Positive Impact of Breastfeeding on Nutritional Status and Metabolic Control in Infants with PKU: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Study Location
2.2. Procedure
2.3. Anthropometric Evaluation
2.4. Blood Phenylalanine Determination
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Metabolic Control
3.2. Anthropometric Outcomes
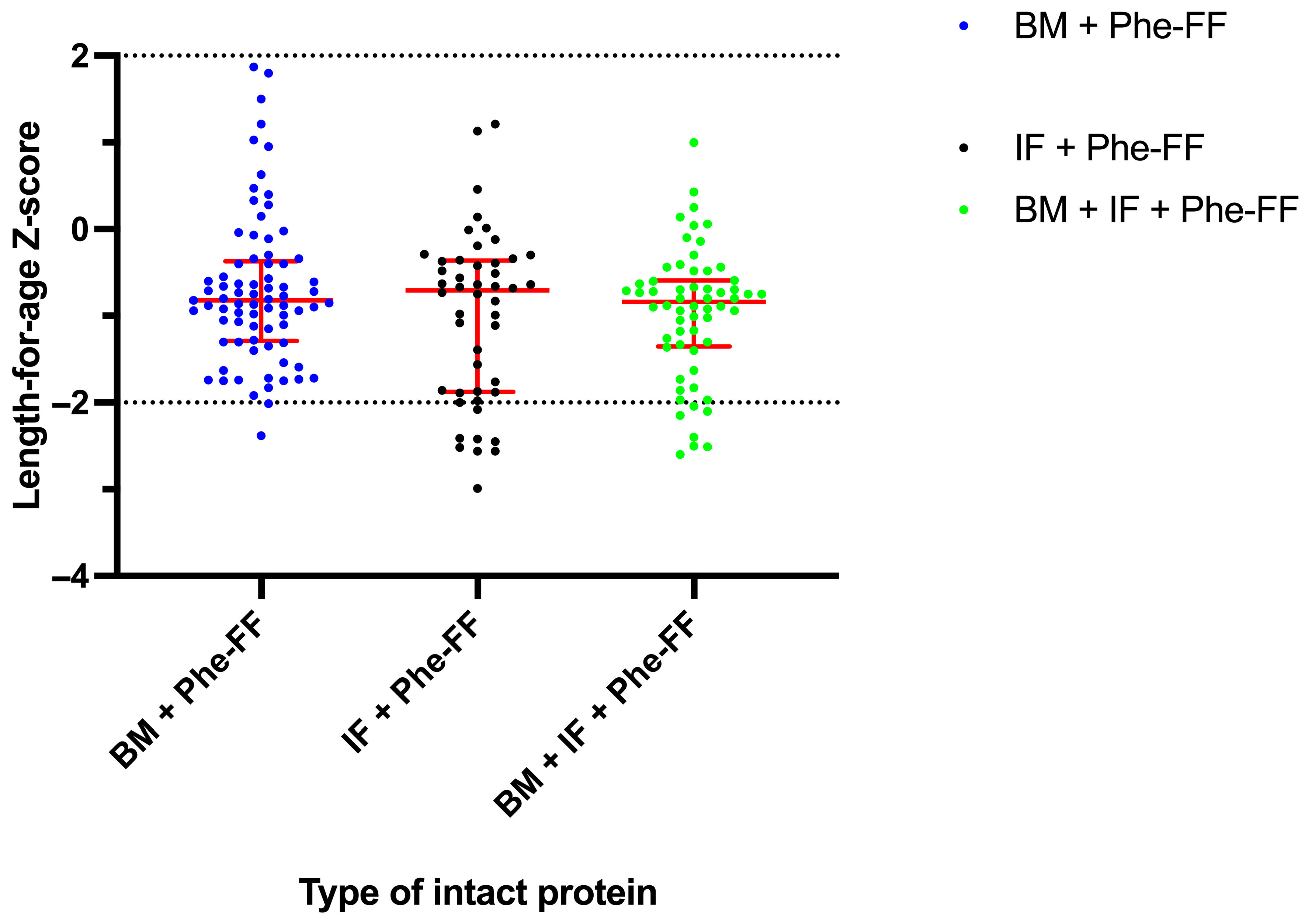
3.2.1. Length-for-Age Z-Score
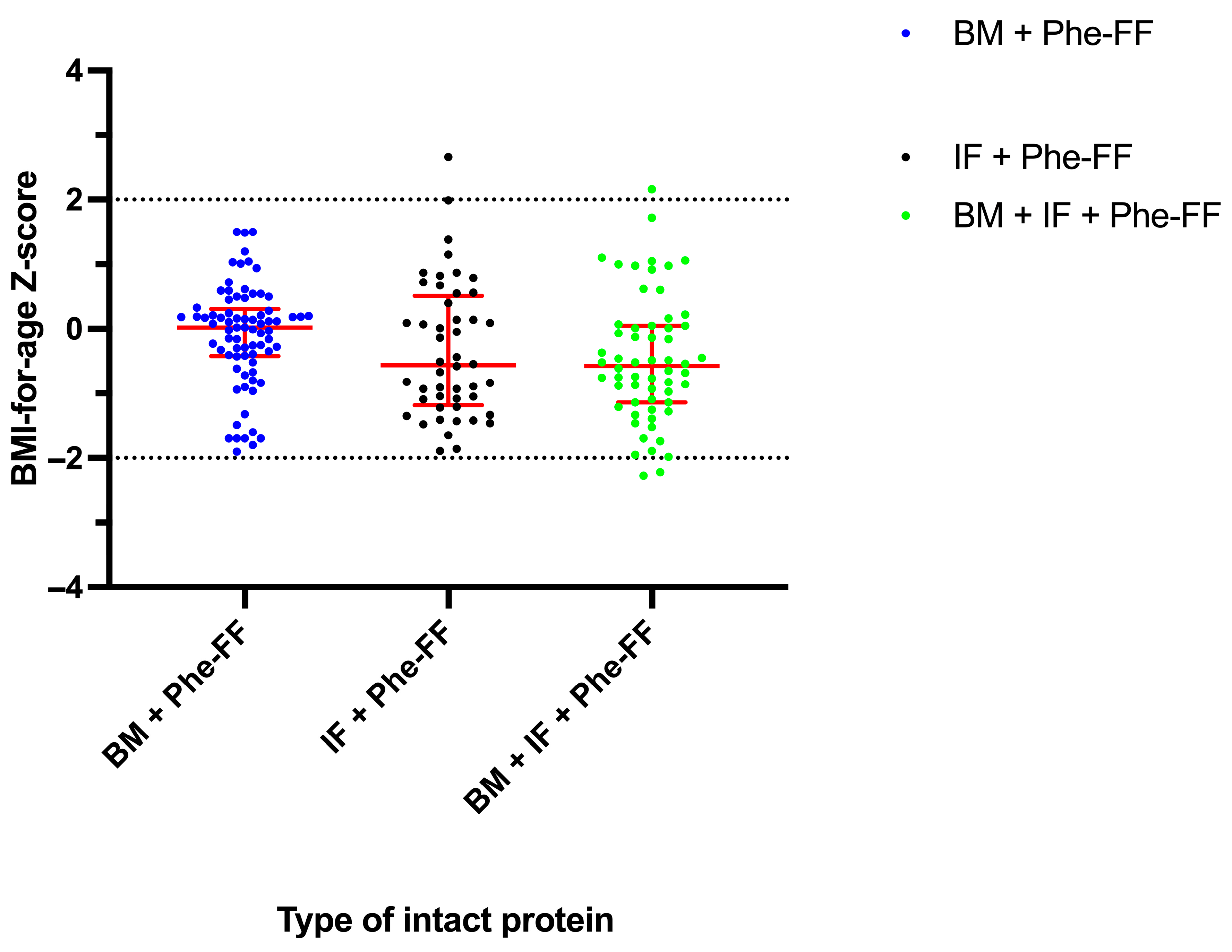
3.2.2. BMI-for-Age Z-Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI. | body mass index |
| HPA. | hyperphenylalaninemia |
| PKU. | phenylketonuria |
| Phe. | phenylalanine |
| WHO. | World Health Organization |
| SERN. | Southeast Regional Genetics Network |
| GMDI. | Genetic Metabolic Dietitians International |
| BH4. | tetrahydrobiopterin |
| LAZ. | length for age Z-Score |
| BAZ. | BMI for age Z-Score |
| BM. | breastmilk |
| IF. | infant formula |
| Phe-FF. | phenylalanine free formula |
| IQR. | interquartile range |
| cPKU. | Classic Phenylketonuria |
| mPKU. | Mild Phenylketonuria |
| MHA. | Mild hyperphenylalaninemia |
References
- van Spronsen, F.J.; van Wegberg, A.M.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017, 5, 743–756. [Google Scholar] [CrossRef]
- Vela-Amieva, M.; Ibarra-González, I.; del Alba Herrera-Pérez, L.; Caamal-Parra, G.; Belmont-Martínez, L.; García-Flores, E.P. Epidemiología de la fenilcetonuria obtenida mediante tamiz neonatal. Acta Pediátr. Méx. 2018, 39, 25S–34S. [Google Scholar] [CrossRef]
- Ibarra-González, I.; Fernández-Lainez, C.; Vela-Amieva, M.; Guillén-López, S.; Belmont-Martínez, L.; López-Mejía, L.; Carrillo-Nieto, R.I.; Guillén-Zaragoza, N.A. A Review of Disparities and Unmet Newborn Screening Needs over 33 Years in a Cohort of Mexican Patients with Inborn Errors of Intermediary Metabolism. Int. J. Neonatal Screen. 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.E.; Berry, S.A.; Bloom, K.; Brown, C.; Burton, B.K.; Demarest, O.M.; Jenkins, G.P.; Malinowski, J.; McBride, K.L.; Mroczkowski, H.J. Phenylalanine hydroxylase deficiency diagnosis and management: A 2023 evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2025, 27, 101289. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.H.; Cunningham, A.C.; Mofidi, S.; Douglas, T.D.; Frazier, D.M.; Hook, D.G.; Jeffers, L.; McCune, H.; Moseley, K.D.; Ogata, B. Updated, web-based nutrition management guideline for PKU: An evidence and consensus based approach. Mol. Genet. Metab. 2016, 118, 72–83. [Google Scholar] [CrossRef]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lőnnerdal, B. Amino acid profiles in term and preterm human milk through lactation: A systematic review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef]
- Genetic Metabolic Dietitians International (GMDI). Metabolic Pro. Available online: https://metabolicpro.org/ (accessed on 27 November 2024).
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef]
- Gertosio, C.; Meazza, C.; Pagani, S.; Bozzola, M. Breastfeeding and its gamut of benefits. Minerva Pediatr. 2015, 68, 201–212. [Google Scholar]
- Banta-Wright, S.A.; Press, N.; Knafl, K.A.; Steiner, R.D.; Houck, G.M. Breastfeeding infants with phenylketonuria in the United States and Canada. Breastfeed. Med. 2014, 9, 142–148. [Google Scholar] [CrossRef]
- Kalvala, J.; Chong, L.; Chadborn, N.; Ojha, S. Breast feeding in infants diagnosed with phenylketonuria (PKU): A scoping review. BMJ Paediatr. Open 2023, 7, e002066. [Google Scholar] [CrossRef]
- Ilgaz, F.; Höller, A.; Marsaux, C.; Banta-Wright, S.; Coşkun, T.; Dingess, K.A.; Jörg-Streller, M.; Newby, C.; Singh, R.; Stahl, B. Human Milk feeding in inherited metabolic disorders: A systematic review of growth, metabolic control, and neurodevelopment outcomes. J. Inherit. Metab. Dis. 2025, 48, e70001. [Google Scholar] [CrossRef]
- Vela-Amieva, M.; Abreu-Gonzalez, M.; Gonzalez-del Angel, A.; Ibarra-Gonzalez, I.; Fernandez-Lainez, C.; Barrientos-Rios, R.; Monroy-Santoyo, S.; Guillén-López, S.; Alcántara-Ortigoza, M. Phenylalanine hydroxylase deficiency in Mexico: Genotype–phenotype correlations, BH4 responsiveness and evidence of a founder effect. Clin. Genet. 2015, 88, 62–67. [Google Scholar] [CrossRef]
- Vela-Amieva, M.; Alcántara-Ortigoza, M.A.; Ibarra-González, I.; González-del Angel, A.; Fernández-Hernández, L.; Guillén-López, S.; López-Mejía, L.; Carrillo-Nieto, R.I.; Belmont-Martínez, L.; Fernández-Lainez, C. An Updated PAH Mutational Spectrum of Phenylketonuria in Mexican Patients Attending a Single Center: Biochemical, Clinical-Genotyping Correlations. Genes 2021, 12, 1676. [Google Scholar] [CrossRef]
- Hillert, A.; Anikster, Y.; Belanger-Quintana, A.; Burlina, A.; Burton, B.K.; Carducci, C.; Chiesa, A.E.; Christodoulou, J.; Đorđević, M.; Desviat, L.R. The genetic landscape and epidemiology of phenylketonuria. Am. J. Hum. Genet. 2020, 107, 234–250. [Google Scholar] [CrossRef]
- SERN/GMDI. Management guidelines portal. The Nutrition Management Guideline for Individuals with Propionic Acidemia. Available online: https://managementguidelines.net/guidelines.php/104/PROP%20Nutrition%20Guidelines/Version%201.2 (accessed on 23 March 2024).
- World Health Organization. Training Course on Child Growth Assessment; WHO: Geneva, Switzerland, 2008; pp. 17–25. [Google Scholar]
- Ibarra-González, I.; Fernández-Lainez, C.; Guillén-López, S.; López-Mejía, L.; Belmont-Martínez, L.; Nieto-Carrillo, R.I.; Vela-Amieva, M. Importance of Studying Older Siblings of Patients Identified by Newborn Screening: A Single-Center Experience in Mexico. J. Inborn Errors Metab. Screen. 2021, 9, e20210001. [Google Scholar] [CrossRef]
- Kose, E.; Aksoy, B.; Kuyum, P.; Tuncer, N.; Arslan, N.; Ozturk, Y. The effects of breastfeeding in infants with phenylketonuria. J. Pediatr. Nurs. 2018, 38, 27–32. [Google Scholar] [CrossRef] [PubMed]
- O’sullivan, E.; Boyle, F.; Mehta, S.; Kearney, J. Breastfeeding improves biochemical control in children with phenylketonuria. FASEB J. 2013, 27, 622. [Google Scholar] [CrossRef]
- Banta-Wright, S.A.; Shelton, K.C.; Lowe, N.D.; Knafl, K.A.; Houck, G.M. Breast-feeding success among infants with phenylketonuria. J. Pediatr. Nurs. 2012, 27, 319–327. [Google Scholar] [CrossRef]
- Moradi, M.; Angali, K.A.; Behzadi, M.H.; Farnoosh, R. The effect of breastfeeding on children’s growth indices up to 6 months: An application of multivariate t linear mixed model. J. Res. Med. Sci. 2023, 28, 31. [Google Scholar] [CrossRef]
- Bell, K.A.; Wagner, C.L.; Feldman, H.A.; Shypailo, R.J.; Belfort, M.B. Associations of infant feeding with trajectories of body composition and growth. Am. J. Clin. Nutr. 2017, 106, 491–498. [Google Scholar] [CrossRef]
- Roldão, C.; Lopes, R.; Silva, J.M.; Neves, N.; Gomes, J.C.; Gavina, C.; Taveira-Gomes, T. Can Breastfeeding Prevent Long-Term Overweight and Obesity in Children? A Population-Based Cohort Study. Nutrients 2024, 16, 2728. [Google Scholar] [CrossRef] [PubMed]
- Zuvadelli, J.; Paci, S.; Salvatici, E.; Giorgetti, F.; Cefalo, G.; Re Dionigi, A.; Rovelli, V.; Banderali, G. Breastfeeding in phenylketonuria: Changing modalities, changing perspectives. Nutrients 2022, 14, 4138. [Google Scholar] [CrossRef] [PubMed]
- Banta-Wright, S.A.; Kodadek, S.M.; Steiner, R.D.; Houck, G.M. Challenges to breastfeeding infants with phenylketonuria. J. Pediatr. Nurs. 2015, 30, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Adams, S.; Ahring, K.; Allen, H.; Almeida, M.; Garcia-Arenas, D.; Arslan, N.; Assoun, M.; Altınok, Y.A.; Barrio-Carreras, D. Early feeding practices in infants with phenylketonuria across Europe. Mol. Genet. Metab. Rep. 2018, 16, 82–89. [Google Scholar] [CrossRef]
- Errico, J.L.; Choi, T.S.; Pacilli, M.; Davidson, Z.E. Supporting breastfeeding when clinical nutrition interventions are required in the paediatric healthcare setting: A systematic review. Int. Breastfeed. J. 2025, 20, 55. [Google Scholar] [CrossRef]

| Patient | Blood Phe Max Historical (μmol/L) | Genotype c.DNA | Protein | Phenotype | Group |
|---|---|---|---|---|---|
| 1 | 448 | c.[434A>T];[809G>A] | p.[Asp145Val];[Arg270Lys] | MHP | BM + Phe-FF (N = 13) |
| 2 | 890 | c.[1162G>A];[1162G>A] | p.[Val388Met];[Val388Met] | mPKU | |
| 3 | 1154 | c.[1066-11G>A];[1162G>A] | p.[?];[Val388Met] | mPKU | |
| 4 | 964 | c.[165del];[208_210del] | p.[Phe55Leufs*6];[Ser70del] | mPKU | |
| 5 | 1008 | c.[809G>A];[1162G>A] | p.[Arg270Lys];[Val388Met] | mPKU | |
| 6 | 1280 | c.[1A>T];[1066-11G>A] | p.[Met1?];[?] | cPKU | |
| 7 | 1900 | c.[1A>T];[1066-11G>A] | p.[Met1?];[?] | cPKU | |
| 8 | 1900 | c.[60+5G>T];[441+5G>T ] | p.[?];[?] | cPKU | |
| 9 | 1454 | c.[809G>A];[809G>A] | p.[Arg270Lys];[Arg270Lys] | cPKU | |
| 10 | 2176 | c.[60+5G>T];[1315+1G>A] | p.[?];[?] | cPKU | |
| 11 | 2506 | c.[1045T>C];[1045T>C] | p.[Ser349Pro];[Ser349Pro] | cPKU | |
| 12 | 1696 | c.[682G>A];[682G>A] | p.[Glu228Lys];[Glu228Lys] | cPKU | |
| 13 | 1252 | c.[649T>C];[649T>C] | p.[Cys217Arg];[Cys217Arg] | cPKU | |
| 14 | 1559 | c.[60+5G>T];[441+5G>T] | p.[?];[?] | mPKU | IF + Phe-FF (N = 11) |
| 15 | 1066 | c.[208_210del];[728G>A] | p.[Ser70del];[Arg243Gln] | cPKU | |
| 16 | 1900 | c.[441+5G>T];[1055del] | p.[?];[Gly352Valfs*48] | cPKU | |
| 17 | 1217 | c.[625_626insC];[625_626insC] | p.[Ile209Thrfs*6];[Ile209Thrfs*6] | cPKU | |
| 18 | 1423 | c.[168+5G>C];[1066-11G>A] | p.[?];[?] | cPKU | |
| 19 | 1527 | c.[1A>T];[1042C>G] | p.[?];[Leu348Val] | cPKU | |
| 20 | 1527 | c.[1A>T];[1042C>G] | p.[?];[Leu348Val] | cPKU | |
| 21 | 1351 | c.[1042C>G;1238G>A];[1042C>G;1238G>A] | p.[Leu348Val;Arg413His];[Leu348Val;Arg413His] | cPKU | |
| 22 | 1715 | c.[60+5G>T];[1315+1G>A] | p.[?];[?] | cPKU | |
| 23 | 1634 | c.[60+5G>T];[1162G>A] | p.[?];[Val388Met] | cPKU | |
| 24 | 1623 | c.[722G>A];[842+1G>A] | p.[Arg241His];[?] | cPKU | |
| 25 | 467 | c.[60+5G>T];[1169A>G] | p.[?];[Glu390Gly] | MHP | BM + IF + Phe-FF (N = 7) |
| 26 | 401 | c.[721C>T];[1066-11G>A] | p.[Arg241Cys];[?] | MHP | |
| 27 | 909 | c.[60+5G>T];[1241A>G] | p.[?];[Tyr414Cys] | mPKU | |
| 28 | 966 | c.[814G>T];[1282C>T] | p.[Gly272*];[Gln428*] | mPKU | |
| 29 | 1753 | c.[791A>G];[1315+5_1315+6insGTGTAACAG] | p.[His264Arg];[?] | cPKU | |
| 30 | 2825 | c.[116_118del];[1045T>C] | p.[Phe39del];[Ser349Pro] | cPKU | |
| 31 | 1325 | c.[60+5G>T];[1066-11G>A] | p.[?];[?] | cPKU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Mejía, L.; Guillén-López, S.; Vela-Amieva, M.; Fernández-Lainez, C.; Castro-Monroy, L. Positive Impact of Breastfeeding on Nutritional Status and Metabolic Control in Infants with PKU: A Retrospective Study. Nutrients 2025, 17, 2851. https://doi.org/10.3390/nu17172851
López-Mejía L, Guillén-López S, Vela-Amieva M, Fernández-Lainez C, Castro-Monroy L. Positive Impact of Breastfeeding on Nutritional Status and Metabolic Control in Infants with PKU: A Retrospective Study. Nutrients. 2025; 17(17):2851. https://doi.org/10.3390/nu17172851
Chicago/Turabian StyleLópez-Mejía, Lizbeth, Sara Guillén-López, Marcela Vela-Amieva, Cynthia Fernández-Lainez, and Lilian Castro-Monroy. 2025. "Positive Impact of Breastfeeding on Nutritional Status and Metabolic Control in Infants with PKU: A Retrospective Study" Nutrients 17, no. 17: 2851. https://doi.org/10.3390/nu17172851
APA StyleLópez-Mejía, L., Guillén-López, S., Vela-Amieva, M., Fernández-Lainez, C., & Castro-Monroy, L. (2025). Positive Impact of Breastfeeding on Nutritional Status and Metabolic Control in Infants with PKU: A Retrospective Study. Nutrients, 17(17), 2851. https://doi.org/10.3390/nu17172851









