Diet and Depression During Peri- and Post-Menopause: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Deviations from the Intended Protocol
2.2. Step 1–Identifying the Research Questions
- What are the characteristics of the available evidence on diet-related variables and depression in peri- and post-menopausal women?
- What are the main findings of the available evidence on diet-related variables and depression in peri- and post-menopausal women?
- What are the main research gaps on the topic of diet-related variables and depression in peri- and post-menopausal women?
2.3. Step 2–Identifying the Relevant Studies
2.4. Step 3–Study Selection
2.4.1. Type of Participants
2.4.2. Type of Exposures and Interventions
2.4.3. Type of Comparators
2.4.4. Type of Outcomes
2.4.5. Type of Study Designs
2.4.6. Selection of Studies
2.5. Step 4–Charting the Data
2.6. Step 5–Collating, Summarizing, and Reporting Results
2.7. Step 6–Methodological Quality Appraisal
- Being at low risk of bias when all domains were rated as such;
- Raising some concerns when at least one domain was rated as such, but no domain was rated as being at high risk of bias;
- Being at high risk of bias when at least one domain was rated as such, or when multiple domains were rated as raising some concerns.
3. Results
3.1. Study Characteristics
3.1.1. Type of Participants
3.1.2. Type of Exposures, Interventions, and Comparators
3.1.3. Type of Outcomes
3.1.4. Type of Study Designs
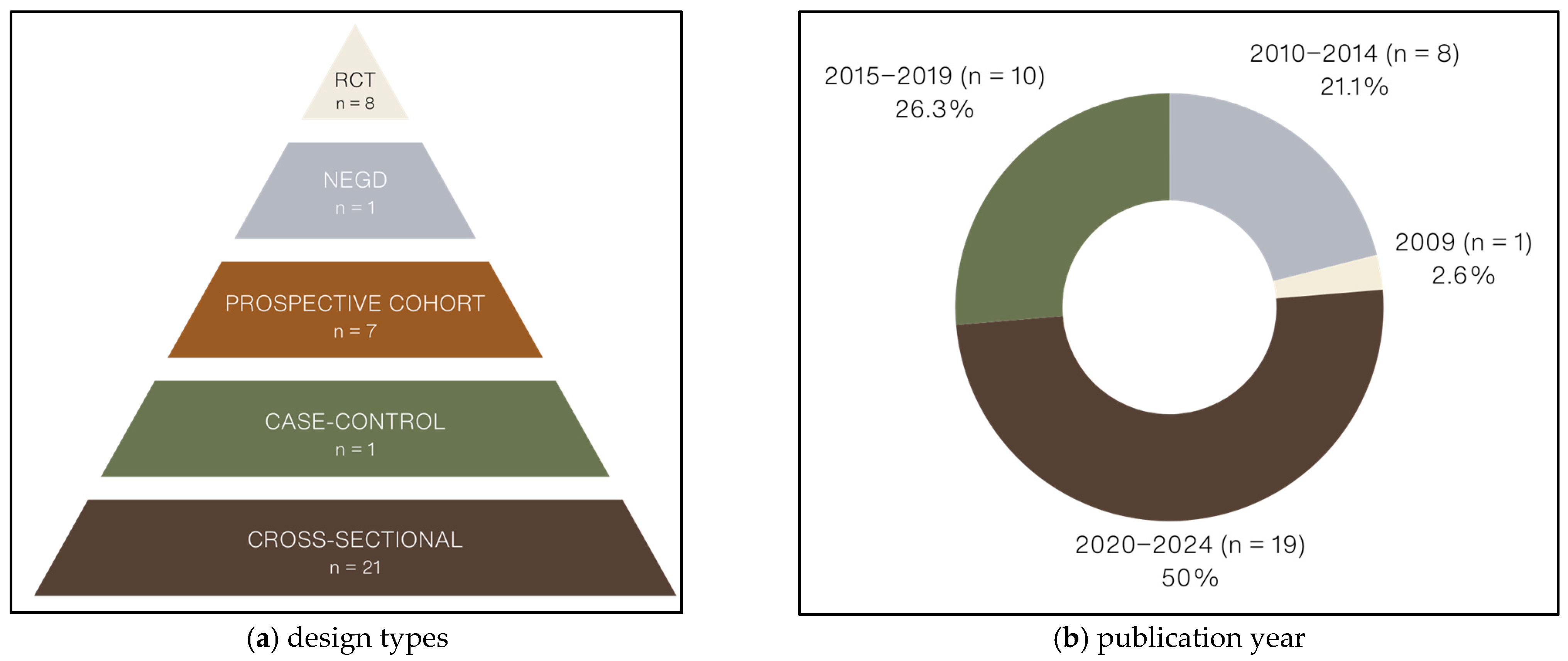
3.1.5. Methodological Quality
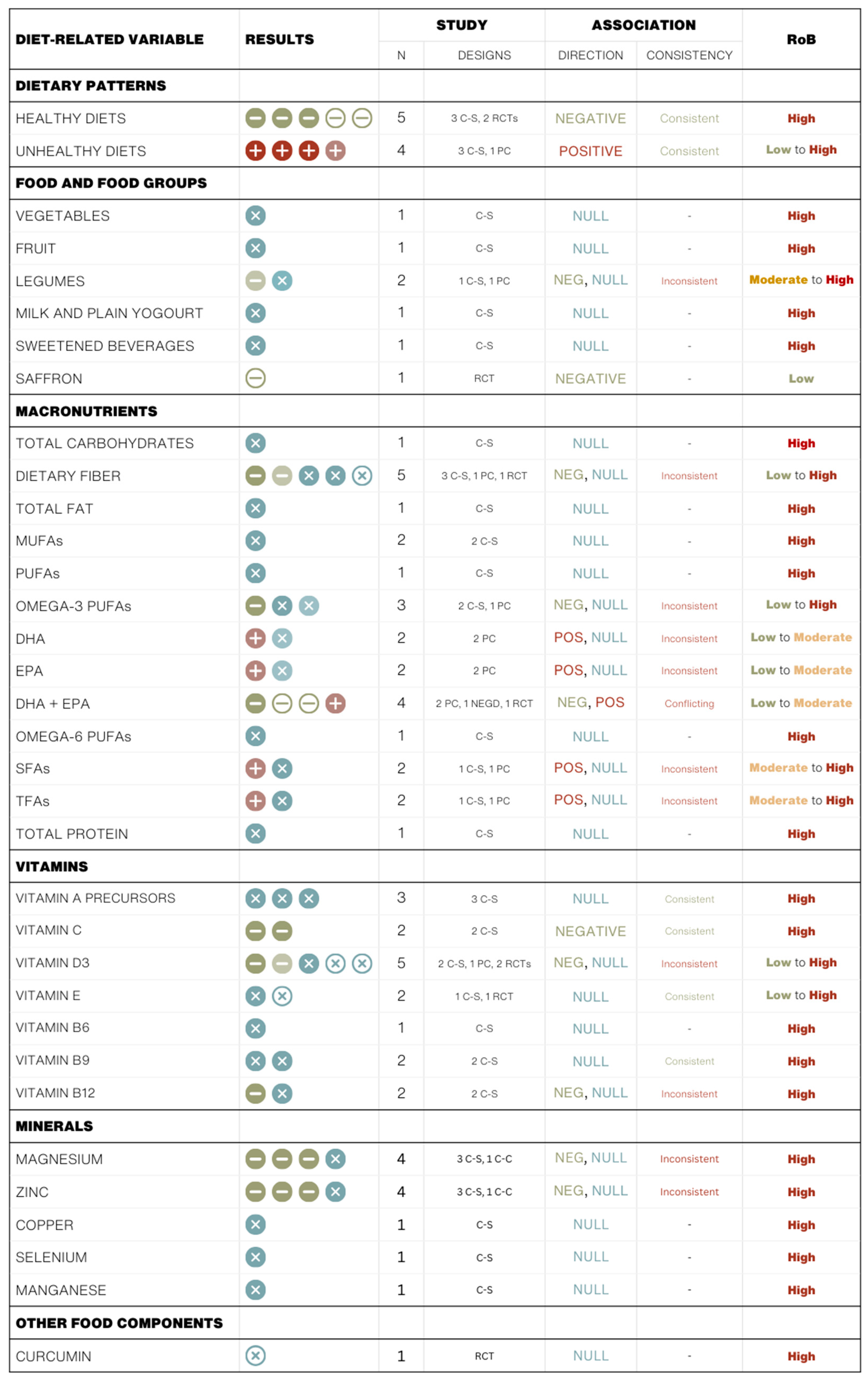
3.2. Summary of Study Findings
3.2.1. Dietary Patterns
| Authors (Year) Country | Population | Exposure | Outcome | Statistical Adjustments | Results | RoB |
|---|---|---|---|---|---|---|
| Abshirini et al. (2019) [93] Iran | n = 175 Post-MP | DTAC Method: FFQ (147 items) and PCA | Depressive symptoms Method: DASS-42 | SECs; MPSs |
| 9/15 |
| Azarmanesh et al. (2022) [94] United States | n = 2392 Post-MP | DII Method: 24 h dietary recall and DII | Depressive symptoms Method: PHQ-9 | SECs; Anthropometrics; Health behaviors |
| 9/15 |
| Chae et al. (2021) [95] South Korea | n = 4150 Post-MP | Omega-3 PUFA intake Method: 24 h dietary recall | Depression dx or symptoms Method: Dx or NR tool | SECs; Anthropometrics; Health behaviors; Diet |
| 9/15 |
| Kim et al. (2021) [96] United States | n = 2858 Post-MP | Dietary fiber intake Method: 24 h dietary recall | Depressive symptoms Method: PHQ-9 | SECs; Anthropometrics; Health behaviors; Chronic diseases |
| 10/15 |
| Kostecka et al. (2022) [114] Poland | n = 191 Peri-MP | Vit. D3 status Method: NR | Depressive symptoms Method: BDI | None reported |
| 8/15 |
| Lee et al. (2023) [109] South Korea | n = 1770 Peri/Post-MN | Vit. B9, A, and E serum levels Method: NR | Depressive symptoms Method: PHQ-9 | SECs; Health behaviors |
| 9/15 |
| Li et al. (2020a) [77] United States | n = 1406 Peri-MN | Omega-3 PUFA intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Diet; SHs |
| 9/15 |
| Li et al. (2020b) [78] United States | n = 2793 Pre/peri-MN | Oleic and linoleic acid intakes Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; MPSs; Anthropometrics; Health behaviors |
| 9/15 |
| Li et al. (2020c) [79] United States | n = 1403 Peri-MN | TFA intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Diet |
| 9/15 |
| Li et al. (2020d) [80] United States | n = 1359 Peri-MN | Mn intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Diet; VMSs |
| 9/15 |
| Li et al. (2020e) [81] United States | n = 1403 Peri-MN | Dietary fiber intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Diet; SHs |
| 9/15 |
| Li et al. (2021) [82] United States | n = 1400 Peri-MN | β-carotene intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Diet; SHs; VMSs |
| 9/15 |
| Li et al. (2022a) [83] United States | n = 3054 Pre/peri-MN | Provit. A intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Diet; SHs |
| 9/15 |
| Li et al. (2022b) [84] United States | n = 3088 Pre/peri-MN | Vit. C intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Health behaviors; Diet; Chronic diseases |
| 9/15 |
| Liao et al. (2019) [97] China | n = 2051 Post-MP | Dietary patterns a posteriori Method: FFQ (100 items) and PCA | Depressive symptoms Method: ZSRDS | SECs; Health behaviors; Diet; Chronic diseases |
| 9/15 |
| Liu et al. (2016) [98] China | n = 1125 Post-MP | Dietary patterns a posteriori Method: FFQ (85 items) and PCA | Depressive symptoms Method: CES-D | SECs; Health behaviors; Diet; Chronic diseases |
| 9/15 |
| Nazari et al. (2019) * [99] Iran | n = 136 Post-MP | Mg and Zn serum levels Method: AAS | Depressive symptoms Method: BDI | NR |
| 7/15 |
| Noll et al. (2022) [100] Brazil | n = 225 Post-MP | Intake of 7 food groups Method: 24 h dietary recall | Depressive symptoms Method: WHQ | SECs; MPSs |
| 9/15 |
| Oldra et al. (2020) [110] Brazil | n = 400 Peri/post-MP | Intake of 19 nutrients Method: 3 d food diary | Depressive symptoms Method: CES-D | NR |
| 9/15 |
| Şengül et al. (2014) [101] Turkey | n = 96 Post-MP | Serum vit. B9 and B12 levels Method: Autoanalyzer | Depressive symptoms Method: CES-D | NR |
| 7/15 |
| Stanisławska et al. (2014) [102] Pomeranian region | n = 171 Post-MN | Plasma Mg and Zn levels Method: AAS | Depressive symptoms Method: BDI | NR |
| 7/15 |
| Wieder-Huszla et al. (2020) [103] Pomeranian region | n = 102 Post-MP | Mg, Zn, Cu, and Se serum levels Method: Mannovette system | Depressive symptoms Method: BDI-II | NR |
| 7/15 |
| Authors (Year) Country | Population | Exposure | Outcome | Statistical Adjustments | Results | RoB |
|---|---|---|---|---|---|---|
| Bertone-Johnson et al. (2011) [87] United States Study duration: 3 y | n = 81,189 Post-MP | Vit. D3 intake Method: FFQ (122 items) | Depressive symptoms Method: 8-BS/AD use | SECs; Anthropometrics; Health behaviors; HT use; Diet; Chronic diseases; Solar irradiance |
| 13/15 |
| Colangelo et al. (2017) [104] United States Study duration: 3.2 y | n = 1616 Post-MP | DHA, EPA, and DHA+EPA intakes Method: FFQ (120 items) | Depressive symptoms Method: CES-D/AD use | SECs; Anthropometrics; Health Behaviors; Diet; Chronic diseases |
| 12/15 |
| Gangwisch et al. (2015) [88] United States Study duration: 3 y | n = 69,954 Post-MP | Dietary glycemic index Added sugar intake Dietary fiber intake Method: FFQ (145 items) | Depressive symptoms Method: 8-BS/AD use | SECs; Anthropometrics; Health behaviors; Social support; Stressful life events; HT use; Diet; Chronic diseases |
| 13/15 |
| Li et al. (2010) [102,111] United States Study duration: 10.6 y | n = 1005 Peri/Post-MP | Weekly legume intake Method: FFQ | Severe depressed mood Method: CES-D/AD use | SECs; Anthropometrics; Health behaviors; Diet; Food Allergies; Chronic diseases |
| 12/15 |
| Li et al. (2020f) [85] United States Study duration: 5 y | n = 2376 Pre/Post-MP | SFA intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | SECs; Anthropometrics; Health behaviors; Chronic stress; AD use; Diet; VMSs; SHs |
| 12/15 |
| Li et al. (2020g) [86] United States Study duration: 5 y | n = 2376 Pre/Post-MP | TFA intake Method: FFQ (103 items) | Depressive symptoms Method: CES-D | Anthropometrics; Health behaviors; Chronic stress; AD use; Diet; SHs |
| 12/15 |
| Persons et al. (2014) [89] United States Study duration: 3 y | n = 7066 Post-MP | Omega-3 PUFA intake DHA, EPA, DHA+EPA intakes RBC omega-3 PUFAs, DHA, and EPA Methods: FFQ (120 items; intake) NR (RBC) | Depressive symptoms Method: 8-BS/AD use | SECs; Health behaviors; HT use; Bilateral oophorectomy; Chronic diseases |
| 13/15 |
| Authors (Year) Country | Study Design | Population | Interventions | Outcome | Results | RoB | |
|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||
| Assaf et al. (2016) [91] United States | Open-label RCT Duration: 1 y n = 48,834 | Post-MP | Low-fat diet | No intervention | Depressive symptoms Method: Modified CES-D |
| High |
| Bertone-Johnson et al. (2012) [90] United States | TB-RCT Duration: 3 y n = 36,282 | Post-MP | Daily vit. D3 (400 IU) + Ca (1000 mg) supplement capsules | Placebo capsule | Depressive symptoms Method: 8-BS/AD use |
| High |
| Farshbaf-Khalili et al. (2022) [105] Iran | TB-RCT Duration: 8 wks. n = 81 | Post-MP | Experimental intervention #1: Daily curcumin (1000 mg) supplement capsules Experimental intervention #2: Daily vit. E (1000 mg) supplement capsules | Placebo capsule | Depressive symptoms Method: GCS |
| Low |
| Freeman et al. (2011) [92] United States | Pre-Post NEGD Duration: 8 wks. n = 20 | Peri/Post-MP | Daily ethyl-DHA (375 mg) + EPA (465 mg) supplement capsules | None | Depressive symptoms Method: MADRS |
| High |
| Kashani et al. (2018) [106] Iran | DB-RCT Duration: 6 wks. n = 56 | Post-MP | Daily saffron (30 mg) supplement capsules | Placebo capsule | Depressive symptoms Method: HDRS |
| High |
| Lucas et al. (2009) [112] Canada | TB-RCT Duration: 8 wks. n = 120 | Peri/Post-MP | Daily ethyl-DHA (150 mg) + EPA (1005 mg) supplement capsules | Placebo capsule | Depressive symptoms Method: HSCL-D-2 and HDRS-21 |
| Low |
| Mason et al. (2016) [107] United States | TB-RCT Duration: 1 y n = 218 | Post-MP | Daily vit. D3 (2000 IU) supplement capsules | Placebo capsule | Depressive symptoms Method: BSI-18 |
| Low |
| Shafie et al. (2022) [74] Iran | TB-RCT Duration: 6 wks. n = 60 | Peri/Post-MP | Prebiotic-enriched yoghurt | Regular yoghurt placebo | Depressive symptoms Method: DASS-21 |
| Low |
| Torres et al. (2012) [108] Australia | Open-label RCT Duration: 14 wks. n = 95 | Post-MP | Experimental intervention #1: DASH diet Experimental intervention #2: Low-fat diet | None | Depressive symptoms Method: 37-item POMS |
| High |
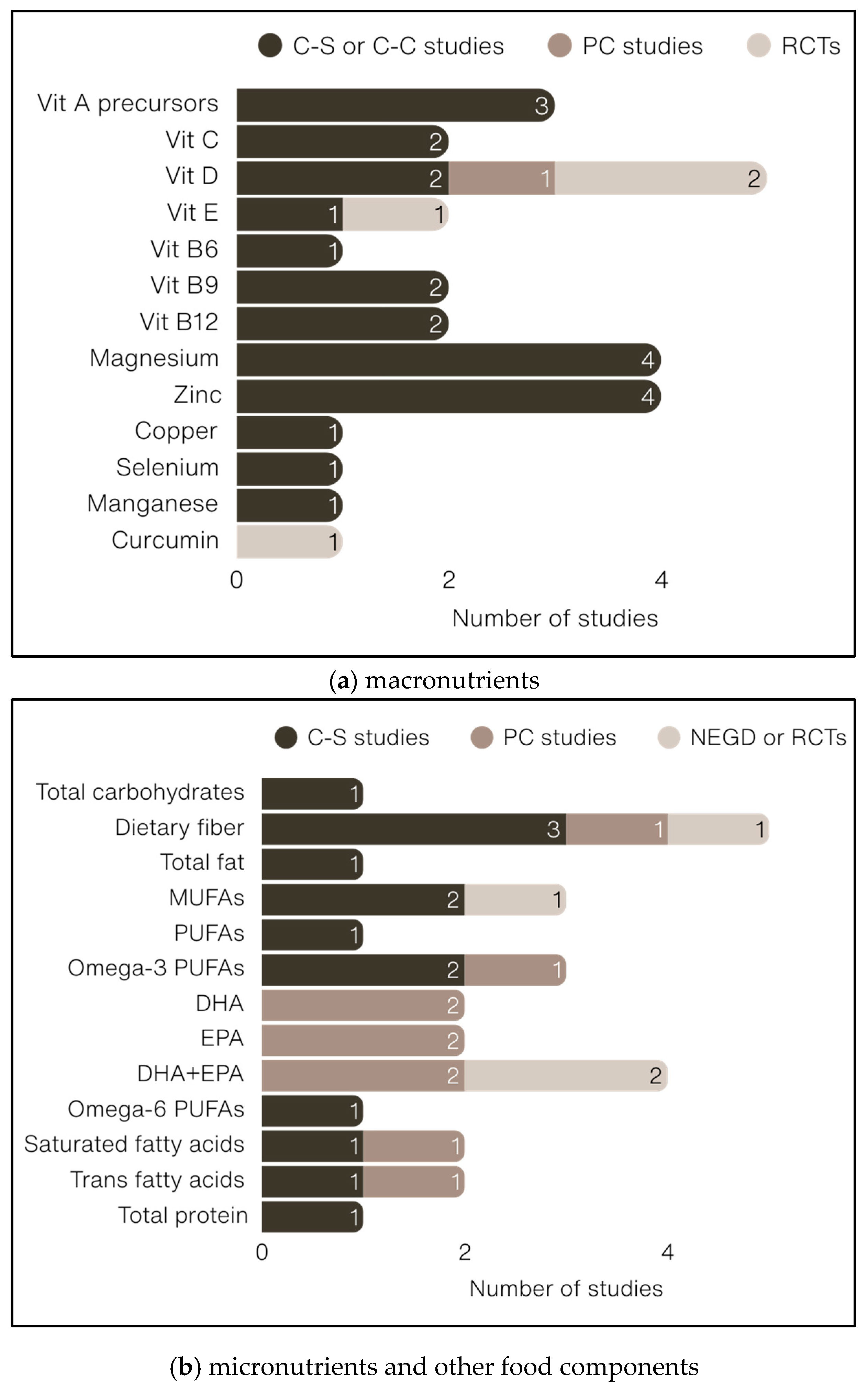
3.2.2. Food and Food Groups
3.2.3. Macronutrients
3.2.4. Micronutrients
3.2.5. Other Food Components
4. Discussion
4.1. Summary of Study Findings
4.2. Findings in Relation to Other Studies
4.3. Strengths and Limitations of the Evidence
4.4. Strengths and Limitations of the Review
4.5. Research Gaps and Future Directions
- Limited interventional research: Few RCTs have specifically examined dietary interventions in peri- and post-menopausal women. Future RCTs are needed to evaluate the effectiveness of dietary strategies for preventing or managing depressive symptoms during and after the menopausal transition.
- Lack of standardization in exposure and outcome measures: Greater consistency is needed in how dietary exposures (e.g., specific dietary patterns, nutrient intake) and depressive outcomes (e.g., validated symptom scales, diagnostic criteria) are defined and measured. Standardization would improve the comparability and synthesis of findings across studies.
- Inadequate consideration of menopause-specific factors: Many studies overlook important contextual variables such as hormonal status, severity of menopausal symptoms, and hormone therapy use. These factors may act as effect modifiers and should be systematically considered in future research to better understand the diet–depression relationship in this population.
- Limited population diversity: Existing studies are often restricted to specific geographic regions or demographically homogeneous cohorts. There is a need for research in more diverse populations to capture how cultural, socioeconomic, and racial/ethnic factors influence dietary intakes and mental health outcomes in midlife women.
- Insufficient investigation of biological mechanisms: Although mechanisms such as sub-chronic inflammation, oxidative stress, and gut microbiota dysbiosis have been proposed, few studies have directly assessed these pathways in peri- and post-menopausal women. Incorporating biomarker analyses and mechanistic approaches into future studies could provide critical insights into how diet influences mental health during midlife.
4.6. Implications for Research and Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDI | Beck’s Depression Inventory |
| BS | Burnam Scale |
| BSI | Brief Symptom Inventory |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| CES-D | Centre for Epidemiologic Studies Depression Scale |
| DASH | Dietary Approach to Stop Hypertension |
| DASS | Depression Anxiety Stress Scale |
| DHA | Docosahexaenoic Acid |
| DII | Dietary Inflammatory Index |
| DTAC | Dietary Total Antioxidant Capacity |
| EPA | Eicosapentaenoic Acid |
| GCS | Greene Climacteric Scale |
| HDRS | Hamilton Depression Rating Scale |
| HPA | Hypothalamic–pituitary–adrenal |
| HSCL-D | Hopkins Symptoms Checklist Depression Scale |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MUFA(s) | Monounsaturated Fatty Acid(s) |
| NEGD | Non-Equivalent Groups Design |
| NHLBI | National Heart, Lung, and Blood Institute |
| PHQ-9 | Patient Health Questionnaire |
| POMS | Profile of Mood State |
| PRISMA | Preferred Reporting Items for Systematic Reviews Meta-Analyses |
| PRISMA-ScR | PRISMA Checklist’s Extension for Scoping Reviews |
| PUFA(s) | Polyunsaturated Fatty Acid(s) |
| RCT(s) | Randomized controlled trial(s) |
| RoB | Risk of Bias |
| SFA(s) | Saturated Fatty Acid(s) |
| SWAN | Studies of Women’s Health Across the Nation |
| TFA(s) | Trans Fatty Acid(s) |
| WHI | Women’s Health Initiative |
| WHQ | Women’s Health Questionnaire |
| ZSRDS | Zung Self-Rating Depression Scale |
References
- Liu, J.; Ning, W.; Zhang, N.; Zhu, B.; Mao, Y. Estimation of the Global Disease Burden of Depression and Anxiety between 1990 and 2044: An Analysis of the Global Burden of Disease Study 2019. Healthcare 2024, 12, 1721. [Google Scholar] [CrossRef]
- Fan, Y.; Fan, A.; Yang, Z.; Fan, D. Global Burden of Mental Disorders in 204 Countries and Territories, 1990–2021: Results from the Global Burden of Disease Study 2021. BMC Psychiatry 2025, 25, 486. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Fahey, P.; Cochrane, B.; Smith, S. Bidirectional Associations Between Clinically Relevant Depression or Anxiety and COPD: A Systematic Review and Meta-Analysis. Chest 2013, 144, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; De Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Rotella, F.; Mannucci, E. Depression as a Risk Factor for Diabetes: A Meta-Analysis of Longitudinal Studies. J. Clin. Psychiatry 2013, 74, 4231. [Google Scholar] [CrossRef]
- Seabury, S.A.; Axeen, S.; Pauley, G.; Tysinger, B.; Schlosser, D.; Hernandez, J.B.; Heun-Johnson, H.; Zhao, H.; Goldman, D.P. Measuring The Lifetime Costs Of Serious Mental Illness And The Mitigating Effects Of Educational Attainment. Behav. Healthc. 2019, 38, 652–659. [Google Scholar] [CrossRef]
- Insel, T.R. Assessing the Economic Costs of Serious Mental Illness. Am. J. Psychiatry 2008, 165, 663–665. [Google Scholar] [CrossRef]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender Differences in Depression in Representative National Samples: Meta-Analyses of Diagnoses and Symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef] [PubMed]
- Douma, S.L.; Husband, C.; O’Donnell, M.E.; Barwin, B.N.; Woodend, A.K. Estrogen-Related Mood Disorders: Reproductive Life Cycle Factors. Adv. Nurs. Sci. 2005, 28, 364–375. [Google Scholar] [CrossRef]
- Sohn, J.H.; Ahn, S.H.; Seong, S.J.; Ryu, J.M.; Cho, M.J. Prevalence, Work-Loss Days and Quality of Life of Community Dwelling Subjects with Depressive Symptoms. J. Korean Med. Sci. 2013, 28, 280–286. [Google Scholar] [CrossRef]
- Alonso, J.; Petukhova, M.; Vilagut, G.; Chatterji, S.; Heeringa, S.; Üstün, T.B.; Alhamzawi, A.O.; Viana, M.C.; Angermeyer, M.; Bromet, E.; et al. Days out of Role Due to Common Physical and Mental Conditions: Results from the WHO World Mental Health Surveys. Mol. Psychiatry 2010, 16, 1234–1246. [Google Scholar] [CrossRef]
- Lerner, D.; Adler, D.A.; Chang, H.; Lapitsky, L.; Hood, M.Y.; Perissinotto, C.; Reed, J.; McLaughlin, T.J.; Berndt, E.R.; Rogers, W.H. Unemployment, Job Retention, and Productivity Loss among Employees with Depression. Psychiatr. Serv. 2004, 55, 1371–1378. [Google Scholar] [CrossRef]
- Beck, A.; Crain, L.A.; Solberg, L.I.; Unützer, J.; Maciosek, M.V.; Whitebird, R.R.; Rossom, R.C. Does Severity of Depression Predict Magnitude of Productivity Loss? Am. J. Care 2014, 20, e294. [Google Scholar]
- Woods, N.F.; Smith-DiJulio, K.; Percival, D.B.; Tao, E.Y.; Mariella, A.; Mitchell, E.S. Depressed Mood during the Menopausal Transition and Early Postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause 2008, 15, 223–232. [Google Scholar] [CrossRef]
- Hickey, M.; Schoenaker, D.A.J.M.; Joffe, H.; Mishra, G.D. Depressive Symptoms across the Menopause Transition: Findings from a Large Population-Based Cohort Study. Menopause 2016, 23, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.S.; Soares, C.N.; Vitonis, A.F.; Otto, M.W.; Harlow, B.L. Risk for New Onset of Depression During the Menopausal Transition: The Harvard Study of Moods and Cycles. Arch. Gen. Psychiatry 2006, 63, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W. Associations of Depression with the Transition to Menopause. Menopause 2010, 17, 823–827. [Google Scholar] [CrossRef]
- Freeman, E.W.; Sammel, M.D.; Lin, H.; Nelson, D.B. Associations of Hormones and Menopausal Status With Depressed Mood in Women With No History of Depression. Arch. Gen. Psychiatry 2006, 63, 375–382. [Google Scholar] [CrossRef]
- Bromberger, J.T.; Schott, L.; Kravitz, H.M.; Joffe, H. Risk Factors for Major Depression during Midlife among a Community Sample of Women with and without Prior Major Depression: Are They the Same or Different? Psychol. Med. 2015, 45, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Kravitz, H.M.; Chang, Y.F.; Cyranowski, J.M.; Brown, C.; Matthews, K.A. Major Depression during and after the Menopausal Transition: Study of Women’s Health Across the Nation (SWAN). Psychol. Med. 2011, 41, 1879–1888. [Google Scholar] [CrossRef]
- de Kruif, M.; Spijker, A.T.; Molendijk, M.L. Depression during the Perimenopause: A Meta-Analysis. J. Affect. Disord. 2016, 206, 174–180. [Google Scholar] [CrossRef]
- Joffe, H.; De Wit, A.; Coborn, J.; Crawford, S.; Freeman, M.; Wiley, A.; Athappilly, G.; Kim, S.; Sullivan, K.A.; Cohen, L.S.; et al. Impact of Estradiol Variability and Progesterone on Mood in Perimenopausal Women With Depressive Symptoms. J. Clin. Endocrinol. Metab. 2020, 105, e642–e650. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, Z.; Lee, S.; Kulkarni, J. The Unique Symptom Profile of Perimenopausal Depression. Clin. Psychol. 2020, 19, 76–84. [Google Scholar] [CrossRef]
- Kulkarni, J.; Gavrilidis, E.; Hudaib, A.R.; Bleeker, C.; Worsley, R.; Gurvich, C. Development and Validation of a New Rating Scale for Perimenopausal Depression—The Meno-D. Transl. Psychiatry 2018, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Thase, M.E.; Entsuah, R.; Cantillon, M.; Kornstein, S.G. Relative Antidepressant Efficacy of Venlafaxine and SSRIs: Sex-Age Interactions. J. Womes Health 2005, 14, 609–616. [Google Scholar] [CrossRef]
- Sell, S.L.; Craft, R.M.; Seitz, P.K.; Stutz, S.J.; Cunningham, K.A.; Thomas, M.L. Estradiol–Sertraline Synergy in Ovariectomized Rats. Psychoneuroendocrinology 2008, 33, 1051–1060. [Google Scholar] [CrossRef]
- Récamier-Carballo, S.; Estrada-Camarena, E.; Reyes, R.; Fernández-Guasti, A. Synergistic Effect of Estradiol and Fluoxetine in Young Adult and Middle-Aged Female Rats in Two Models of Experimental Depression. Behav. Brain Res. 2012, 233, 351–358. [Google Scholar] [CrossRef]
- Graziottin, A.; Serafini, A. Depression and the Menopause: Why Antidepressants Are Not Enough? Menopause Int. 2009, 15, 76–81. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and Veganism Compared with Mental Health and Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Yao, Z.; Zhang, T.; Li, Z. Dietary Inflammatory Potential and the Incidence of Depression and Anxiety: A Meta-Analysis. J. Health Popul. Nutr. 2022, 41, 24. [Google Scholar] [CrossRef]
- Bizzozero-Peroni, B.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R.; Jiménez-López, E.; Núñez de Arenas-Arroyo, S.; Saz-Lara, A.; Díaz-Goñi, V.; Mesas, A.E. The Impact of the Mediterranean Diet on Alleviating Depressive Symptoms in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2024, 83, 29–39. [Google Scholar] [CrossRef]
- Soltani, S.; Sangsefidi, Z.S.; Asoudeh, F.; Torabynasab, K.; Zeraattalab-Motlagh, S.; Hejazi, M.; Khalighi Sikaroudi, M.; Meshkini, F.; Razmpoosh, E.; Abdollahi, S. Effect of Low-Fat Diet on Depression Score in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Nutr. Rev. 2025, 83, e741–e750. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Travica, N.; Dissanayaka, T.; Ashtree, D.N.; Gauci, S.; Lotfaliany, M.; O’neil, A.; Jacka, F.N.; Marx, W. Ultra-Processed Food Consumption and Mental Health: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2022, 14, 2568. [Google Scholar] [CrossRef]
- Mazloomi, S.N.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghavami, A.; Zarpoosh, M.; Mohammadi, H.; Wong, A.; Nordvall, M.; Kermani, M.A.H.; et al. The Association of Ultra-Processed Food Consumption with Adult Mental Health Disorders: A Systematic Review and Dose-Response Meta-Analysis of 260,385 Participants. Nutr. Neurosci. 2023, 26, 913–931. [Google Scholar] [CrossRef] [PubMed]
- Werneck, A.O.; Steele, E.M.; Delpino, F.M.; Lane, M.M.; Marx, W.; Jacka, F.N.; Stubbs, B.; Touvier, M.; Srour, B.; Louzada, M.L.; et al. Adherence to the Ultra-Processed Dietary Pattern and Risk of Depressive Outcomes: Findings from the NutriNet Brasil Cohort Study and an Updated Systematic Review and Meta-Analysis. Clin. Nutr. 2024, 43, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mardi, P.; Hejrani, B.; Mahdavi, F.S.; Ghoreshi, B.; Gohari, K.; Heidari-Beni, M.; Qorbani, M. Association between Junk Food Consumption and Mental Health Problems in Adults: A Systematic Review and Meta-Analysis. BMC Psychiatry 2024, 24, 438. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-Analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A Meta-Analysis of Blood Cytokine Network Alterations in Psychiatric Patients: Comparisons between Schizophrenia, Bipolar Disorder and Depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of Central Inflammation in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Studies Examining Cerebrospinal Fluid, Positron Emission Tomography and Post-Mortem Brain Tissue. Brain Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liao, X.; Chen, J.; He, T.; Lai, S.; Jia, Y. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS ONE 2015, 10, e0138904. [Google Scholar] [CrossRef]
- Jimenez-Fernandez, S.; Gurpegui, M.; Diaz-Atienza, F.; Perez-Costillas, L.; Gerstenberg, M.; Correll, C.U. Oxidative Stress and Antioxidant Parameters in Patients With Major Depressive Disorder Compared to Healthy Controls Before and After Antidepressant Treatment: Results From a Meta-Analysis. J. Clin. Psychiatry 2015, 76, 13705. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W.J.H. Is Depression Associated with Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and Hypothalamic-Pituitary-Adrenal Activation: A Quantitative Summary of Four Decades of Research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Duran, N.L.; Kovacs, M.; George, C.J. Hypothalamic–Pituitary–Adrenal Axis Dysregulation in Depressed Children and Adolescents: A Meta-Analysis. Psychoneuroendocrinology 2009, 34, 1272–1283. [Google Scholar] [CrossRef]
- Lin, P.Y.; Tseng, P.T. Decreased Glial Cell Line-Derived Neurotrophic Factor Levels in Patients with Depression: A Meta-Analytic Study. J. Psychiatr. Res. 2015, 63, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Duman, R.; Sanacora, G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol. Psychiatry 2008, 64, 527–532. [Google Scholar] [CrossRef]
- Amirkhanzadeh Barandouzi, Z.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- van Zonneveld, S.M.; van den Oever, E.J.; Haarman, B.C.M.; Grandjean, E.L.; Nuninga, J.O.; van de Rest, O.; Sommer, I.E.C. An Anti-Inflammatory Diet and Its Potential Benefit for Individuals with Mental Disorders and Neurodegenerative Diseases—A Narrative Review. Nutrients 2024, 16, 2646. [Google Scholar] [CrossRef]
- Yin, Y.; Ju, T.; Zeng, D.; Duan, F.; Zhu, Y.; Liu, J.; Li, Y.; Lu, W. “Inflamed” Depression: A Review of the Interactions between Depression and Inflammation and Current Anti-Inflammatory Strategies for Depression. Pharmacol. Res. 2024, 207, 107322. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2007, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for Conducting Systematic Scoping Reviews. Int. J. Evid. Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Duquet, M.; Prud’homme, D.; Giroux, I. Diet and Depression during Peri- and Post-Menopause: A Scoping Review Protocol. Methods Protoc. 2023, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Savovic, J.; Page, M.; Elbers, R. Chapter 8: Assessing Risk of Bias in a Randomized Trial [Last Updated October 2019]. In Cochrane Handbook for Systematic Reviews of Interventions, version 6.5; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane Collaboration: London, UK, 2024; Available online: www.cochrane.org/handbook (accessed on 5 May 2025).
- National Heart, Lung, and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies; National Institutes of Health: Bethesda, MD, USA, 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 5 May 2025).
- National Heart, Lung, and Blood Institute. Quality Assessment Tool for Case-Control Studies; National Institutes of Health: Bethesda, MD, USA, 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 5 May 2025).
- Jokar, A.; Farahi, F. Effect of Vitamin C on Depression in Menopausal Women with Balanced Diet: A Randomized Clinical Trial. Iran. J. Obstet. Gynecol. Infertil. 2014, 17, 18–23. [Google Scholar] [CrossRef]
- Shahraeini, M.; Shourab, N.J.; Javan, R.; Shakeri, M.T. Effect of Food-Based Strategies of Iranian Traditional Medicine on Women’s Quality of Life during Menopause. Iran. J. Obstet. Gynecol. Infertil. 2021, 23, 67–75. [Google Scholar] [CrossRef]
- Chocano-Bedoya, P.; O’Reilly, E.; Lucas, M.; Mirzaei, F.; Okereke, O.; Fung, T.; Hu, F.; Ascherio, A. Dietary Patterns and Depression in the Nurses’ Health Study. Am. J. Epidemiol. 2012, 175, S75. [Google Scholar] [CrossRef]
- Pasco, J.A.; Williams, L.J.; Brennan-Olsen, S.L.; Berk, M.; Jacka, F.N. Milk Consumption and the Risk for Incident Major Depressive Disorder. Psychother. Psychosom. 2015, 84, 384–386. [Google Scholar] [CrossRef]
- Crawford, G.B.; Khedkar, A.; Flaws, J.A.; Sorkin, J.D.; Gallicchio, L. Depressive Symptoms and Self-Reported Fast-Food Intake in Midlife Women. Prev. Med. 2011, 52, 254–257. [Google Scholar] [CrossRef]
- Bae, Y.J.; Kim, S.K. Low Dietary Calcium Is Associated with Self-Rated Depression in Middle-Aged Korean Women. Nutr. Res. Pract. 2012, 6, 527–533. [Google Scholar] [CrossRef]
- Shivappa, N.; Schoenaker, D.A.J.M.; Hebert, J.R.; Mishra, G.D. Association between Inflammatory Potential of Diet and Risk of Depression in Middle-Aged Women: The Australian Longitudinal Study on Women’s Health. Brit. J. Nutr. 2016, 116, 1077–1086. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Suzuki, R.; Kato, K.; Hirose, A.; Miyasaka, N. Depressive Symptoms in Middle-Aged and Elderly Women Are Associated with a Low Intake of Vitamin B6: A Cross-Sectional Study. Nutrients 2020, 12, 3437. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, D.K.; Kim, B.; Na, K.S.; Lee, C.H.; Son, Y.D.; Lee, H.J. The Association between Omega-3 Fatty Acid Intake and Human Brain Connectivity in Middle-Aged Depressed Women. Nutrients 2020, 12, 2191. [Google Scholar] [CrossRef] [PubMed]
- Shon, J.; Seong, Y.; Choi, Y.; Kim, Y.; Cho, M.S.; Ha, E.; Kwon, O.; Kim, Y.; Park, Y.J.; Kim, Y. Meal-Based Intervention on Health Promotion in Middle-Aged Women: A Pilot Study. Nutrients 2023, 15, 2108. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, S.J.; Lee, H.J. Healthy and Unhealthy Dietary Patterns of Depressive Symptoms in Middle-Aged Women. Nutrients 2024, 16, 776. [Google Scholar] [CrossRef]
- Carels, R.A.; Darby, L.A.; Cacciapaglia, H.M.; Douglass, O.M. Reducing Cardiovascular Risk Factors in Postmenopausal Women through a Lifestyle Change Intervention. J. Womens Health 2004, 13, 412–426. [Google Scholar] [CrossRef]
- Palacios, S.; Mustata, C.; Rizo, J.M.; Regidor, P.A. Improvement in Menopausal Symptoms with a Nutritional Product Containing Evening Primrose Oil, Hop Extract, Saffron, Tryptophan, Vitamins B6, D3, K2, B12, and B9. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8180–8189. [Google Scholar] [CrossRef] [PubMed]
- Kachko, V.A.; Shulman, L.P.; Kuznetsova, I.V.; Uspenskaya, Y.B.; Burchakov, D.I. Clinical Evaluation of Effectiveness and Safety of Combined Use of Dietary Supplements Amberen® and Smart B® in Women with Climacteric Syndrome in Perimenopause. Adv. Ther. 2024, 41, 3183–3195. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; He, D.; Zhang, D.; Liu, X. Association between Different Triglyceride Glucose Index-Related Indicators and Depression in Premenopausal and Postmenopausal Women: NHANES, 2013–2016. J. Affect. Disord. 2024, 360, 297–304. [Google Scholar] [CrossRef]
- Shafie, M.; Homayouni Rad, A.; Mirghafourvand, M. Effects of Prebiotic-Rich Yogurt on Menopausal Symptoms and Metabolic Indices in Menopausal Women: A Triple-Blind Randomised Controlled Trial. Int. J. Food Sci. Nutr. 2022, 73, 693–704. [Google Scholar] [CrossRef]
- Haghshenas, N.; Baharanchi, F.H.; Melekoglu, E.; Sohouli, M.H.; Shidfar, F. Comparison of Predictive Effect of the Dietary Inflammatory Index and Empirically Derived Food-Based Dietary Inflammatory Index on the Menopause-Specific Quality of Life and Its Complications. BMC Womens Health 2023, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Khosravi, S.; Hedayati, M.; Aslani, Z.; Soleymani, M.; Sotoudeh, G. Dietary Total Antioxidant Capacity Is Inversely Related to Menopausal Symptoms: A Cross-Sectional Study among Iranian Postmenopausal Women. Nutrition 2018, 55–56, 161–167. [Google Scholar] [CrossRef]
- Li, D.; Liang, H.; Tong, Y.; Li, Y. Association of Dietary N-3 Polyunsaturated Fatty Acids Intake with Depressive Symptoms in Midlife Women. J. Affect. Disord. 2020, 261, 164–171. [Google Scholar] [CrossRef]
- Li, D.; Tong, Y.; Li, Y. Associations between Dietary Oleic Acid and Linoleic Acid and Depressive Symptoms in Perimenopausal Women: The Study of Women’s Health Across the Nation. Nutrition 2020, 71, 110602. [Google Scholar] [CrossRef]
- Li, D.; Tong, Y.; Li, Y. Associations of Dietary Trans Fatty Acid Intake with Depressive Symptoms in Midlife Women. J. Affect. Disord. 2020, 260, 194–199. [Google Scholar] [CrossRef]
- Li, D.; Wu, Q.; Xu, W.; Zheng, H.; Tong, Y.; Li, Y. Dietary Manganese Intake Is Inversely Associated with Depressive Symptoms in Midlife Women: A Cross-Sectional Study. J. Affect. Disord. 2020, 276, 914–919. [Google Scholar] [CrossRef]
- Li, D.; Tong, Y.; Li, Y. Dietary Fiber Is Inversely Associated With Depressive Symptoms in Premenopausal Women. Front. Neurosci. 2020, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, Q.; Tong, Y.; Zheng, H.; Li, Y. Dietary Beta-Carotene Intake Is Inversely Associated with Anxiety in US Midlife Women. J. Affect. Disord. 2021, 287, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, H.; Tong, Y.; Li, Y. Associations of Dietary Provitamin A Carotenoid Intake with Depressive Symptoms in Midlife Women: Results from the Study of Women’s Health Across the Nation. J. Affect. Disord. 2022, 317, 91–97. [Google Scholar] [CrossRef]
- Li, D.; Xu, W.; Wu, Q.; Zheng, H.; Li, Y. Ascorbic Acid Intake Is Inversely Associated with Prevalence of Depressive Symptoms in US Midlife Women: A Cross-Sectional Study. J. Affect. Disord. 2022, 299, 498–503. [Google Scholar] [CrossRef]
- Li, D.; Liang, H.; Tong, Y.; Zheng, H.; Li, Y. Association between Saturated Fatty Acid Intake and Depressive Symptoms in Midlife Women: A Prospective Study. J. Affect. Disord. 2020, 267, 17–22. [Google Scholar] [CrossRef]
- Li, D.; Zheng, H.; Tong, Y.; Li, Y. Prospective Association between Trans Fatty Acid Intake and Depressive Symptoms: Results from the Study of Women’s Health across the Nation. J. Affect. Disord. 2020, 264, 256–262. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Brunner, R.L.; Michael, Y.L.; Larson, J.C.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Liu, S.; et al. Vitamin D Intake from Foods and Supplements and Depressive Symptoms in a Diverse Population of Older Women. Am. J. Clin. Nutr. 2011, 94, 1104–1112. [Google Scholar] [CrossRef]
- Gangwisch, J.E.; Hale, L.; Garcia, L.; Malaspina, D.; Opler, M.G.; Payne, M.E.; Rossom, R.C.; Lane, D. High Glycemic Index Diet as a Risk Factor for Depression: Analyses from the Women’s Health Initiative. Am. J. Clin. Nutr. 2015, 102, 454–463. [Google Scholar] [CrossRef]
- Persons, J.E.; Robinson, J.G.; Ammann, E.M.; Coryell, W.H.; Espeland, M.A.; Harris, W.S.; Manson, J.E.; Fiedorowicz, J.G. Omega-3 Fatty Acid Biomarkers and Subsequent Depressive Symptoms. Int. J. Geriatr. Psychiatry 2014, 29, 747–757. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R.; Powers, S.I.; Spangler, L.; Larson, J.; Michael, Y.L.; Millen, A.E.; Bueche, M.N.; Salmoirago-Blotcher, E.; Wassertheil-Smoller, S.; Brunner, R.L.; et al. Vitamin D Supplementation and Depression in the Women’s Health Initiative Calcium and Vitamin D Trial. Am. J. Epidemiol. 2012, 176, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Assaf, A.R.; Beresford, S.A.A.; Risica, P.M.; Aragaki, A.; Brunner, R.L.; Bowen, D.J.; Naughton, M.; Rosal, M.C.; Snetselaar, L.; Wenger, N. Low-Fat Dietary Pattern Intervention and Health-Related Quality of Life: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. J. Acad. Nutr. Diet. 2016, 116, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.P.; Hibbeln, J.R.; Silver, M.; Hirschberg, A.M.; Wang, B.; Yule, A.M.; Petrillo, L.F.; Pascuillo, E.; Economou, N.I.; Joffe, H.; et al. Omega-3 Fatty Acids for Major Depressive Disorder Associated with the Menopausal Transition: A Preliminary Open Trial. Menopause 2011, 18, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Mozaffari, H.; Aslani, Z.; Soleymani, M.; Entezarian, M.; Sotoudeh, G. Dietary Total Antioxidant Capacity Is Inversely Associated with Depression, Anxiety and Some Oxidative Stress Biomarkers in Postmenopausal Women: A Cross-Sectional Study. Ann. Gen. Psychiatry 2019, 18, 3. [Google Scholar] [CrossRef]
- Azarmanesh, D.; Bertone-Johnson, E.R.; Pearlman, J.; Liu, Z.; Carbone, E.T. Association of the Dietary Inflammatory Index with Depressive Symptoms among Pre-and Post-Menopausal Women: Findings from the National Health and Nutrition Examination Survey (NHANES) 2005–2010. Nutrients 2022, 14, 1980. [Google Scholar] [CrossRef]
- Chae, M.; Park, K. Association between Dietary Omega-3 Fatty Acid Intake and Depression in Postmenopausal Women. Nutr. Res. Pract. 2021, 15, 468–478. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, M.; Kim, S.; Shin, W.Y.; Kim, J.H. Inverse Association between Dietary Fiber Intake and Depression in Premenopausal Women: A Nationwide Population-Based Survey. Menopause 2021, 28, 150–156. [Google Scholar] [CrossRef]
- Liao, K.; Gu, Y.; Liu, M.; Fu, J.; Wang, X.; Yang, G.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; et al. Association of Dietary Patterns with Depressive Symptoms in Chinese Postmenopausal Women. Brit. J. Nutr. 2019, 122, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Ho, S.C.; Xie, Y.J.; Chen, Y.J.; Chen, Y.M.; Chen, B.; Yeung-Shan Wong, S.; Chan, D.; Ka Man Wong, C.; He, Q.; et al. Associations between Dietary Patterns and Psychological Factors: A Cross-Sectional Study among Chinese Postmenopausal Women. Menopause 2016, 23, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Nazari, F.; Hekmat, K.; Iravani, M.; Haghighizadeh, M.H.; Boostani, H. The Relationship Between Serum Zinc and Magnesium Levels and Depression in Postmenopausal Women. J. Biochem. Technol. 2019, 10, 109–114. [Google Scholar] [CrossRef]
- Noll, P.R.e.S.; Noll, M.; Zangirolami-Raimundo, J.; Baracat, E.C.; da Costa Louzada, M.L.; Soares Júnior, J.M.; Sorpreso, I.C.E. Life Habits of Postmenopausal Women: Association of Menopause Symptom Intensity and Food Consumption by Degree of Food Processing. Maturitas 2022, 156, 1–11. [Google Scholar] [CrossRef]
- Şengül, Ö.; Uygur, D.; Güleç, M.; Dilbaz, B.; Şimşek, E.M.; Göktolga, Ü. The Comparison of Folate and Vitamin B12 Levels between Depressive and Nondepressive Postmenopausal Women. Turk. J. Med. Sci. 2014, 44, 611–615. [Google Scholar] [CrossRef]
- Stanisławska, M.; Szkup-Jabłońska, M.; Jurczak, A.; Wieder-Huszla, S.; Samochowiec, A.; Jasiewicz, A.; Noceń, I.; Augustyniuk, K.; Brodowska, A.; Karakiewicz, B.; et al. The Severity of Depressive Symptoms vs. Serum Mg and Zn Levels in Postmenopausal Women. Biol. Trace Elem. Res. 2014, 157, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Wieder-Huszla, S.; Zabielska, P.; Kotwas, A.; Owsianowska, J.; Karakiewicz-Krawczyk, K.; Kowalczyk, R.; Jurczak, A. The Severity of Depressive and Anxiety Symptoms in Postmenopausal Women Depending on Their Magnesium, Zinc, Selenium and Copper Levels. J. Elem. 2020, 25, 1305–1317. [Google Scholar] [CrossRef]
- Colangelo, L.A.; Ouyang, P.; Golden, S.H.; Szklo, M.; Gapstur, S.M.; Vaidya, D.; Liu, K. Do Sex Hormones or Hormone Therapy Modify the Relation of N-3 Fatty Acids with Incident Depressive Symptoms in Postmenopausal Women? The MESA Study. Psychoneuroendocrinology 2017, 75, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf-Khalili, A.; Ostadrahimi, A.; Mirghafourvand, M.; Ataei-Almanghadim, K.; Dousti, S.; Iranshahi, A.M. Clinical Efficacy of Curcumin and Vitamin e on Inflammatory-Oxidative Stress Biomarkers and Primary Symptoms of Menopause in Healthy Postmenopausal Women: A Triple-Blind Randomized Controlled Trial. J. Nutr. Metab. 2022, 2022, 6339715. [Google Scholar] [CrossRef] [PubMed]
- Kashani, L.; Esalatmanesh, S.; Eftekhari, F.; Salimi, S.; Foroughifar, T.; Etesam, F.; Safiaghdam, H.; Moazen-Zadeh, E.; Akhondzadeh, S. Efficacy of Crocus Sativus (Saffron) in Treatment of Major Depressive Disorder Associated with Post-Menopausal Hot Flashes: A Double-Blind, Randomized, Placebo-Controlled Trial. Arch. Gynecol. Obstet. 2018, 297, 717–724. [Google Scholar] [CrossRef]
- Mason, C.; de Dieu Tapsoba, J.; Duggan, C.; Wang, C.Y.; Korde, L.; McTiernan, A. Repletion of Vitamin D Associated with Deterioration of Sleep Quality among Postmenopausal Women. Prev. Med. 2016, 93, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.J.; Nowson, C.A. A Moderate-Sodium DASH-Type Diet Improves Mood in Postmenopausal Women. Nutrition 2012, 28, 896–900. [Google Scholar] [CrossRef]
- Lee, S.M.; Baek, J.C. Serum Vitamin Levels, Cardiovascular Disease Risk Factors, and Their Association with Depression in Korean Women: A Cross-Sectional Study of a Nationally Representative Sample. Medicina 2023, 59, 2183. [Google Scholar] [CrossRef]
- Oldra, C.M.; Benvegnú, D.M.; Silva, D.R.P.; Wendt, G.W.; Vieira, A.P. Relationships between Depression and Food Intake in Climacteric Women. Climacteric 2020, 23, 474–481. [Google Scholar] [CrossRef]
- Li, Y.; Dai, Q.; Tedders, S.H.; Arroyo, C.; Zhang, J. Legume Consumption and Severe Depressed Mood, the Modifying Roles of Gender and Menopausal Status. Public Health Nutr. 2010, 13, 1198–1206. [Google Scholar] [CrossRef]
- Lucas, M.; Asselin, G.; Mérette, C.; Poulin, M.J.; Dodin, S. Ethyl-Eicosapentaenoic Acid for the Treatment of Psychological Distress and Depressive Symptoms in Middle-Aged Women: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Am. J. Clin. Nutr. 2009, 89, 641–651. [Google Scholar] [CrossRef]
- Shafie, M.; Homayouni Rad, A.; Mohammad-Alizadeh-Charandabi, S.; Mirghafourvand, M. The Effect of Probiotics on Mood and Sleep Quality in Postmenopausal Women: A Triple-Blind Randomized Controlled Trial. Clin. Nutr. ESPEN 2022, 50, 15–23. [Google Scholar] [CrossRef]
- Kostecka, D.; Schneider-Matyka, D.; Barczak, K.; Starczewska, M.; Szkup, M.; Ustianowski, P.; Brodowski, J.; Grochans, E. The Effect of Vitamin D Levels on Lipid, Glucose Profiles and Depression in Perimenopausal Women. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3493–3505. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Dinu, M.; Nucci, D.; Eussen, S.J.P.M.; Amerio, A.; Schram, M.T.; Schaper, N.; Odone, A. Association between Dietary Patterns and Depression: An Umbrella Review of Meta-Analyses of Observational Studies and Intervention Trials. Nutr. Rev. 2023, 81, 346–359. [Google Scholar] [CrossRef]
- Appleton, K.M.; Boxall, L.R.; Adenuga-Ajayi, O.; Seyar, D.F. Does Fruit and Vegetable Consumption Impact Mental Health? Systematic Review and Meta-Analyses of Published Controlled Intervention Studies. Brit. J. Nutr. 2024, 131, 163–173. [Google Scholar] [CrossRef]
- Aslam, H.; Lotfaliany, M.; So, D.; Berding, K.; Berk, M.; Rocks, T.; Hockey, M.; Jacka, F.N.; Marx, W.; Cryan, J.F.; et al. Fiber Intake and Fiber Intervention in Depression and Anxiety: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Controlled Trials. Nutr. Rev. 2024, 82, 1678–1695. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.N. Menopause and Mood: The Role of Estrogen in Midlife Depression and Beyond. Psychiatr. Clin. North Am. 2023, 46, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, X.; Tang, C. Estrogen-Immuno-Neuromodulation Disorders in Menopausal Depression. J. Neuroinflammation 2024, 21, 159. [Google Scholar] [CrossRef]
- Peters, B.A.; Santoro, N.; Kaplan, R.C.; Qi, Q. Spotlight on the Gut Microbiome in Menopause: Current Insights. Int. J. Womens Health 2022, 14, 1059. [Google Scholar] [CrossRef]
- Nieto, M.R.; Rus, M.J.; Areal-Quecuty, V.; Lubián-López, D.M.; Simon-Soro, A. Menopausal Shift on Women’s Health and Microbial Niches. Women’s Health 2025, 3, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, J.; Yang, Y.; Ye, D.; Li, Y.; Li, D.; Wang, J. Estradiol Metabolism by Gut Microbiota in Women’s Depression Pathogenesis: Inspiration from Nature. Front. Psychiatry 2025, 16, 1505991. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodnaruc, A.M.; Duquet, M.; Prud’homme, D.; Giroux, I. Diet and Depression During Peri- and Post-Menopause: A Scoping Review. Nutrients 2025, 17, 2846. https://doi.org/10.3390/nu17172846
Bodnaruc AM, Duquet M, Prud’homme D, Giroux I. Diet and Depression During Peri- and Post-Menopause: A Scoping Review. Nutrients. 2025; 17(17):2846. https://doi.org/10.3390/nu17172846
Chicago/Turabian StyleBodnaruc, Alexandra M., Miryam Duquet, Denis Prud’homme, and Isabelle Giroux. 2025. "Diet and Depression During Peri- and Post-Menopause: A Scoping Review" Nutrients 17, no. 17: 2846. https://doi.org/10.3390/nu17172846
APA StyleBodnaruc, A. M., Duquet, M., Prud’homme, D., & Giroux, I. (2025). Diet and Depression During Peri- and Post-Menopause: A Scoping Review. Nutrients, 17(17), 2846. https://doi.org/10.3390/nu17172846







