |
| Il-6 | Interleukin-6 |
| Ccl2 | Chemokine (C-C motif) ligand 2 |
| Actb | Actin, beta |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase |
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2022, 31, 17–27. [Google Scholar] [CrossRef]
- Hydes, T.; Alam, U.; Cuthbertson, D.J. The Impact of Macronutrient Intake on Non-alcoholic Fatty Liver Disease (NAFLD): Too Much Fat, Too Much Carbohydrate, or Just Too Many Calories? Front. Nutr. 2021, 8, 640557. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, D.; Ji, W.; Lu, Z.; Huang, C.; Zhao, J.; Xiao, T.; Wang, D.; Kong, Y.; Jia, J.; et al. Global and Chinese burden of non-alcoholic fatty liver disease in chronic liver disease: Findings from the Global Burden of Disease Study 2021. Chin. Med. J. 2025, 138, 1741–1751. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated mechanisms of MASLD pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Ferré, P.; Foufelle, F. Hepatic steatosis: A role for lipogenesis and the transcription factor SREBP-1c. Diabetes Obes. Metab. 2010, 12, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.X.; Baker, S.S.; Liu, W.S.; Tao, M.H.; Patel, R.; Nowak, N.J.; Baker, R.D. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: Combined effects of pathways on steatosis. Metab. Clin. Exp. 2011, 60, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyoshi, H.; Yasui, K.; Harano, Y.; Endo, M.; Tsuji, K.; Minami, M.; Itoh, Y.; Okanoue, T.; Yoshikawa, T. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res. 2009, 39, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Simancas-Racines, D.; Annunziata, G.; Verde, L.; Fasci-Spurio, F.; Reytor-Gonzalez, C.; Muscogiuri, G.; Frias-Toral, E.; Barrea, L. Nutritional Strategies for Battling Obesity-Linked Liver Disease: The Role of Medical Nutritional Therapy in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Management. Curr. Obes. Rep. 2025, 14, 7. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, J.; Xie, K.; Tang, C.; Gan, C.; Gao, J. MASLD development: From molecular pathogenesis toward therapeutic strategies. Chin. Med. J. 2025, 138, 1807–1824. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Charatcharoenwitthaya, P.; Treeprasertsuk, S.; Benson, J.T.; Enders, F.B.; Angulo, P. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut 2009, 58, 1538–1544. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Eng. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.Y.; Chen, S.M.; Ji, X.G.; Shen, X.; You, J.S.; Liang, X.Y.; Yin, H.; Zhao, L.M. Current innovations in nutraceuticals and functional foods for intervention of non-alcoholic fatty liver disease. Pharmacol. Res. 2021, 166, 105517. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Liu, J.; Wood, C.; Gagnon, C.; Cober, E.R.; Fregeau-Reid, J.A.; Gleddie, S.; Xiao, C.W. The alpha’ subunit of beta-conglycinin and various glycinin subunits of soy are not required to modulate hepatic lipid metabolism in rats. Eur. J. Nutr. 2018, 57, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Yang, X.Q.; Swallah, M.S.; Fu, H.L.; Ji, L.; Meng, X.Z.; Yu, H.S.; Lyu, B. Soy protein isolate: An overview on foaming properties and air-liquid interface. Int. J. Food Sci. Technol. 2022, 57, 188–200. [Google Scholar] [CrossRef]
- Fu, H.; Shan, D.; Li, J.; Swallah, M.S.; Yang, X.; Ji, L.; Wang, S.; Gong, H.; Lyu, B.; Yu, H. Potential functionality of beta-conglycinin with subunit deficiencies: Soy protein may regulate glucose and lipid metabolism. Food Funct. 2022, 13, 12291–12302. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Ma, L.K.; Ji, J.; Huo, R.C.; Dong, S.; Bai, Y.F.; Hua, L.L.; Lei, J.; Tian, S.S.; Wang, M.N.; et al. Protective effect of soy isolate protein against streptozotocin induced gestational diabetes mellitus via TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 2023, 168, 115688. [Google Scholar] [CrossRef]
- Hakkak, R.; Zeng, H.W.; Dhakal, I.B.; Korourian, S. Short- and Long-Term Soy Diet Versus Casein Protects Liver Steatosis Independent of the Arginine Content. J. Med. Food 2015, 18, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Kozaczek, M.; Bottje, W.; Kong, B.; Dridi, S.; Albataineh, D.; Lassiter, K.; Hakkak, R. Long-Term Soy Protein Isolate Consumption Reduces Liver Steatosis Through Changes in Global Transcriptomics in Obese Zucker Rats. Front. Nutr. 2020, 7, 607970. [Google Scholar] [CrossRef]
- Kozaczek, M.; Bottje, W.; Kong, B.; Albataineh, D.; Hakkak, R. A Comparison of Short- and Long-Term Soy Protein Isolate Intake and Its Ability to Reduce Liver Steatosis in Obese Zucker Rats Through Modifications of Genes Involved in Inflammation and Lipid Transport. J. Med. Food 2021, 24, 1010–1016. [Google Scholar] [CrossRef]
- Liu, S.S.; Luo, T.T.; Song, Y.R.; Ren, H.B.; Qiu, Z.D.; Ma, C.X.; Tian, Y.S.; Wu, Q.; Wang, F.; Krishnan, H.B.; et al. Hypocholesterolemic effects of soy protein isolates from soybeans differing in 7S and 11S globulin subunits vary in rats fed a high cholesterol diet. J. Funct. Foods 2022, 99, 105347. [Google Scholar] [CrossRef]
- Shan, D.D.; Yu, H.S.; Lyu, B.; Fu, H.L. Soybean β-Conglycinin: Structure Characteristic, Allergenicity, Plasma Lipid-Controlling, Prevention of Obesity and Non-alcoholic Fatty Liver Disease. Curr. Protein Pept. Sci. 2021, 22, 831–847. [Google Scholar] [CrossRef]
- Song, B.; Qiu, Z.D.; Li, M.X.; Luo, T.T.; Wu, Q.; Krishnan, H.B.; Wu, J.J.; Xu, P.F.; Zhang, S.Z.; Liu, S.S. Breeding of ‘DND358’: A new soybean cultivar for processing soy protein isolate with a hypocholesterolemic effect similar to that of fenofibrate. J. Funct. Foods 2022, 90, 104979. [Google Scholar] [CrossRef]
- Liu, Y.W.; Yang, J.; Lei, L.; Wang, L.J.; Wang, X.B.; Ma, K.Y.; Yang, X.Q.; Chen, Z.Y. 7S protein is more effective than total soybean protein isolate in reducing plasma cholesterol. J. Funct. Foods 2017, 36, 18–26. [Google Scholar] [CrossRef]
- Duranti, M.; Lovati, M.R.; Dani, V.; Barbiroli, A.; Scarafoni, A.; Castiglioni, S.; Ponzone, C.; Morazzoni, P. The α′ subunit from soybean 7S globulin lowers plasma lipids and upregulates liver β-VLDL receptors in rat feed a hypercholesterolemic diet. J. Nutr. 2004, 134, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, C.; Duranti, M.; Eberini, I.; Scharnag, H.; März, W.; Castiglioni, S.; Lovati, M.R. Subcellular localization of soybean 7S globulin in HepG2 cells and LDL receptor up-regulation by its α′ constituent subunit. J. Nutr. 2003, 133, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Cabanos, C.; Kato, N.; Amari, Y.; Fujiwara, K.; Ohno, T.; Shimizu, K.; Goto, T.; Shimada, M.; Kuroda, M.; Masuda, T.; et al. Development of a novel transgenic rice with hypocholesterolemic activity via high-level accumulation of the α’ subunit of soybean β-conglycinin. Transgenic Res. 2014, 23, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, R.; Yang, Z.; Zhang, K.; Xing, J. Mechanism of Metabolic Dysfunction-associated Steatotic Liver Disease: Important role of lipid metabolism. J. Clin. Transl. Hepatol. 2024, 12, 815–826. [Google Scholar] [CrossRef]
- Scorletti, E.; Carr, R.M.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef]
- Parks, E.J.; Manrique, C.M.; Syed-Abdul, M.M.; Gaballah, A.H.; Hammoud, G.M.; Buckley, D.; Duke, G.; McCulloch, W.; Kemble, G. Pharmacologic inhibition of FASN reverses diet-induced steatohepatitis in mice and inhibits lipogenesis in humans. Hepatology 2017, 66, 1045a. [Google Scholar]
- Lawitz, E.J.; Coste, A.; Poordad, F.; Alkhouri, N.; Loo, N.; McColgan, B.J.; Tarrant, J.M.; Nguyen, T.; Han, L.; Chung, C.H.; et al. Acetyl-CoA carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2018, 16, 1983–1991.e3. [Google Scholar] [CrossRef] [PubMed]
- Esler, W.P.; Bence, K.K. Metabolic Targets in Nonalcoholic Fatty Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Jegatheesan, P.; Beutheu, S.; Ventura, G.; Nubret, E.; Sarfati, G.; Bergheim, I.; De Bandt, J.P. Citrulline and Nonessential Amino Acids Prevent Fructose-Induced Nonalcoholic Fatty Liver Disease in Rats. J. Nutr. 2015, 145, 2273–2279. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Zhang, J.; Zeng, F.Y.; Zhu, Y.Z. Roles of the peroxisome proliferator-activated receptors (PPARs) in the pathogenesis of nonalcoholic fatty liver disease (NAFLD). Pharmacol. Res. 2023, 192, 106786. [Google Scholar] [CrossRef]
- Veiga, F.M.; Graus-Nunes, F.; Rachid, T.L.; Barreto, A.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Anti-obesogenic effects of WY14643 (PPAR-agonist): Hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie 2017, 140, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M. Lipid Metabolism in Metabolic-Associated Steatotic Liver Disease (MASLD). Metabolites 2024, 14, 12. [Google Scholar] [CrossRef]
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Konishi, T. Tofu (soybean curd) lowers serum lipid levels and modulates hepatic gene expression involved in lipogenesis primarily through its protein, not isoflavone, component in rats. J. Agric. Food Chem. 2011, 59, 8976–8984. [Google Scholar] [CrossRef]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in serum triacylglycerol and visceral fat mediated by dietary soybean beta-conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Two Peptides from Soy β-Conglycinin Induce a Hypocholesterolemic Effect in HepG2 Cells by a Statin-Like Mechanism: Comparative in Vitro and in Silico Modeling Studies. J. Agric. Food Chem. 2015, 63, 7945–7951. [Google Scholar] [CrossRef]
- Consonni, A.; Lovati, M.R.; Manzoni, C.; Pizzagalli, A.; Morazzoni, P.; Duranti, M. Cloning, yeast expression, purification and biological activity of a truncated form of the soybean 7S globulin α′ subunit involved in Hep G2 cell cholesterol homeostasis. J. Nutr. Biochem. 2010, 21, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Hummelgaard, S.; Vilstrup, J.P.; Gustafsen, C.; Glerup, S.; Weyer, K. Targeting PCSK9 to tackle cardiovascular disease. Pharmacol. Ther. 2023, 249, 108480. [Google Scholar] [CrossRef]
- Schwärzler, J.; Grabherr, F.; Grander, C.; Adolph, T.E.; Tilg, H. The pathophysiology of MASLD: An immunometabolic perspective. Expert Rev. Clin. Immunol. 2024, 20, 375–386. [Google Scholar] [CrossRef]
- Anand, P.K. Lipids, inflammasomes, metabolism, and disease. Immunol. Rev. 2020, 297, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.T.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.H.; Brickey, W.J.; Ting, J.P.Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.; Jung, F.; Baumann, A.; Brandt, A.; Staltner, R.; Sanchez, V.; Bergheim, I. TNFalpha is a key trigger of inflammation in diet-induced non-obese MASLD in mice. Redox Biol. 2023, 66, 102870. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.C.; Landes, S.G.; Nguyen, V.; Novobrantseva, T.I.; Hahn, Y.S. Kuppfer Cells Trigger Nonalcoholic Steatohepatitis Development in Diet-induced Mouse Model through Tumor Necrosis Factor-α Production. J. Biol. Chem. 2012, 287, 40161–40172. [Google Scholar] [CrossRef]
- Pan, J.J.; Ou, Z.B.; Cai, C.; Li, P.Z.; Gong, J.P.; Ruan, X.Z.; He, K. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell. Immunol. 2018, 332, 111–120. [Google Scholar] [CrossRef]
- Wan, P.; Yang, G.; Cheng, Q.; Zhang, X.L.; Yue, Z.Y.; Li, M.R.; Liu, C.L.; Yi, Q.; Jia, Y.L.; Liu, J.B.; et al. The role of inflammasome in chronic viral hepatitis. Front. Cell. Infect. Microbiol. 2024, 14, 1382029. [Google Scholar] [CrossRef]
- Tans, R.; Bande, R.; van Rooij, A.; Molloy, B.J.; Stienstra, R.; Tack, C.J.; Wevers, R.A.; Wessels, H.J.C.T.; Gloerich, J.; van Gool, A.J. Evaluation of cyclooxygenase oxylipins as potential biomarker for obesity- associated adipose tissue inflammation and type 2 diabetes using targeted multiple reaction monitoring mass spectrometry. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102157. [Google Scholar] [CrossRef]
- Chan, P.C.; Liao, M.T.; Hsieh, P.S. The Dualistic Effect of COX-2-Mediated Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 3115. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.Z.; Song, Y.Y.; Chen, L.; Hu, J.J.; Meng, Y.; Chen, X.; Li, S.; Zheng, G.H.; Qiu, Z.P. Celecoxib attenuates hepatosteatosis by impairing de novo lipogenesis via Akt-dependent lipogenic pathway. J. Cell. Mol. Med. 2022, 26, 3995–4006. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, W.; Abd El-Aty, A.M.; Hacimuftuoglu, A.; Jeong, J.H.; Jung, T.W. Valdecoxib attenuates lipid-induced hepatic steatosis through autophagy-mediated suppression of endoplasmic reticulum stress. Biochem. Pharmacol. 2022, 199, 115022. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, J.; Desouza, C.; Thakare, R.; Alnouti, Y.; Saraswathi, V. Global deletion of COX-2 attenuates hepatic inflammation but impairs metabolic homeostasis in diet-induced obesity. J. Lipid Res. 2025, 66, 100823. [Google Scholar] [CrossRef]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, A.G.; Meng, C.Z.; Li, Z.; He, P.L. Quantification of lectin in soybeans and soy products by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2021, 1185, 122987. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Das, D.; Kabir, M.E.; Sarkar, S.; Wann, S.B.; Kalita, J.; Manna, P. Antidiabetic potential of soy protein/peptide: A therapeutic insight. Int. J. Biol. Macromol. 2022, 194, 276–288. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, W.S.; Kim, C.H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef]
- Li, T.N.; Zhang, X.R.; Ren, Y.Y.; Zeng, Y.J.; Huang, Q.W.; Wang, C. Antihypertensive effect of soybean bioactive peptides: A review. Curr. Opin. Pharmacol. 2022, 62, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Rupasinghe, S.G.; Schuler, M.A.; de Mejia, E.G. Peptides from purified soybean β-conglycinin inhibit fatty acid synthase by interaction with the thioesterase catalytic domain. FEBS J. 2010, 277, 1481–1493. [Google Scholar] [CrossRef]
- Macchi, C.; Greco, M.F.; Ferri, N.; Magni, P.; Arnoldi, A.; Corsini, A.; Sirtori, C.R.; Ruscica, M.; Lammi, C. Impact of Soy β-Conglycinin Peptides on PCSK9 Protein Expression in HepG2 Cells. Nutrients 2022, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- de Souza, S.M.A.; Hernández-Ledesma, B.; de Souza, T.L.F. Lunasin as a Promising Plant-Derived Peptide for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 9548. [Google Scholar] [CrossRef] [PubMed]
| Gene | Species | Forward Primer | Reverse Primer |
|---|---|---|---|
| Srebf1 | rat | TCTTGACCGACATCGAAGACAT | CTGTCTCACCCCCAGCATAG |
| Acaca | rat | GCGGCTCTGGAGGTATATGT | GGGATGTTCCCTCTGTTTGGA |
| Fasn | rat | GCCTAACACCTCTGTGCAGT | GGCAATACCCGTTCCCTGAA |
| Pcsk9 | rat | TTTCCACAGACAGGCGAGC | CCTTGACAGTTGAGCACACG |
| Pparα | rat | CTGAACATTGGCGTTCGCAG | TTCAGTCTTGGCTCGCCTC |
| Hmgcr | rat | TGCAGAGCGATCAGTCTTGG | AATCTGCTCGTGCTGTCGAA |
| Nlrp3 | rat | CTGCAGAGCCTACAGTTGGG | ACCCTACACTAAAAGCGCCC |
| Tnf-α | rat | GATCGGTCCCAACAAGGAGG | CTTGGTGGTTTGCTACGACG |
| Il-1β | rat | GGCTTCCTTGTGCAAGTGTC | AGTCAAGGGCTTGGAAGCAA |
| Il-6 | rat | GCCCACCAGGAACGAAAGTC | ACTGGCTGGAAGTCTCTTGCG |
| Ccl2 | rat | TTAATGCCCCACTCACCTGC | GAGCTTGGTGACAAATACTACAGC |
| Actb | rat | TGCTATGTTGCCCTAGACTTCG | GTTGGCATAGAGGTCTTTACGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Guo, S.; Lai, X.; Xiao, Q.; Wu, X.; Pang, J.; Pei, L.; Gu, Y.; Zhang, X.; Yang, L. α-Lack-SPI Alleviates MASLD in Rats via Regulating Hepatic Lipid Accumulation and Inflammation. Nutrients 2025, 17, 2918. https://doi.org/10.3390/nu17182918
Chen M, Guo S, Lai X, Xiao Q, Wu X, Pang J, Pei L, Gu Y, Zhang X, Yang L. α-Lack-SPI Alleviates MASLD in Rats via Regulating Hepatic Lipid Accumulation and Inflammation. Nutrients. 2025; 17(18):2918. https://doi.org/10.3390/nu17182918
Chicago/Turabian StyleChen, Mingtao, Shanshan Guo, Xuye Lai, Qiyao Xiao, Xueqian Wu, Jinzhu Pang, Lei Pei, Yingying Gu, Xuguang Zhang, and Lili Yang. 2025. "α-Lack-SPI Alleviates MASLD in Rats via Regulating Hepatic Lipid Accumulation and Inflammation" Nutrients 17, no. 18: 2918. https://doi.org/10.3390/nu17182918
APA StyleChen, M., Guo, S., Lai, X., Xiao, Q., Wu, X., Pang, J., Pei, L., Gu, Y., Zhang, X., & Yang, L. (2025). α-Lack-SPI Alleviates MASLD in Rats via Regulating Hepatic Lipid Accumulation and Inflammation. Nutrients, 17(18), 2918. https://doi.org/10.3390/nu17182918






