Status of Common Water-Soluble Vitamins in Medical Students: A Cross-Sectional Analysis with Implications for Targeted Nutritional Screening Programs
Highlights
- This first comprehensive assessment reveals that 10% of Middle Eastern medical students exhibit suboptimal levels of water-soluble vitamins, despite their educational exposure to health and nutrition topics.
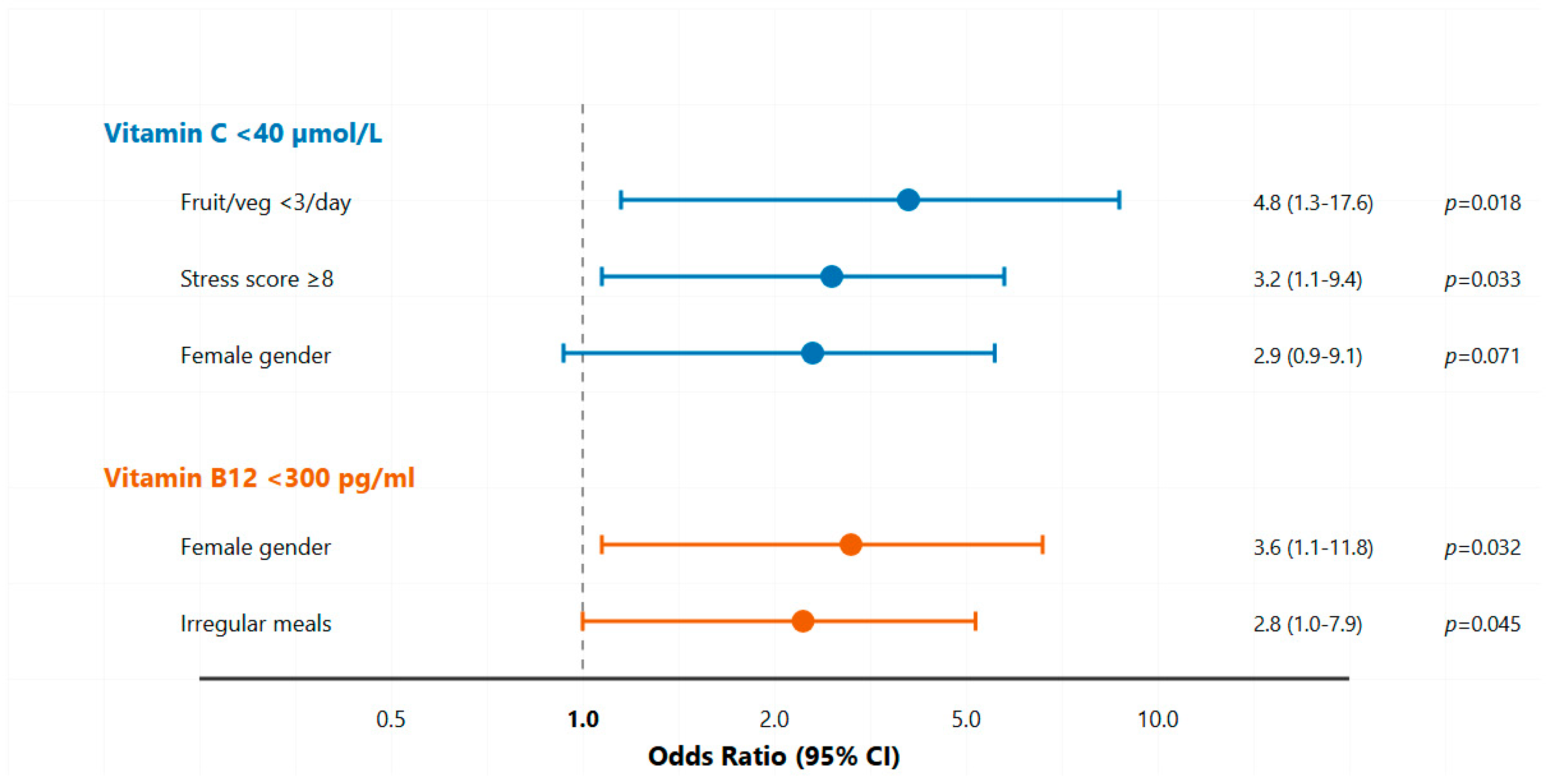
- Students with an inadequate fruit and vegetable intake were five times more likely to have vitamin C insufficiency, while high academic stress was associated with a threefold increased risk.
- All cases of vitamin C insufficiency occurred exclusively among female students, who also demonstrated a 3.6-fold higher risk of vitamin B12 insufficiency compared to males.
- Vitamin-deficient students experienced 40% more fatigue, twice the rate of respiratory infections, and impaired concentration in comparison to peers with normal vitamin levels.
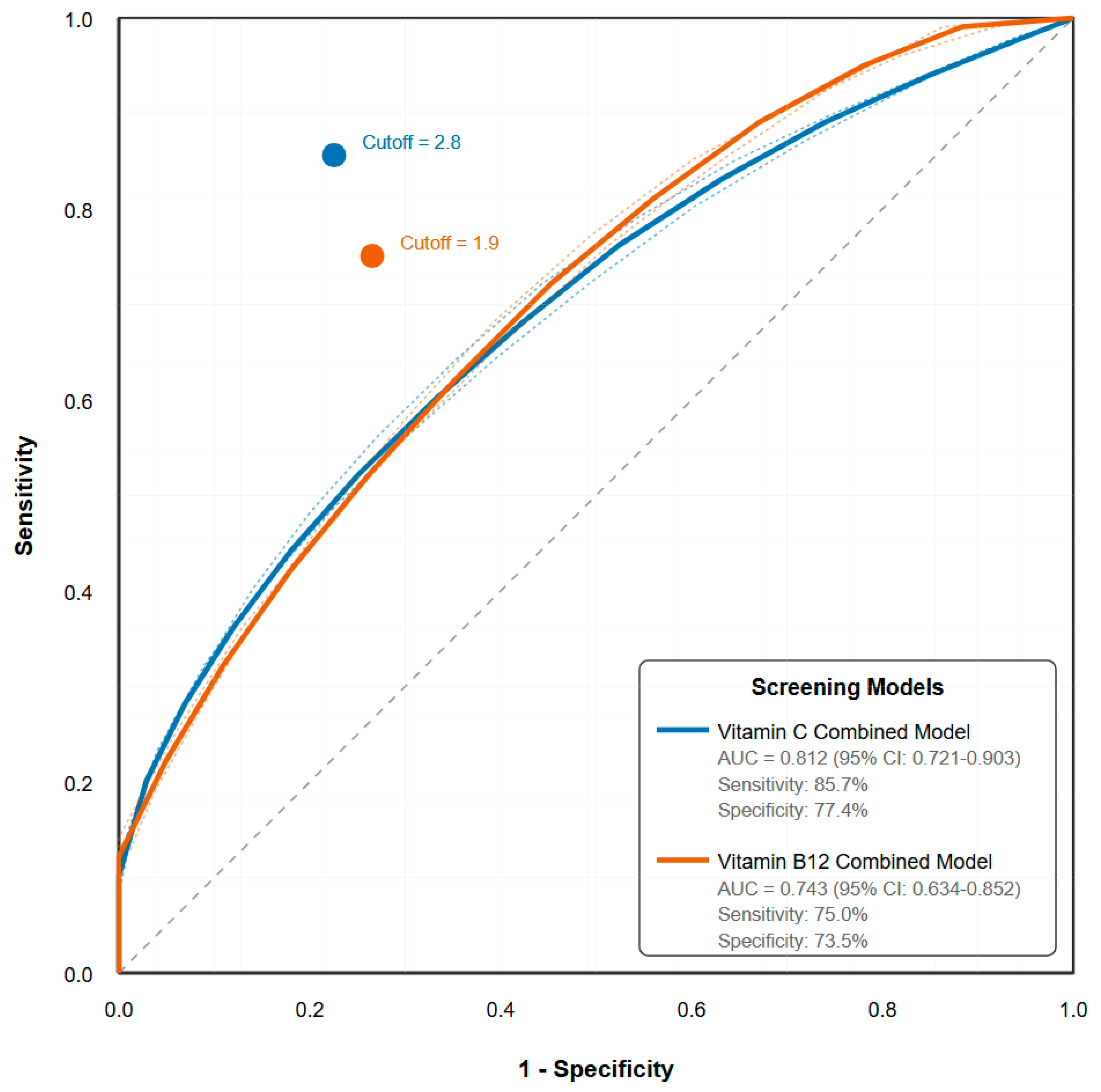
- A screening tool based on diet and stress achieved 98% accuracy in ruling out vitamin C insufficiency, offering a practical tool for university health services.
Abstract
1. Introduction
1.1. Water-Soluble Vitamins: Biochemical Roles and Clinical Significance
1.2. Nutritional Vulnerabilities and Global Prevalence
1.3. Study Rationale and Objectives
Primary Objective
2. Materials and Methods
2.1. Study Design and Ethical Considerations
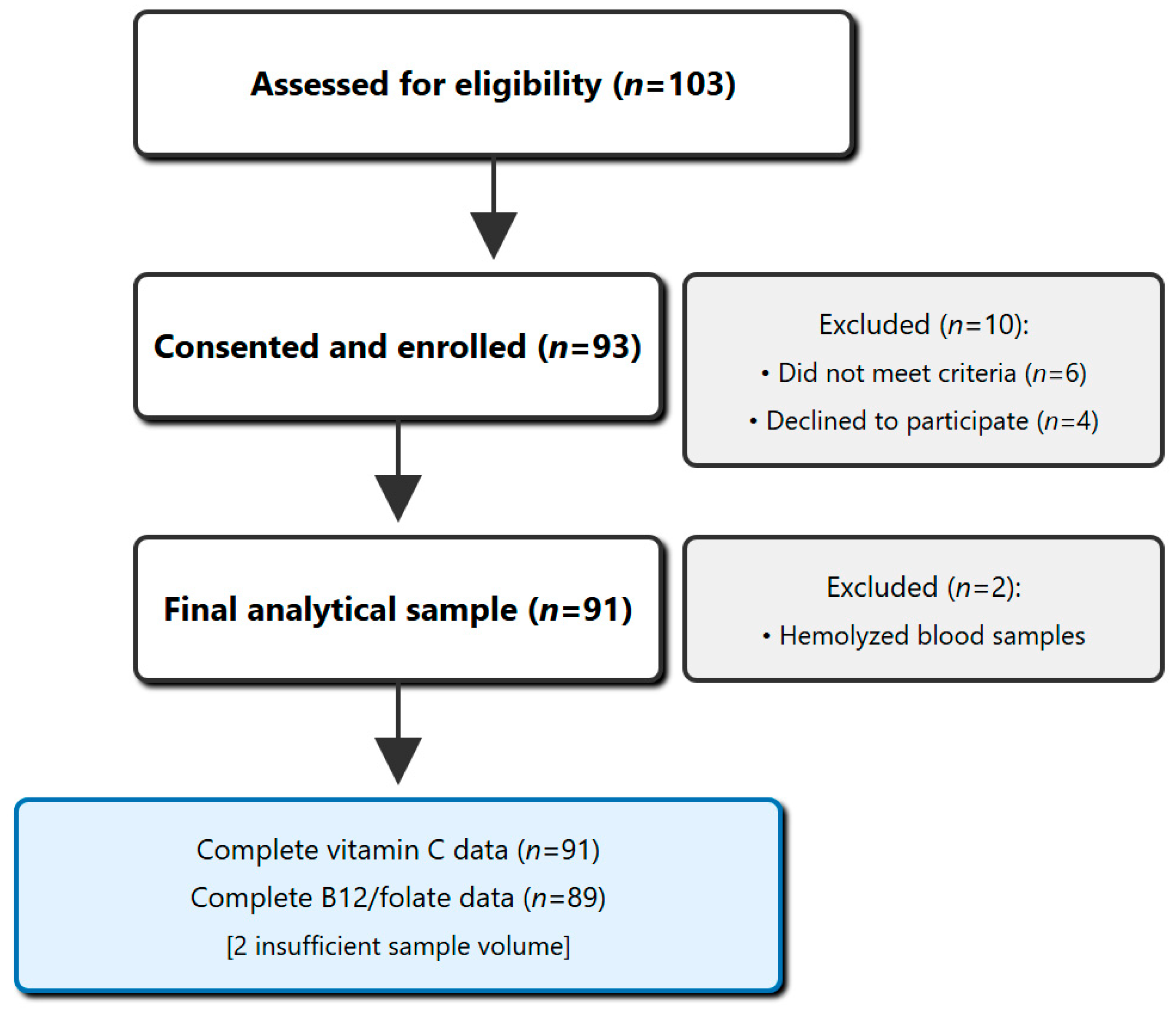
2.2. Participant Recruitment and Selection
2.2.1. Sampling Strategy
2.2.2. Participant Selection
2.3. Sample Size Calculation
2.4. Data Collection Procedures
2.4.1. Questionnaire Administration
Demographic and Socioeconomic Information
Relevant Medical History
Lifestyle Assessment
Dietary Assessment
2.4.2. Anthropometric Measurements
- Height: Wall-mounted stadiometer (SECA 213, SECA GmbH & Co. KG, Hamburg, Germany), measured to nearest 0.1 cm
- Weight: Calibrated digital scale (TANITA BC-418, TANITA Corporation, Tokyo, Japan), measured to nearest 0.1 kg
- BMI: Calculated as weight (kg)/height2 (m2)
- Waist circumference: Non-stretchable tape at midpoint between lowest rib and iliac crest
- Hip circumference: Maximum circumference over buttocks
- Waist-to-hip ratio: Calculated for central adiposity assessment
- Body composition: Bioelectrical impedance analysis (TANITA BC-418)
2.5. Laboratory Analyses
2.5.1. Pre-Analytical Sample Handling and Stability
2.5.2. Vitamin Assays
2.6. Statistical Analysis
2.6.1. Data Management
2.6.2. Statistical Methods
3. Results
3.1. Study Population Characteristics
3.2. Water-Soluble Vitamin Status
3.3. Gender-Specific Patterns
3.4. Correlational Analyses
3.5. Multivariate Predictors of Vitamin Status
3.6. Diagnostic Accuracy of Screening Parameters
3.7. Clinical and Academic Correlates
4. Discussion
4.1. Principal Findings and Clinical Significance
4.2. Vitamin C: Stress, Diet, and Academic Performance
4.3. Vitamin B12: Gender Disparities and Subclinical Implications
4.4. Implications for a Targeted Screening Framework
4.5. Strengths and Methodological Rigor
4.6. Limitations and Future Directions
4.7. Public Health and Policy Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón-Ospina, C.A.; Nava-Mesa, M.O. B Vitamins in the nervous system: Current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci. Ther. 2020, 26, 5–13. [Google Scholar] [CrossRef]
- Huskisson, E.; Maggini, S.; Ruf, M. The role of vitamins and minerals in energy metabolism and well-being. J. Int. Med. Res. 2007, 35, 277–289. [Google Scholar] [CrossRef]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef]
- Waterland, R.A. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Nutr. 2019, 39, 263–288. [Google Scholar]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 deficiency. BMJ 2014, 349, g5226. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., III; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Stover, P.J. Physiology of folate and vitamin B12 in health and disease. Nutr. Rev. 2004, 62, S3–S12. [Google Scholar] [CrossRef]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Yahia, N.; Wang, D.; Rapley, M.; Dey, R. Assessment of weight status, dietary habits and beliefs, physical activity, and nutritional knowledge among university students. Perspect. Public Health 2016, 136, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, M.; Mantzorou, M.; Serdari, A.; Bonotis, K.; Vasios, G.; Pavlidou, E.; Trifonos, C.; Vadikolias, K.; Petridis, D.; Giaginis, C. Evaluating Mediterranean diet adherence in university student populations: Does this dietary pattern affect students’ academic performance and mental health? Int. J. Health Plann. Manage. 2020, 35, 5–21. [Google Scholar] [CrossRef]
- Racette, S.B.; Deusinger, S.S.; Strube, M.J.; Highstein, G.R.; Deusinger, R.H. Changes in weight and health behaviors from freshman through senior year of college. J. Nutr. Educ. Behav. 2008, 40, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Dyrbye, L.N.; Thomas, M.R.; Shanafelt, T.D. Systematic review of depression, anxiety, and other indicators of psychological distress among U.S. and Canadian medical students. Acad. Med. 2006, 81, 354–373. [Google Scholar] [CrossRef]
- Bergmann, N.; Gyntelberg, F.; Faber, J. The appraisal of chronic stress and the development of the metabolic syndrome: A systematic review of prospective cohort studies. Endocr. Connect. 2014, 3, R55–R80. [Google Scholar] [CrossRef]
- Hope, V.; Henderson, M. Medical student depression, anxiety and distress outside North America: A systematic review. Med. Educ. 2014, 48, 963–979. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Fayet-Moore, F.; Petocz, P.; Samman, S. Micronutrient Status in Female University Students: Iron, Zinc, Copper, Selenium, Vitamin B12 and Folate. Nutrients 2014, 6, 5103–5116. [Google Scholar] [CrossRef]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B(12) deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The Prevalence of Micronutrient Deficiencies and Inadequacies in the Middle East and Approaches to Interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef]
- Basalamah, M.A.; Ibrahim, M.O.; Qutob, M.S.; Al-Jedani, S.T.; Al-Hawsawi, L.M.; Mahrous, R.M.; Abdel-Salam, O.H. Vitamin B12 status among asymptomatic young adult females and its association with some anthropometric and biochemical parameters: A cross-sectional study from Makkah (cobalamin deficiency in young adult females). Medicine 2023, 102, e35838. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO STEPS Surveillance Manual: The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Moritz, B.; Schmitz, A.E.; Rodrigues, A.L.S.; Dafre, A.L.; Cunha, M.P. The role of vitamin C in stress-related disorders. J. Nutr. Biochem. 2020, 85, 108459. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.-L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef]
- Stover, P.J. Vitamin B12 and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 24–27. [Google Scholar] [CrossRef]
| Category | Specific Exclusions |
|---|---|
| Medical Conditions | Diabetes, thyroid disorders, malabsorption syndromes, chronic kidney/liver disease |
| Medications | Proton pump inhibitors, metformin, isoniazid, phenytoin, phenobarbital |
| Physiological State | Pregnancy, lactation, acute illness within the preceding four weeks |
| Dietary Habits | Strict veganism, diagnosed eating disorders, regular alcohol consumption |
| Anthropometrics | Body Mass Index (BMI) < 18.5 or >30 kg/m2 |
| Other | Were being treated for a previously diagnosed vitamin deficiency, current participation in another nutritional study |
| Characteristic | Total (n = 91) | Males (n = 27) | Females (n = 64) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean ± SD | 19.8 ± 1.4 | 20.1 ± 1.5 | 19.7 ± 1.3 | 0.234 |
| Academic year, n (%) | ||||
| - Year 1–2 | 51 (56.0) | 14 (51.9) | 37 (57.8) | |
| - Year 3–4 | 30 (33.0) | 10 (37.0) | 20 (31.3) | |
| - Year 5–6 | 10 (11.0) | 3 (11.1) | 7 (10.9) | |
| Anthropometrics | ||||
| BMI (kg/m2), mean ± SD | 23.2 ± 2.9 | 24.3 ± 3.1 | 22.7 ± 2.7 | 0.018 * |
| Waist circumference (cm) | 76.4 ± 9.8 | 84.2 ± 8.1 | 73.1 ± 8.6 | <0.001 *** |
| Body fat (%), mean ± SD | 24.8 ± 7.3 | 18.6 ± 5.2 | 27.4 ± 6.8 | <0.001 *** |
| Lifestyle Factors | ||||
| Physical activity (min/week) | 142 ± 86 | 184 ± 92 | 124 ± 78 | 0.002 ** |
| Sleep duration (hours/night) | 6.4 ± 1.2 | 6.6 ± 1.1 | 6.3 ± 1.2 | 0.268 |
| Stress score (0–10) | 7.1 ± 1.6 | 6.6 ± 1.7 | 7.3 ± 1.5 | 0.049 * |
| Study hours/week | 42.3 ± 12.1 | 39.8 ± 11.4 | 43.3 ± 12.3 | 0.213 |
| Dietary Patterns | ||||
| Fruit/vegetable servings/day | 2.9 ± 1.4 | 3.0 ± 1.5 | 2.8 ± 1.4 | 0.547 |
| Fast food meals/week | 3.3 ± 2.0 | 3.7 ± 2.2 | 3.1 ± 1.9 | 0.201 |
| Skip breakfast ≥ 3/week, n (%) | 38 (41.8) | 9 (33.3) | 29 (45.3) | 0.292 |
| Coffee intake (cups/day) | 2.1 ± 1.3 | 2.3 ± 1.4 | 2.0 ± 1.2 | 0.314 |
| Vitamin | Mean ± SD | Median (IQR) | Reference Range | Deficiency n (%) | Insufficiency n (%) | Normal n (%) |
|---|---|---|---|---|---|---|
| Cobalamin (pg/mL) | 485.3 ± 165.0 | 446.4 (375.2–569.2) | 300–900 | 0 (0.0) | 8 (9.0) | 81 (91.0) * |
| Folate (ng/mL) | 14.1 ± 4.9 | 13.1 (10.4–16.1) | >5 | 0 (0.0) | 0 (0.0) | 89 (100.0) * |
| Vitamin C (µmol/L) | 56.7 ± 14.8 | 55.1 (49.6–62.0) | 40–75 | 2 (2.2) | 5 (5.5) | 84 (92.3) |
| Vitamin | Males (n = 27) | Females (n = 64) | p-Value | Effect Size (Cohen’s d) |
|---|---|---|---|---|
| Cobalamin (pg/mL) | 524.6 ± 178.3 | 468.7 ± 157.2 | 0.146 | 0.33 |
| Folate (ng/mL) | 13.4 ± 4.7 | 14.3 ± 5.0 | 0.431 | 0.18 |
| Vitamin C (µmol/L) | 60.2 ± 15.9 | 55.2 ± 14.1 | 0.142 | 0.33 |
| B12 < 300 pg/mL, n (%) | 1 (3.7) | 7 (10.9) | 0.433 ‡ | - |
| Vit C < 40 µmol/L, n (%) | 0 (0.0) | 7 (10.9) | 0.096 ‡ | - |
| Variable | Vitamin C | Vitamin B12 | Folate |
|---|---|---|---|
| Dietary Factors | |||
| Fruit/vegetable intake | 0.412 *** | 0.156 | 0.234 * |
| Fast food frequency | −0.287 ** | −0.143 | −0.089 |
| Breakfast regularity | 0.198 * | 0.167 | 0.156 |
| Lifestyle Factors | |||
| Physical activity | 0.156 | 0.098 | 0.123 |
| Sleep duration | 0.167 | 0.145 | 0.134 |
| Stress score | −0.241 * | −0.089 | −0.098 |
| Anthropometrics | |||
| BMI | −0.198 * | −0.067 | 0.045 |
| Body fat % | −0.234 * | −0.098 | −0.056 |
| Predictors | Coefficient | Standard Error | p-Value | Adjusted Odds Ratio | 95% Confidence Interval for Odds Ratio | |
|---|---|---|---|---|---|---|
| Model 1: Vitamin C < 40 µmol/L | ||||||
| Fruit/vegetable < 3 servings/day | 1.57 | 0.66 | 0.018 * | 4.8 | 1.3–17.6 | |
| Stress score ≥ 8 | 1.16 | 0.54 | 0.033 * | 3.2 | 1.1–9.4 | |
| Female gender | 1.06 | 0.58 | 0.071 | 2.9 | 0.9–9.1 | |
| Model 2: Vitamin B12 < 300 pg/mL | ||||||
| Female gender | 1.28 | 0.60 | 0.032 * | 3.6 | 1.1–11.8 | |
| Irregular meals | 1.03 | 0.51 | 0.045 * | 2.8 | 1.0–7.9 | |
| Vegetarian tendency | 0.83 | 0.56 | 0.138 | 2.3 | 0.8–6.7 | |
| Screening Tool | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC (95% CI) |
|---|---|---|---|---|---|
| For Vitamin C Deficiency | |||||
| Combined model | 85.7 | 77.4 | 28.6 | 98.1 | 0.812 (0.721–0.903) |
| For B12 Insufficiency | |||||
| Combined model | 75.0 | 73.5 | 20.0 | 97.1 | 0.743 (0.634–0.852) |
| Outcome | Normal Vitamins | Any Suboptimal | p-Value |
|---|---|---|---|
| Fatigue score (0–10) | 5.2 ± 1.8 | 7.4 ± 1.5 | <0.001 |
| URI episodes/year | 1.8 ± 1.1 | 3.0 ± 1.4 | 0.003 |
| Concentration difficulty, n (%) | 28 (35.0) | 9 (75.0) | 0.009 |
| Academic performance (self-rated) | 7.2 ± 1.3 | 6.1 ± 1.5 | 0.012 |
| Tier | Identification of Students | Proposed Action |
|---|---|---|
| Tier 1: Universal Prevention | All students | Promote access to healthy food options; Provide an educational module on nutrition during academic stress. |
| Tier 2: Targeted Risk Assessment | Students with key risk factors (poor diet, high stress, female gender, etc.) | Administer risk questionnaire; Perform targeted biochemical screening based on risk profile |
| Tier 3: Comprehensive Management | Students with confirmed deficiencies from Tier 2 testing. | Conduct complete micronutrient panel; Provide individualized dietary plan and counseling; Consider supplementation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agha, A.; Yasin, J.; Sharma, C.; Alkaabi, J. Status of Common Water-Soluble Vitamins in Medical Students: A Cross-Sectional Analysis with Implications for Targeted Nutritional Screening Programs. Nutrients 2025, 17, 2862. https://doi.org/10.3390/nu17172862
Agha A, Yasin J, Sharma C, Alkaabi J. Status of Common Water-Soluble Vitamins in Medical Students: A Cross-Sectional Analysis with Implications for Targeted Nutritional Screening Programs. Nutrients. 2025; 17(17):2862. https://doi.org/10.3390/nu17172862
Chicago/Turabian StyleAgha, Adnan, Javed Yasin, Charu Sharma, and Juma Alkaabi. 2025. "Status of Common Water-Soluble Vitamins in Medical Students: A Cross-Sectional Analysis with Implications for Targeted Nutritional Screening Programs" Nutrients 17, no. 17: 2862. https://doi.org/10.3390/nu17172862
APA StyleAgha, A., Yasin, J., Sharma, C., & Alkaabi, J. (2025). Status of Common Water-Soluble Vitamins in Medical Students: A Cross-Sectional Analysis with Implications for Targeted Nutritional Screening Programs. Nutrients, 17(17), 2862. https://doi.org/10.3390/nu17172862








