Effects of Raspberry Leaf Tea Polyphenols on Postprandial Glucose and Insulin Responses in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Brewing Conditions and Polyphenol Analysis of Raspberry Leaf Tea
2.2. In Human Study
2.2.1. Study Participants
2.2.2. Sample Size Determination
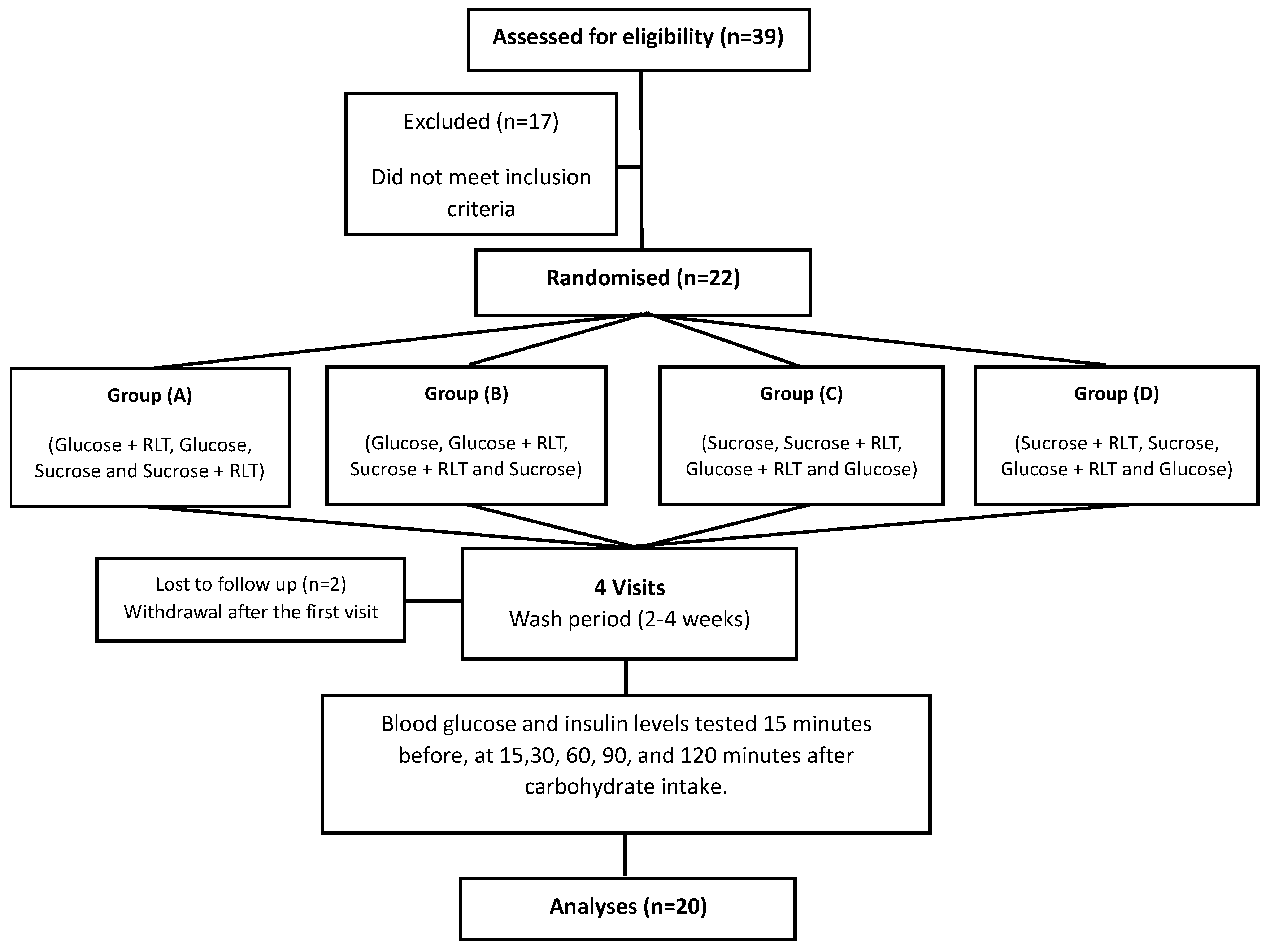
2.2.3. Study Design
2.2.4. Study Products
2.2.5. Procedures for Each Visit
2.2.6. Blood Sample Collection, Processing, and Biochemical Analysis
2.2.7. Statistical Analysis
3. Results
3.1. LC–MS/MS Analysis of Polyphenol Compounds in Raspberry Leaf Samples
3.2. Basic Characteristics of Study Participants
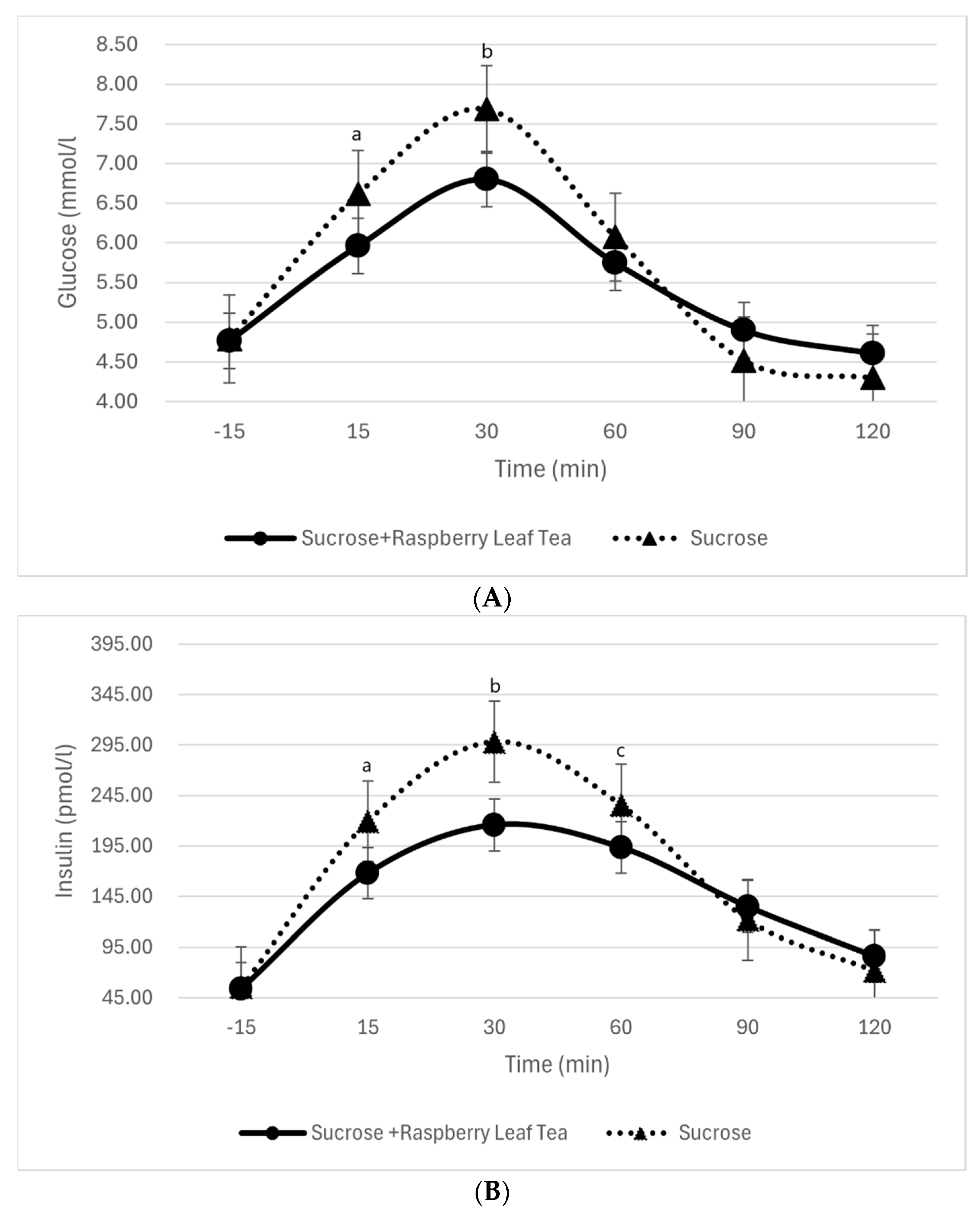
3.3. Blood Glucose and Insulin Levels Before and During Sucrose Intake with or Without RL Tea
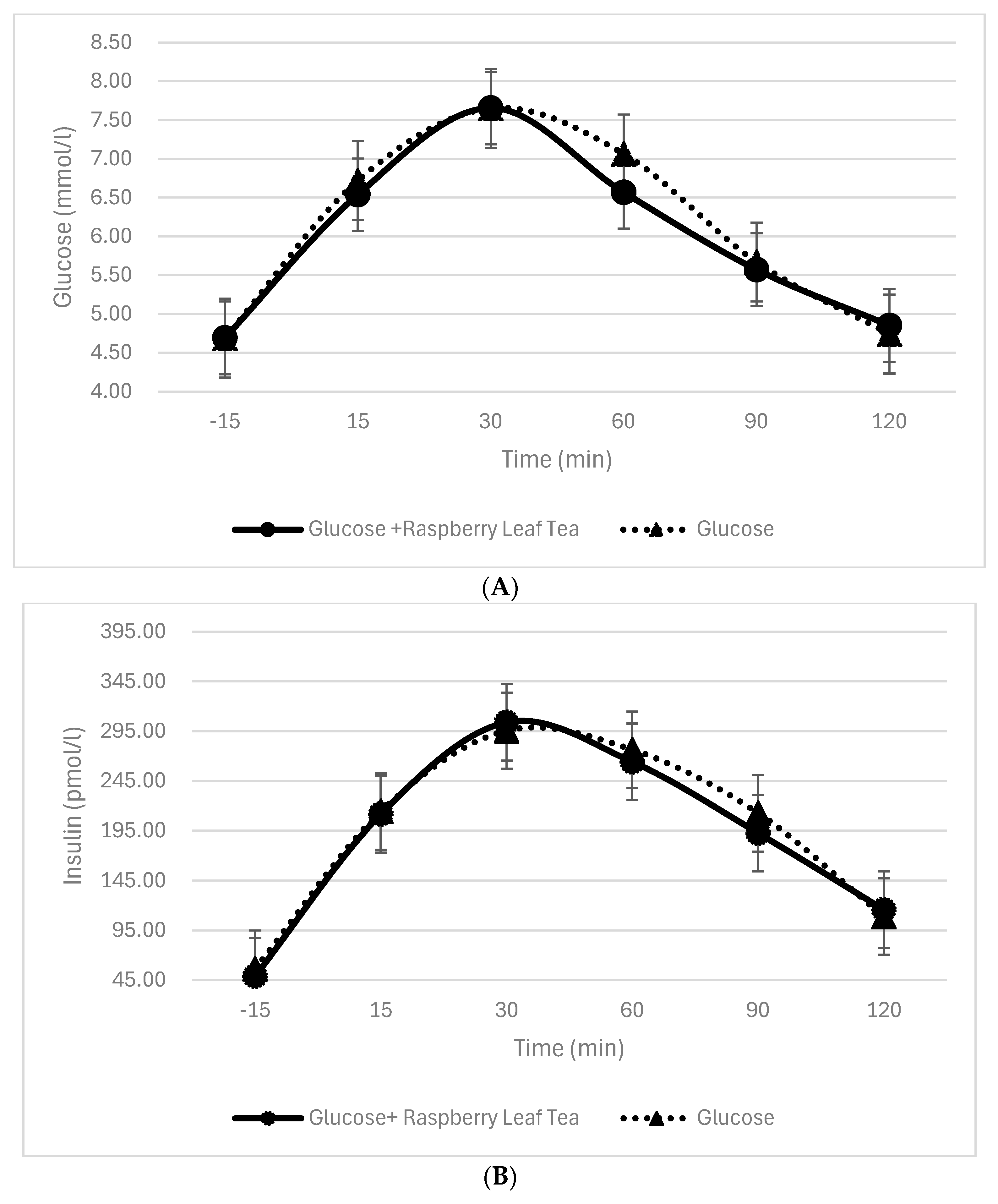
3.4. Blood Glucose and Insulin Levels Before and During Glucose Intake with or Without RL Tea
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes–global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107. [Google Scholar] [CrossRef]
- Yen, F.S.; Qin, C.S.; Xuan, S.T.S.; Ying, P.J.; Le, H.Y.; Darmarajan, T.; Gunasekaran, B.; Salvamani, S.; Wang, H. Hypoglycemic Effects of Plant Flavonoids: A Review. Evid.-Based Complement. Altern. Med. 2021, 2021, 2057333. [Google Scholar]
- World Health Organization. Diabetes. World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 19 January 2025).
- Mayfield, J.A. Diagnosis and classification of diabetes mellitus: New criteria. Am. Fam. Physician 1998, 58, 1355. [Google Scholar]
- Aslan, M.; Orhan, D.D.; Orhan, N.; Sezik, E.; Yesilada, E. In vivo antidiabetic and antioxidant potential of Helichrysum plicatum ssp. plicatum capitulums in streptozotocin-induced-diabetic rats. J. Ethnopharmacol. 2007, 109, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3; US Department of Agriculture: Washington, DC, USA, 2018; Volume 173.
- Younger, K.M. Antioxidant, anti-inflammatory, anti-carcinogenic and anti-diabetic. Nutr. Res. Rev. 2010, 23, 181–183. [Google Scholar] [CrossRef]
- Tsai, Y.D.; Hsu, H.F.; Chen, Z.H.; Wang, Y.T.; Huang, S.H.; Chen, H.J.; Wang, C.P.; Wang, S.W.; Chang, C.C.; Houng, J.Y. Antioxidant, anti-inflammatory, and anti-proliferative activities of extracts from different parts of farmed and wild Glossogyne tenuifolia. Ind. Crops Prod. 2014, 57, 98–105. [Google Scholar] [CrossRef]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Liu, Y.J.; Cai, L.B.; Xu, F.R.; Xie, T.; He, Q.Q. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Davey, R.J.; Murray, K.; Radavelli-Bagatini, S.; Bondonno, C.P.; Blekkenhorst, L.C.; Sim, M.; Magliano, D.J.; Daly, R.M.; Shaw, J.E.; et al. Associations between fruit intake and risk of diabetes in the AusDiab cohort. J. Clin. Endocrinol. Metab. 2021, 106, e4097–e4108. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef]
- Adu, M.D.; Bondonno, C.P.; Parmenter, B.H.; Sim, M.; Davey, R.J.; Murray, K.; Radavelli-Bagatini, S.; Magliano, D.J.; Daly, R.M.; Shaw, J.E.; et al. Association between non-tea flavonoid intake and risk of type 2 diabetes: The Australian diabetes, obesity and lifestyle study. Food Funct. 2022, 13, 4459–4468. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef]
- Almoosawi, S.; Fyfe, L.; Ho, C.; Al-Dujaili, E. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br. J. Nutr. 2010, 103, 842–850. [Google Scholar] [CrossRef]
- Dall’Asta, M.; Bayle, M.; Neasta, J.; Scazzina, F.; Bruni, R.; Cros, G.; Del Rio, D.; Oiry, C. Protection of pancreatic β-cell function by dietary polyphenols. Phytochem. Rev. 2015, 14, 933–959. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food polyphenols and type II diabetes mellitus: Pharmacology and mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-Anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.W.; Hsuan, C.Y.; Feng, H.J. Characterization of maltase and sucrase inhibitory constituents from Rhodiola crenulata. Foods 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.L.; Shen, J.P.; Chen, J.F.; Gao, Y.T.; Xiang, J.J.; Ye, B.D.; Zhou, Y.H. Effect of Chinese medicine treatment based on pattern identification on cellular immunophenotype of myelodysplastic syndrome. Chin. J. Integr. Med. 2017, 23, 469–473. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Louadj, L.; Rizzo, P.; Poiana, M.; Sicari, V. Packaging and storage condition affect the physicochemical properties of red raspberries (Rubus idaeus L.; cv. Erika). Food Control 2019, 97, 105–113. [Google Scholar] [CrossRef]
- Alice, L.A.; Campbell, C.S. Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Am. J. Bot. 1999, 86, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Guo, H.; Xu, W.B.; Ge, J.; Li, X.; Alimu, M.; He, D.J. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, M. A Handbook of Scientific Information on Widely Used Plant Drugs: Companion to Vol. 1 of the British Herbal Pharmacopoeia; Peter, R., Ed.; British Herbal Medicine Association: Exeter, UK, 1992; Volume 239, p. 45. ISBN 0-903032-09-0. [Google Scholar]
- Patel, A.V.; Rojas-Vera, J.; Dacke, C.G. Therapeutic constituents and actions of Rubus species. Curr. Med. Chem. 2004, 11, 1501–1512. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Determination of Flavonoids, Tannins and Ellagic acid in leaves from Rubus L. species. Arch. Pharmacal Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and human health: A review. J. Agric. Food Chem. 2004, 58, 3871–3883. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical composition, antioxidant and antimicrobial activity of raspberry, blackberry, and raspberry-blackberry hybrid leaf buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Alkhudaydi, H.M.S.; Muriuki, E.N.; Spencer, J.P. Determination of the Polyphenol Composition of Raspberry Leaf Using LC-MS/MS. Molecules 2025, 30, 970. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J.; Kostrzewa-Nowak, D.; Tarasiuk, J. In vitro antileukaemic activity of extracts from berry plant leaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006, 236, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic-Andjelkovic, A.S.; Andjelkovic, M.Z.; Radovanovic, A.N.; Radovanovic, B.C.; Randjelovic, V. Phenol composition, radical scavenging activity and antimicrobial activity of berry leaf extracts. Bulg. Chem. Commun. 2016, 48, 27–32. [Google Scholar]
- Yang, J.; Cui, J.; Han, H.; Chen, J.; Yao, J.; Liu, Y. Determination of active compounds in raspberry leaf extracts and the effects of extract intake on mice. Food Sci. Technol. 2020, 40, 124–131. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdyl̷o, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef]
- Luo, T.; Chen, S.; Zhang, H.; Jia, S.; Wang, J. Phytochemical composition and potential biological activities assessment of raspberry leaf extracts from nine different raspberry species and raspberry leaf tea. J. Berry Res. 2020, 10, 295–309. [Google Scholar] [CrossRef]
- Costea, T.; Lupu, A.R.; Vlase, L.; Nencu, I.; Gird, C.E. Phenolic content and antioxidant activity of a raspberry leaf dry extract. Rom. Biotechnol. Lett. 2016, 21, 11346–11356. [Google Scholar]
- Pavlović, A.V.; Papetti, A.; Zagorac, D.Č.D.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Mitić, M.; Mitić, J.; Nikolić, J.; Mašković, P. Classification of fruit tree leaves acording to phenolic profile using principal component analysis. In Proceedings of the 1st International Symposium on Biotechnology, Čačak, Serbia, 17–18 March 2023. [Google Scholar]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus L.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Guo, Q.; Gao, X.; Ma, Q.; Xue, Z.; Ferri, N.; Zhang, M.; Chen, H. Identification of ellagitannins in the unripe fruit of Rubus Chingii Hu and evaluation of its potential antidiabetic activity. J. Agric. Food Chem. 2019, 67, 7025–7039. [Google Scholar] [CrossRef]
- Yang, J.; Hao, Y.; Li, N.; Wang, C.; Liu, Y. Metabolic and microbial modulation of phenolic compounds from raspberry leaf extract under in vitro digestion and fermentation. Int. J. Food Sci. Technol. 2021, 56, 5168–5177. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Hummer, K.E. Rubus pharmacology: Antiquity to the present. HortScience 2010, 45, 1587–1591. [Google Scholar] [CrossRef]
- Zou, T.; Wang, B.; Yang, Q.; de Avila, J.M.; Zhu, M.J.; You, J.; Chen, D.; Du, M. Raspberry promotes brown and beige adipocyte development in mice fed high-fat diet through activation of AMP-activated protein kinase (AMPK) α1. J. Nutr. Biochem. 2018, 55, 157–164. [Google Scholar] [CrossRef]
- Cheang, K.I.; Nguyen, T.T.; Karjane, N.W.; Salley, K.E. Raspberry leaf and hypoglycemia in gestational diabetes mellitus. Obstet. Gynecol. 2016, 128, 1421–1424. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.; Bignon, C.; Sulzenbacher, G.; Henrissat, B.; Czjzek, M. The three-dimensional structure of invertase (β-fructosidase) from Thermotoga maritima reveals a bimodular arrangement and an evolutionary relationship between retaining and inverting glycosidases. J. Biol. Chem. 2004, 279, 18903–18910. [Google Scholar] [CrossRef]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Roșcan, A.G.; Ifrim, I.L.; Patriciu, O.I.; Fînaru, A.L. Exploring the Therapeutic Value of Some Vegetative Parts of Rubus and Prunus: A Literature Review on Bioactive Profiles and Their Pharmaceutical and Cosmetic Interest. Molecules 2025, 30, 3144. [Google Scholar] [CrossRef] [PubMed]
- Grafia, A.L.; Gonzalez, N.; Pacheco, C.; Razuc, M.F.; Acebal, C.C.; López, O.V. Eco-Friendly Packaging for Functional Food. Processes 2025, 13, 2027. [Google Scholar] [CrossRef]
- Van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; Van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols–Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. Acute intake of quercetin from onion skin extract does not influence postprandial blood pressure and endothelial function in overweight-to-obese adults with hypertension: A randomized, double-blind, placebo-controlled, crossover trial. Eur. J. Nutr. 2017, 56, 1347–1357. [Google Scholar] [CrossRef]
- Ghazaee, H.; Raouf Sheibani, A.; Mahdian, H.; Gholami, S.; Askari, V.R.; Baradaran Rahimi, V. Ellagic acid as potential therapeutic compound for diabetes and its complications: A systematic review from bench to bed. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 9345–9366. [Google Scholar] [CrossRef] [PubMed]
- Törrönen, R.; Kolehmainen, M.; Sarkkinen, E.; Mykkänen, H.; Niskanen, L. Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am. J. Clin. Nutr. 2012, 96, 527–533. [Google Scholar] [CrossRef]
- Isono, Y.; Watanabe, H.; Kumada, M.; Takara, T.; Iio, S.I. Black tea decreases postprandial blood glucose levels in healthy humans and contains high-molecular-weight polyphenols that inhibit α-glucosidase and α-amylase in vitro: A randomized, double blind, placebo-controlled, crossover trial. Funct. Foods Health Dis. 2021, 11, 222–237. [Google Scholar] [CrossRef]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G.; Arroo, R.; Xiao, J.; Sari, S. Advances in the natural α-glucosidase inhibitors. Efood 2023, 4, e112. [Google Scholar] [CrossRef]
- Patil, S.B.; Gadad, P.C. Chlorogenic Acid, a Potential Glucose-6-Phosphatase Inhibitor: An Approach to Develop a Pre-Clinical Glycogen Storage Disease Type I Model. Indian J. Pharm. Educ. Res. 2024, 58, s372–s381. [Google Scholar] [CrossRef]
- Shi, R.; Zhou, N.; Zhang, H.; Gong, M.; Han, L. Bioaffinity ultrafiltration coupled with HPLC-ESI-MS/MS for screening potential α-glucosidase inhibitors from pomegranate peel. Front. Nutr. 2022, 9, 1014862. [Google Scholar] [CrossRef]
- Xing, X.; Chun, C.; Qiang, H.; Xiong, F.; Rui-Hai, L. Investigation into the mechanisms of quercetin-3-O-glucuronide inhibiting α-glucosidase activity and non-enzymatic glycation by spectroscopy and molecular docking. Food Funct. 2021, 12, 7825–7835. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as human intestinal α-glucosidase inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of human and rat sucrase and maltase activities to assess antiglycemic potential: Optimization of the assay using acarbose and polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Zhang, X. Effects of Ellagic Acid on Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. J. Nutr. Metab. 2024, 2024, 5558665. [Google Scholar] [CrossRef] [PubMed]
- Lanza, U.; Alongi, M.; Frossi, B.; Pucillo, C.; Anese, M.; Nicoli, M.C. Investigating the hypoglycaemic potential of processed apple and acarbose combination in vitro, ex vivo, and in vivo: The role of quercetin-3-glucoside in steering α-glucosidase inhibition. Food Funct. 2025, 16, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid regulating glucose and lipids metabolism: A review. Evid.-Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, H.; Burger, H.J.; Below, P.; Schubert, G.; Rippel, R.; Schindler, P.W.; Paulus, E.; Herling, A.W. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J. Med. Chem. 1997, 40, 137–145. [Google Scholar] [CrossRef]
- Alkhudaydi, H.M.S.; Spencer, J.P. A comparison of the effects of green tea and cocoa on glycaemic control and insulin sensitivity in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Nutr. Healthy Aging 2024, 9, 17–36. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Leyva, M.J.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef]
- Curtis, P.J.; Van Der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome—Results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.R.; Mariappan, N.; Stull, A.J.; Francis, J. Blueberry supplementation attenuates oxidative stress within monocytes and modulates immune cell levels in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Food Funct. 2017, 8, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Champagne, C.M.; Gupta, A.K.; Boston, R.; Beyl, R.A.; Johnson, W.D.; Cefalu, W.T. Blueberries improve endothelial function, but not blood pressure, in adults with metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2015, 7, 4107–4123. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef]
- Rostami, A.; Khalili, M.; Haghighat, N.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Ebrahimpour-Koujan, S.; Eghtesadi, M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015, 11, 21. [Google Scholar]
- Dicks, L.; Kirch, N.; Gronwald, D.; Wernken, K.; Zimmermann, B.F.; Helfrich, H.P.; Ellinger, S. Regular intake of a usual serving size of flavanol-rich cocoa powder does not affect cardiometabolic parameters in stably treated patients with type 2 diabetes and hypertension—A double-blinded, randomized, placebo-controlled trial. Nutrients 2018, 10, 1435. [Google Scholar] [CrossRef]
- Jafarirad, S.; Ayoobi, N.; Karandish, M.; Jalali, M.T.; Haghighizadeh, M.H.; Jahanshahi, A. Dark chocolate effect on serum adiponectin, biochemical and inflammatory parameters in diabetic patients: A randomized clinical trial. Int. J. Prev. Med. 2018, 9, 86. [Google Scholar] [CrossRef]
- Fakhari, M.; Fakhari, M.; BamBaeichi, E. The effects of pilates and flavanol-rich dark chocolate consumption on the total antioxidant capacity, glycemic control and BMI in diabetic females with neuropathy complications. J. Bodyw. Mov. Ther. 2021, 26, 294–299. [Google Scholar] [CrossRef]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients: A double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Mellor, D.D.; Sathyapalan, T.; Kilpatrick, E.S.; Beckett, S.; Atkin, S.L. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in Type 2 diabetes patients. Diabet. Med. 2010, 27, 1318–1321. [Google Scholar] [CrossRef]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef]
- Hua, C.; Liao, Y.; Lin, S.; Tsai, T.; Huang, C.; Chou, P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebocontrolled clinical trial. Altern. Med. Rev. 2011, 16, 157–163. [Google Scholar]
- Mousavi, A.; Vafa, M.; Neyestani, T.; Khamseh, M.; Hoseini, F. The effects of green tea consumption on metabolic and anthropometric indices in patients with type 2 diabetes. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 1080–1086. [Google Scholar]
- Lasaite, L.; Spadiene, A.; Savickiene, N.; Skesters, A.; Silova, A. The effect of Ginkgo biloba and Camellia sinensis extracts on psychological state and glycemic control in patients with type 2 diabetes mellitus. Nat. Prod. Commun. 2014, 9, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Huang, C.J.; Huang, L.H.; Chen, I.J.; Chiu, J.P.; Hsu, C.H. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: A randomized, double-blinded, and placebo-controlled trial. PLoS ONE 2014, 9, e91163. [Google Scholar] [CrossRef]
- Mirzaei, K.; Hossein-Nezhad, A.; Karimi, M.; Hosseinzadeh-Attar, M.J.; Jafari, N.; Najmafshar, A.; Larijani, B. Effect of green tea extract on bone turnover markers in type 2 diabetic patients; a double-blind, placebo-controlled clinical trial study. DARU J. Pharm. Sci. 2015, 17 (Suppl. 1), 38–44. [Google Scholar]
- Hosseini, S.A.; Alipour, M.; Zakerkish, M.; Cheraghian, B.; Ghandil, P. The Gene-Treatment Interaction of FTO-rs9939609 Gene Polymorphism and Epigallocatechin-GallateIntervention on Anthropometric Indices, Fasting Blood Sugar and Insulin Resistance/Sensitivity in Patients with Type 2 Diabetes Mellitus. Iran. Red Crescent Med. J. 2018, 20, e82228. [Google Scholar]
- Stote, K.S.; Wilson, M.M.; Hallenbeck, D.; Thomas, K.; Rourke, J.M.; Sweeney, M.I.; Gottschall-Pass, K.T.; Gosmanov, A.R. Effect of blueberry consumption on cardiometabolic health parameters in men with type 2 diabetes: An 8-week, double-blind, randomized, placebo-controlled trial. Curr. Dev. Nutr. 2020, 4, nzaa030. [Google Scholar] [CrossRef] [PubMed]
- Fukino, Y.; Shimbo, M.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. J. Nutr. Sci. Vitaminol. 2005, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Fernández, P.; Trujillo-Quiros, J.; Pascoe-González, S.; Trujillo-Rangel, W.A.; Cardona-Müller, D.; Ramos-Becerra, C.G.; Barocio-Pantoja, M.; Rodríguez-de la Cerda, M.; Nerida Sanchez-Rodriguez, E.; Cardona-Munoz, E.G.; et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2019, 70, 977–985. [Google Scholar] [CrossRef]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; de Faria, J.M.L. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef]
- Fukino, Y.; Ikeda, A.; Maruyama, K.; Aoki, N.; Okubo, T.; Iso, H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur. J. Clin. Nutr. 2008, 62, 953–960. [Google Scholar] [CrossRef]
- Ryu, O.H.; Lee, J.; Lee, K.W.; Kim, H.Y.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Effects of green tea consumption on inflammation, insulin resistance and pulse wave velocity in type 2 diabetes patients. Diabetes Res. Clin. Pract. 2006, 71, 356–358. [Google Scholar] [CrossRef]
- Ghadimi, M.; Foroughi, F.; Hashemipour, S.; Nooshabadi, M.R.; Ahmadi, M.H.; Yari, M.G.; Kavianpour, M.; Haghighian, H.K. Decreased insulin resistance in diabetic patients by influencing Sirtuin1 and Fetuin-A following supplementation with ellagic acid: A randomized controlled trial. Diabetol. Metab. Syndr. 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Ghadimi, M.; Foroughi, F.; Hashemipour, S.; Rashidi Nooshabadi, M.; Ahmadi, M.H.; Ahadi Nezhad, B.; Khadem Haghighian, H. Randomized double-blind clinical trial examining the Ellagic acid effects on glycemic status, insulin resistance, antioxidant, and inflammatory factors in patients with type 2 diabetes. Phytother. Res. 2021, 35, 1023–1032. [Google Scholar] [CrossRef]
- Ghadimi, M.; Hashemipour, S.; Nooshabadi, M.R.; Kavianpour, M.; Haghighian, H.K. The effect of Ellagic acid on sleep quality in patients with type 2 diabetes: A randomized double blind clinical trial. Int. J. Diabetes Dev. Ctries. 2021, 41, 29–36. [Google Scholar] [CrossRef]
- Yari, M.G.; Foroughi, F.; Hashemipour, S.; Nooshabadi, M.R.; Ahmadi, M.H.; Nezhad, B.A.; Khadem, H. Effects of ellagic acid supplementation on glycemic index and insulin resistance in type 2 diabetics: A double blind randomized clinical trial. Koomesh 2021, 23, 582–590. [Google Scholar]
- Hidalgo-Lozada, G.M.; Villarruel-López, A.; Martínez-Abundis, E.; Vázquez-Paulino, O.; González-Ortiz, M.; Pérez-Rubio, K.G. Ellagic acid effect on the components of metabolic syndrome, insulin sensitivity and insulin secretion: A randomized, double-blind, placebo-controlled clinical trial. J. Clin. Med. 2022, 11, 5741. [Google Scholar] [CrossRef] [PubMed]
- Immanuel, J.; Kongbrailatpam, T.; Rajagopal, R.; Simmons, D. Evaluation of the analytical and clinical accuracy of four blood glucose meters in pregnant women with hyperglycaemia. Diabetes Obes. Metab. 2025, 27, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Arias-Rivera, S.; Raurell-Torredà, M.; Fernández-Castillo, R.J.; Campos-Asensio, C.; Thuissard-Vasallo, I.J.; Andreu-Vázquez, C.; Rodríguez-Delgado, M.E. Blood glucose monitoring in critically ill adult patients: Type of sample and method of analysis. Systematic review and meta-analysis. In Enfermería Intensiva (English Ed.); Elsevier: Amsterdam, The Netherlands, 2024; Volume 35, pp. 45–72. [Google Scholar]
| Compound Identity | Content, mg/10 g |
|---|---|
| Epigallocatechin | 0.02 ± 0.00 |
| Epicatechin gallate | 0.18 ± 0.14 |
| Cyanidin Chloride | 0.34 ± 0.05 |
| Catechin | 0.06 ± 0.01 |
| Vanillin | 0.06 ± 0.00 |
| Epicatechin | 0.36 ± 0.00 |
| Naringenin | 0.02 ± 0.00 |
| Hesperetin | 0.02 ± 0.00 |
| Verbascoside | 0.02 ± 0.00 |
| Chlorogenic Acid | 2.21 ± 0.09 |
| Neochlorogenic Acid | 0.05 ± 0.00 |
| Cryptochloroqenic Acid | 0.09 ± 0.01 |
| Quercetin | 0.00 ± 0.00 |
| Myricetin | 0.01 ± 0.00 |
| P-Coumaric Aicd | 0.09 ± 0.00 |
| Salicylic Acid | 0.03 ± 0.01 |
| Vanillic Acid | 0.15 ± 0.02 |
| Mcoumaric | 0.07 ± 0.00 |
| Ocoumaric | 0.01 ± 0.00 |
| Isoferulic Acid | 0.03 ± 0.00 |
| Ellagic Acid | 38.03 ± 3.59 |
| Ferulic Acid | 0.04 ± 0.00 |
| Phloridizin | 0.03 ± 0.05 |
| Gallic Acid | 0.68 ± 0.02 |
| 3.4-dihydroxybenzoic acid | 0.18 ± 0.00 |
| 2,3-Dihydroxybenzoic Acid | 0.25 ± 0.01 |
| Caffeic Acid | 0.35 ± 0.01 |
| Luteolin-7-O-glucoside | 0.09 ± 0.01 |
| Quercetin-3-O-glucuronide | 6.47 ± 0.43 |
| Quercetin-3-O-rutinoside | 0.24 ± 0.01 |
| Quercetin 3-O-galactoside | 0.14 ± 0.00 |
| Kaempferol-3-O-glucoside | 0.08 ± 0.01 |
| Quercetin-3-glucoside | 0.14 ± 0.00 |
| Kaempferol-O-rutinoside | 0.02 ± 0.00 |
| Petunidin Chloride | 0.01 ± 0.00 |
| Gallocatechin | 0.00 ± 0.00 |
| Total of Ellagitannin content (mg/10 g) | 38.03 ± 3.59 |
| Total of Flavonoid content (mg/10 g) | 7.76 ± 0.49 |
| Total of Phenolic Acid content (mg/10 g) | 4.78 ± 0.15 |
| Total of Polyphenol content (mg/10 g) | 50.56 ± 3.00 |
| Characteristic | N | Mean ± SD | 95% CI | Range | Median |
|---|---|---|---|---|---|
| Age, year | 20 | 34.86 ± 45.94 | 34.86–45.94 | 62–22 | 39.5 |
| Height, cm | 20 | 164.98 ± 174.92 | 164.98–174.92 | 192–153 | 170.5 |
| Weight, kg | 20 | 64.17 ± 76.9 | 64.17–76.9 | 104.5–53 | 63.7 |
| BMI, kg/m2 | 20 | 22.82 ± 25.77 | 22.82–25.77 | 30.1–20 | 23.3 |
| Fat % | 20 | 20.81 ± 26.95 | 20.81–26.95 | 40–9.9 | 23.5 |
| Fasting Blood Glucose (mmol/L) | 20 | 4.43 ± 4.89 | 4.43–4.89 | 5.68–2.95 | 4.75 |
| Fasting Blood Insulin (Pmol/L) | 20 | 41.91 ± 59.74 | 41.91–59.74 | 91.8–21.6 | 44.7 |
| HOMA-IR | 20 | 1.43 ± 2.07 | 1.43–2.07 | 2.93–0.71 | 1.52 |
| Systolic blood pressure, mmHg | 20 | 115.1 ± 125.6 | 115.1–125.6 | 147–100 | 119 |
| Diastolic blood pressure, mmHg | 20 | 72.38 ± 79.82 | 72.38–79.82 | 96–65 | 73 |
| Food Group | Change in Postprandial Blood Glucose Versus 15 min Before Carbohydrates Intake (mmol/L and %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 15 min | % | 30 min | % | 60 min | % | 90 min | % | 120 min | % | |
| Glucose | 20 | 2.03 ± 0.95 | 43.30 | 2.98 ± 1.46 | 63.57 | 2.40 ± 1.72 | 51.13 | 1.02 ± 1.53 | 21.74 | −0.031.05 | −0.57 |
| Glucose + RLT | 20 | 1.93 ± 0.72 | 41.47 | 3.00 ± 1.01 | 64.59 | 2.06 ± 1.97 | 44.24 | 0.95 ± 1.80 | 20.43 | 0.03 ± 1.64 | 0.64 |
| p | 0.64 | 0.94 | 0.36 | 0.78 | 0.79 | ||||||
| Sucrose | 20 | 1.83 ± 0.87 | 38.32 | 2.90 ± 0.95 | 60.69 | 1.29 ± 1.38 | 26.95 | −0.27 ± 0.84 | −5.70 | −0.96 ± 2.18 | −20.10 |
| Sucrose + RLT | 20 | 1.19 ± 0.88 | 25.59 | 2.03 ± 1.05 | 43.57 | 1.23 ± 1.29 | 26.36 | 0.13 ± 0.76 | 2.85 | −0.16 ± 0.77 | −3.44 |
| p | 0.001 | 0.0004 | 0.23 | 0.06 | 0.16 | ||||||
| Food Group | Change in Postprandial Blood Insulin Versus 15 min Before Carbohydrates Intake (pmol/L and %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 15 min | % | 30 min | % | 60 min | % | 90 min | % | 120 min | % | |
| Glucose | 20 | 159.90 ± 139.24 | 287.71 | 239.88 ± 95.30 | 431.62 | 214.48 ± 114.60 | 385.92 | 157.15 ± 146.51 | 282.77 | 53.47 ± 76.64 | 96.21 |
| Glucose + RLT | 20 | 162.79 ± 78.23 | 331.75 | 254.78 ± 103.43 | 519.22 | 215.10 ± 144.40 | 438.34 | 143.63 ± 130.22 | 292.69 | 61.34 ± 94.16 | 125.01 |
| p | 0.93 | 0.50 | 0.98 | 0.45 | 0.44 | ||||||
| Sucrose | 20 | 164.07 ± 125.70 | 296.71 | 242.90 ± 125.01 | 439.28 | 180.25 ± 94.02 | 325.98 | 66.62 ± 58.57 | 120.48 | 16.40 ± 32.78 | 29.66 |
| Sucrose + RLT | 20 | 113.90 ± 59.58 | 211.05 | 161.76 ± 91.96 | 299.74 | 139.44 ± 75.96 | 258.39 | 80.84 ±58.40 | 149.79 | 31.87 ± 43.97 | 59.06 |
| p | 0.01 | 0.0008 | 0.02 | 0.21 | 0.12 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhudaydi, H.M.S.; Spencer, J.P.E. Effects of Raspberry Leaf Tea Polyphenols on Postprandial Glucose and Insulin Responses in Healthy Adults. Nutrients 2025, 17, 2849. https://doi.org/10.3390/nu17172849
Alkhudaydi HMS, Spencer JPE. Effects of Raspberry Leaf Tea Polyphenols on Postprandial Glucose and Insulin Responses in Healthy Adults. Nutrients. 2025; 17(17):2849. https://doi.org/10.3390/nu17172849
Chicago/Turabian StyleAlkhudaydi, Hind Mesfer S., and Jeremy P. E. Spencer. 2025. "Effects of Raspberry Leaf Tea Polyphenols on Postprandial Glucose and Insulin Responses in Healthy Adults" Nutrients 17, no. 17: 2849. https://doi.org/10.3390/nu17172849
APA StyleAlkhudaydi, H. M. S., & Spencer, J. P. E. (2025). Effects of Raspberry Leaf Tea Polyphenols on Postprandial Glucose and Insulin Responses in Healthy Adults. Nutrients, 17(17), 2849. https://doi.org/10.3390/nu17172849






