Impact of Arabinoxylan Consumption on Glycemic Control: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Risk of Bias Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics
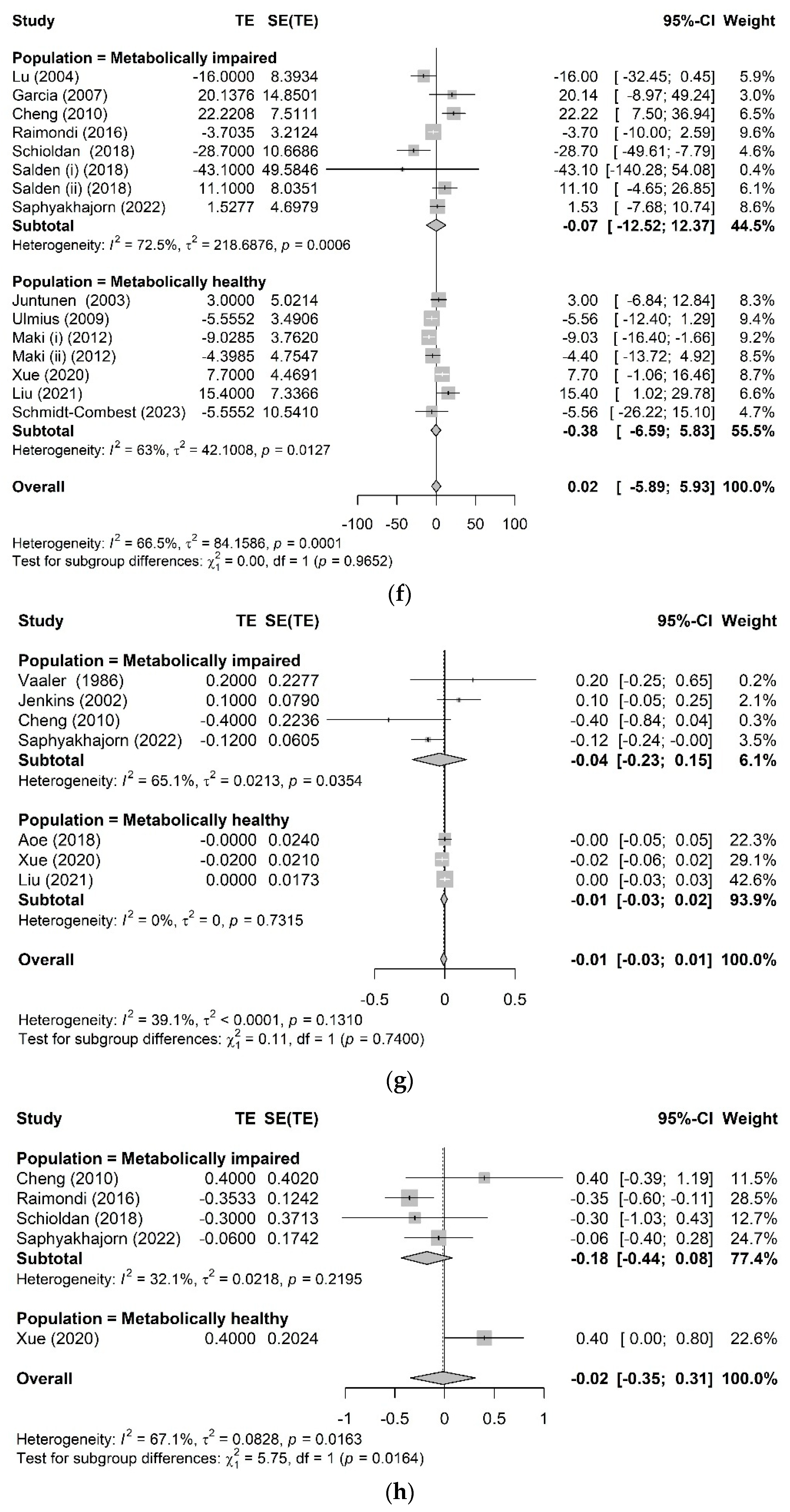
3.3. Effects of AX on Glycemic Control
3.3.1. Postprandial Glycemic Response
3.3.2. Chronic Glycemic Control
3.3.3. Other Metabolic Health-Related Biomarkers
3.4. Publication Bias and Sensitivity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.-P. Metabolic syndrome. Nat. Rev. Dis. Primers 2024, 10, 77. [Google Scholar] [CrossRef]
- Tarekegn, E.T.; Gobezie, M.Y.; Haile, M.B.; Zerga, A.A. Glycemic control and associated factors among type 2 diabetes patients attending at Dessie comprehensive specialized hospital outpatient department. Sci. Rep. 2025, 15, 9286. [Google Scholar] [CrossRef]
- Lipinski, B. Pathophysiology of oxidative stress in diabetes mellitus. J. Diabetes Complicat. 2001, 15, 203–210. [Google Scholar] [CrossRef]
- King, G.L.; Wakasaki, H. Theoretical mechanisms by which hyperglycemia and insulin resistance could cause cardiovascular diseases in diabetes. Diabetes Care 1999, 22, C31. [Google Scholar] [PubMed]
- Gerich, J.E. The importance of tight glycemic control. Am. J. Med. 2005, 118, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Williams, K.; DeFronzo, R.; Stern, M. Risk of Progression to Type 2 Diabetes Based on Relationship Between Postload Plasma Glucose and Fasting Plasma Glucose. Diabetes Care 2006, 29, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- Yosef, T.; Nureye, D.; Tekalign, E. Poor Glycemic Control and Its Contributing Factors Among Type 2 Diabetes Patients at Adama Hospital Medical College in East Ethiopia. Diabetes Metab. Syndr. Obes. 2021, 14, 3273–3280. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation—Current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Qi, X.; Al-Ghazzewi, F.H.; Tester, R.F. Dietary fiber, gastric emptying, and carbohydrate digestion: A mini-review. Starch-Stärke 2018, 70, 1700346. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D.; Ding, Y.; Zhang, J.; Ding, Y.; Lyu, F. Effect and mechanism of insoluble dietary fiber on postprandial blood sugar regulation. Trends Food Sci. Technol. 2024, 146, 104354. [Google Scholar] [CrossRef]
- Schulze, M.B.; Schulz, M.; Heidemann, C.; Schienkiewitz, A.; Hoffmann, K.; Boeing, H. Fiber and magnesium intake and incidence of type 2 diabetes: A prospective study and meta-analysis. Arch. Intern. Med. 2007, 167, 956–965. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Kumanyika, S.K.; Lemaitre, R.N.; Olson, J.L.; Burke, G.L.; Siscovick, D.S. Cereal, fruit, and vegetable fiber intake and the risk of cardiovascular disease in elderly individuals. JAMA 2003, 289, 1659–1666. [Google Scholar] [CrossRef]
- Scazzina, F.; Siebenhandl-Ehn, S.; Pellegrini, N. The effect of dietary fibre on reducing the glycaemic index of bread. Br. J. Nutr. 2013, 109, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Möhlig, M.; Schöfl, C.; Arafat, A.M.; Otto, B.; Viehoff, H.; Koebnick, C.; Kohl, A.; Spranger, J.; Pfeiffer, A.F. Cereal fiber improves whole-body insulin sensitivity in overweight and obese women. Diabetes Care 2006, 29, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.; Mai, V.; Pfeiffer, R.; Kahle, L.; Remaley, A.; Rosen, C.; Lanza, E.; Schatzkin, A. The effects of a high-fruit and-vegetable, high-fiber, low-fat dietary intervention on serum concentrations of insulin, glucose, IGF-I and IGFBP-3. Eur. J. Clin. Nutr. 2008, 62, 186–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-related effects and improving extractability of cereal arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831. [Google Scholar] [CrossRef]
- Moreira, F.D.; Mendes, G.F.; Nascimento, G.D.; Reis, C.E.; Gallassi, A.D.; Welker, A.F. Postprandial hyperglycemia in patients with type 2 diabetes is reduced by raw insoluble fiber: A randomized trial. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2673–2679. [Google Scholar] [CrossRef]
- Hartvigsen, M.; Lærke, H.; Overgaard, A.; Holst, J.J.; Bach Knudsen, K.; Hermansen, K. Postprandial effects of test meals including concentrated arabinoxylan and whole grain rye in subjects with the metabolic syndrome: A randomised study. Eur. J. Clin. Nutr. 2014, 68, 567–574. [Google Scholar] [CrossRef]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; O’Dea, K. Arabinoxylan fibre improves metabolic control in people with Type II diabetes. Eur. J. Clin. Nutr. 2004, 58, 621–628. [Google Scholar] [CrossRef]
- Cheng, H.-H.; Huang, H.-Y.; Chen, Y.-Y.; Huang, C.-L.; Chang, C.-J.; Chen, H.-L.; Lai, M.-H. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann. Nutr. Metab. 2010, 56, 45–51. [Google Scholar] [CrossRef]
- Neyrinck, A.M.; Hiel, S.; Bouzin, C.; Campayo, V.G.; Cani, P.D.; Bindels, L.B.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with bifidogenic properties abolishes metabolic disorders induced by western diet in mice. Nutr. Diabetes 2018, 8, 15. [Google Scholar] [CrossRef]
- Feng, C.; Cai, C.; Deehan, E.C.; Jiang, S.; Yang, M.; Weng, Z.; Long, J.; Li, G.; Li, J.; Liu, J. Evaluating the effects of intrinsic and isolated arabinoxylans on human gut microbiota and short-chain fatty acids: A systematic review and meta-analysis. Trends Food Sci. Technol. 2025, 156, 104837. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0; The Cochrane Collaboration: Oxford, UK, 2011. [Google Scholar]
- Hamberg, O.; Rumessen, J.; Gudmand-Høyer, E. Blood glucose response to pea fiber: Comparisons with sugar beet fiber and wheat bran. Am. J. Clin. Nutr. 1989, 50, 324–328. [Google Scholar] [CrossRef]
- Cherbut, C.; Des Varannes, S.B.; Schnee, M.; Rival, M.; Galmiche, J.; Delort-Laval, J. Involvement of small intestinal motility in blood glucose response to dietary fibre in man. Br. J. Nutr. 1994, 71, 675–685. [Google Scholar] [CrossRef]
- Lia, A.; Andersson, H.; Mekki, N.; Juhel, C.; Senft, M.; Lairon, D. Postprandial lipemia in relation to sterol and fat excretion in ileostomy subjects given oat-bran and wheat test meals. Am. J. Clin. Nutr. 1997, 66, 357–365. [Google Scholar] [CrossRef]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; Mascara, T.; O’Dea, K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am. J. Clin. Nutr. 2000, 71, 1123–1128. [Google Scholar] [CrossRef]
- Juntunen, K.S.; Laaksonen, D.E.; Autio, K.; Niskanen, L.K.; Holst, J.J.; Savolainen, K.E.; Liukkonen, K.-H.; Poutanen, K.S.; Mykkänen, H.M. Structural differences between rye and wheat breads but not total fiber content may explain the lower postprandial insulin response to rye bread. Am. J. Clin. Nutr. 2003, 78, 957–964. [Google Scholar] [CrossRef]
- Möhlig, M.; Koebnick, C.; Weickert, M.; Lueder, W.; Otto, B.; Steiniger, J.; Twilfert, M.; Meuser, F.; Pfeiffer, A.; Zunft, H. Arabinoxylan-enriched meal increases serum ghrelin levels in healthy humans. Horm. Metab. Res. 2005, 37, 303–308. [Google Scholar] [CrossRef]
- Tapola, N.; Karvonen, H.; Niskanen, L.; Mikola, M.; Sarkkinen, E. Glycemic responses of oat bran products in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 255–261. [Google Scholar] [CrossRef]
- Hlebowicz, J.; Jönsson, J.M.; Lindstedt, S.; Björgell, O.; Darwich, G.; Almér, L.-O. Effect of commercial rye whole-meal bread on postprandial blood glucose and gastric emptying in healthy subjects. Nutr. J. 2009, 8, 26. [Google Scholar] [CrossRef]
- Ulmius, M.; Johansson, A.; Önning, G. The influence of dietary fibre source and gender on the postprandial glucose and lipid response in healthy subjects. Eur. J. Nutr. 2009, 48, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Selinheimo, E.; Schwab, U.; Katina, K.; Lehtinen, P.; Mykkänen, H.; Kolehmainen, M.; Poutanen, K. Sourdough fermentation of wholemeal wheat bread increases solubility of arabinoxylan and protein and decreases postprandial glucose and insulin responses. J. Cereal Sci. 2010, 51, 152–158. [Google Scholar] [CrossRef]
- Afaghi, A.; Ranjbari Omidi, B.; Sarreshtehdari, M.; Ghanei, L.; Alipour, M.; Azadmehr, A.; Emam Jomeh, M.; Safari-Varyani, A. Effect of wheat bran on postprandial glucose response in subjects with impaired fasting glucose. Curr. Top. Nutraceutical Res. 2011, 9, 35. [Google Scholar]
- Juvonen, K.R.; Salmenkallio-Marttila, M.; Lyly, M.; Liukkonen, K.-H.; Lähteenmäki, L.; Laaksonen, D.E.; Uusitupa, M.; Herzig, K.-H.; Poutanen, K.; Karhunen, L.J. Semisolid meal enriched in oat bran decreases plasma glucose and insulin levels, but does not change gastrointestinal peptide responses or short-term appetite in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 748–756. [Google Scholar] [CrossRef]
- Brennan, M.A.; Derbyshire, E.J.; Brennan, C.S.; Tiwari, B.K. Impact of dietary fibre-enriched ready-to-eat extruded snacks on the postprandial glycaemic response of non-diabetic patients. Mol. Nutr. Food Res. 2012, 56, 834–837. [Google Scholar] [CrossRef]
- Lappi, J.; Aura, A.-M.; Katina, K.; Nordlund, E.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Comparison of postprandial phenolic acid excretions and glucose responses after ingestion of breads with bioprocessed or native rye bran. Food Funct. 2013, 4, 972–981. [Google Scholar] [CrossRef]
- Hartvigsen, M.; Gregersen, S.; Laerke, H.; Holst, J.J.; Bach Knudsen, K.; Hermansen, K. Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: A randomized study. Eur. J. Clin. Nutr. 2014, 68, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Mykkänen, H.; Knudsen, K.E.B.; Kirjavainen, P.; Katina, K.; Pihlajamäki, J.; Poutanen, K.; Kolehmainen, M. Postprandial glucose metabolism and SCFA after consuming wholegrain rye bread and wheat bread enriched with bioprocessed rye bran in individuals with mild gastrointestinal symptoms. Nutr. J. 2014, 13, 104. [Google Scholar] [CrossRef]
- Lafond, D.W.; Greaves, K.A.; Maki, K.C.; Leidy, H.J.; Romsos, D.R. Effects of Two Dietary Fibers as Part of Ready-to-Eat Cereal (RTEC) Breakfasts on Perceived Appetite and Gut Hormones in Overweight Women. Nutrients 2015, 7, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Giulia Falchi, A.; Grecchi, I.; Muggia, C.; Palladini, G.; Perlini, S. Effects of a bioavailable arabinoxylan-enriched white bread flour on postprandial glucose response in normoglycemic subjects. J. Diet. Suppl. 2016, 13, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Shi, L.; Webb, D.-L.; Hellström, P.M.; Risérus, U.; Landberg, R. Effects of whole-grain rye porridge with added inulin and wheat gluten on appetite, gut fermentation and postprandial glucose metabolism: A randomised, cross-over, breakfast study. Br. J. Nutr. 2016, 116, 2139–2149. [Google Scholar] [CrossRef]
- Shi, L.; Brunius, C.; Lindelöf, M.; Shameh, S.A.; Wu, H.; Lee, I.; Landberg, R.; Moazzami, A.A. Targeted metabolomics reveals differences in the extended postprandial plasma metabolome of healthy subjects after intake of whole-grain rye porridges versus refined wheat bread. Mol. Nutr. Food Res. 2017, 61, 1600924. [Google Scholar] [CrossRef]
- Camps, S.G.; Lim, J.; Ishikado, A.; Inaba, Y.; Suwa, M.; Matsumoto, M.; Henry, C.J. Co-ingestion of rice bran soymilk or plain soymilk with white bread: Effects on the glycemic and insulinemic response. Nutrients 2018, 10, 449. [Google Scholar] [CrossRef]
- Ullah, H.; Esposito, C.; Piccinocchi, R.; De Lellis, L.F.; Santarcangelo, C.; Minno, A.D.; Baldi, A.; Buccato, D.G.; Khan, A.; Piccinocchi, G. Postprandial glycemic and insulinemic response by a Brewer’s spent grain extract-based food supplement in subjects with slightly impaired glucose tolerance: A monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. Nutrients 2022, 14, 3916. [Google Scholar] [CrossRef]
- Åberg, S.; Webb, D.-L.; Nordin, E.; Hellström, P.M.; Landberg, R. Postprandial Effects of Four Test Meals Containing Wholegrain Rye or Refined Wheat Foods on Circulating Incretins, Ghrelin, Glucose, and Inflammatory Markers. J. Nutr. 2025, 155, 185–196. [Google Scholar] [CrossRef]
- Ponzo, V.; Ojeda-Mercado, D.; Finocchiaro, C.; Goitre, I.; Favaro, E.; Lamberti, L.; Bo, S. The effects of a fibre-enriched bakery product on glucose, insulin values and appetite. A pilot randomised cross-over trial. Int. J. Food Sci. Nutr. 2024, 75, 407–415. [Google Scholar] [CrossRef]
- Xu, Y.; Leong, Z.N.; Zhang, W.; Jin, X.; Kong, J.W.; Chan, G.C.T.; Kim, J.E. Impact of Brewers’ Spent Grain-Containing Biscuit on Postprandial Glycaemic Response in Individuals with Metabolic Syndrome: A Crossover Randomised Controlled Trial. Nutrients 2024, 16, 909. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Story, L.; Sieling, B.; Chen, W.L.; Petro, M.S.; Story, J. Hypocholesterolemic effects of oat-bran or bean intake for hypercholesterolemic men. Am. J. Clin. Nutr. 1984, 40, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Vaaler, S.; Hanssen, K.; Dahl-Jørgensen, K.; Frølich, W.; Aaseth, J.; Ødegaard, B.; AagenaE, Ø. Diabetic control is improved by guar gum and wheat bran supplementation. Diabet. Med. 1986, 3, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Martini, M.C.; Axelsen, M.; Faulkner, D.; Vidgen, E.; Parker, T.; Lau, H.; Connelly, P.W. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care 2002, 25, 1522–1528. [Google Scholar] [CrossRef]
- Juntunen, K.S.; Laaksonen, D.E.; Poutanen, K.S.; Niskanen, L.K.; Mykkänen, H.M. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am. J. Clin. Nutr. 2003, 77, 385–391. [Google Scholar] [CrossRef]
- McIntosh, G.H.; Noakes, M.; Royle, P.J.; Foster, P.R. Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men. Am. J. Clin. Nutr. 2003, 77, 967–974. [Google Scholar] [CrossRef]
- Garcia, A.; Otto, B.; Reich, S.; Weickert, M.; Steiniger, J.; Machowetz, A.; Rudovich, N.; Möhlig, M.; Katz, N.; Speth, M. Arabinoxylan consumption decreases postprandial serum glucose, serum insulin and plasma total ghrelin response in subjects with impaired glucose tolerance. Eur. J. Clin. Nutr. 2007, 61, 334–341. [Google Scholar] [CrossRef]
- Maki, K.C.; Gibson, G.R.; Dickmann, R.S.; Kendall, C.W.C.; Chen, C.Y.O.; Costabile, A.; Comelli, E.M.; McKay, D.L.; Almeida, N.G.; Jenkins, D.; et al. Digestive and physiologic effects of a wheat bran extract, arabino-xylan-oligosaccharide, in breakfast cereal. J. Nutr. 2012, 28, 1115–1121. [Google Scholar] [CrossRef]
- Raimondi de Souza, S.; Moraes de Oliveira, G.M.; Raggio Luiz, R.; Rosa, G. Effects of oat bran and nutrition counseling on the lipid and glucose profile and anthropometric parameters of hypercholesterolemia patients. Nutr. Hosp. 2016, 33, 123–130. [Google Scholar] [CrossRef]
- Aoe, S.; Nakamura, F.; Fujiwara, S. Effect of Wheat Bran on Fecal Butyrate-Producing Bacteria and Wheat Bran Combined with Barley on Bacteroides Abundance in Japanese Healthy Adults. Nutrients 2018, 10, 1980. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; Wilms, E.; Truchado, P.; Vilchez-Vargas, R.; Pieper, D.H.; Jáuregui, R.; Marzorati, M.; van de Wiele, T.; Possemiers, S.; et al. Reinforcement of intestinal epithelial barrier by arabinoxylans in overweight and obese subjects: A randomized controlled trial: Arabinoxylans in gut barrier. Clin. Nutr. 2018, 37, 471–480. [Google Scholar] [CrossRef]
- Schioldan, A.G.; Gregersen, S.; Hald, S.; Bjørnshave, A.; Bohl, M.; Hartmann, B.; Holst, J.J.; Stødkilde-Jørgensen, H.; Hermansen, K. Effects of a diet rich in arabinoxylan and resistant starch compared with a diet rich in refined carbohydrates on postprandial metabolism and features of the metabolic syndrome. Eur. J. Nutr. 2018, 57, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Hermes, G.D.A.; Emanuel, E.C.; Holst, J.J.; Zoetendal, E.G.; Smidt, H.; Troost, F.; Schaap, F.G.; Damink, S.O.; Jocken, J.W.E.; et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: A randomized controlled trial in healthy adults with a slow gut transit. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Liu, Y.; Iversen, K.N.; Mazidi, M.; Qu, Z.; Dong, C.; Jin, T.; Hallmans, G.; Åman, P.; Johansson, A.; et al. Impact of a Fermented High-Fiber Rye Diet on Helicobacter pylori and Cardio-Metabolic Risk Factors: A Randomized Controlled Trial Among Helicobacter pylori-Positive Chinese Adults. Front. Nutr. 2020, 7, 608623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, K.; Iversen, K.N.; Qu, Z.; Dong, C.; Jin, T.; Hallmans, G.; Åman, P.; Johansson, A.; He, G.; et al. The effects of fermented rye products on gut microbiota and their association with metabolic factors in Chinese adults—An explorative study. Food Funct. 2021, 12, 9141–9150. [Google Scholar] [CrossRef]
- Schmidt-Combest, S.; Warren, C.; Grams, M.; Wang, W.; Miketinas, D.; Patterson, M. Evaluation of brewers’ spent grain on cardiovascular disease risk factors in adults: Lessons learned from a pilot study. Bioact. Carbohydr. Diet. Fibre. 2023, 30, 100367. [Google Scholar] [CrossRef]
- Saphyakhajorn, W.; Sirirat, R.; Sapwarobol, S. Effect of defatted rice bran supplementation on metabolic parameters and inflammatory status in overweight/obese adults with hypercholesterolemia: A randomized, placebo-controlled intervention. BMC Nutr. 2022, 8, 94. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Shoaibinobarian, N.; Zamani, E.; Salari, A.; Mahdavi-Roshan, M.; Porteghali, P.; Ahmadnia, Z. Supplementing the standard diet with brown rice bran powder might effectively improve the metabolic syndrome characteristics and antioxidant status: An open label randomized controlled trial. Food Funct. 2025, 16, 750–762. [Google Scholar] [CrossRef]
- Ohara, I.; Tabuchi, R.; Onai, K.; Econ, M.H. Effects of modified rice bran on serum lipids and taste preference in streptozotocin-induced diabetic rats. Nutr. Res. 2000, 20, 59–68. [Google Scholar] [CrossRef]
- Lærke, H.N.; Pedersen, C.; Mortensen, M.A.; Theil, P.K.; Larsen, T.; Knudsen, K.E.B. Rye bread reduces plasma cholesterol levels in hypercholesterolaemic pigs when compared to wheat at similar dietary fibre level. J. Sci. Food Agric. 2008, 88, 1385–1393. [Google Scholar] [CrossRef]
- Kim, S.M.; Rico, C.W.; Lee, S.C.; Kang, M.Y. Modulatory Effect of Rice Bran and Phytic Acid on Glucose Metabolism in High Fat-Fed C57BL/6N Mice. J. Clin. Biochem. Nutr. 2010, 47, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Van Hée, V.F.; Piront, N.; De Backer, F.; Toussaint, O.; Cani, P.D.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2012, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, M.L.; Jeppesen, P.B.; Lærke, H.N.; Njabe, E.N.; Knudsen, K.E.B.; Hermansen, K. Concentrated arabinoxylan in wheat bread has beneficial effects as rye breads on glucose and changes in gene expressions in insulin-sensitive tissues of Zucker diabetic fatty (ZDF) rats. J. Agric. Food Chem. 2013, 61, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Alauddin, M.; Shirakawa, H.; Koseki, T.; Kijima, N.; Ardiansyah; Budijanto, S.; Islam, J.; Goto, T.; Komai, M. Fermented rice bran supplementation mitigates metabolic syndrome in stroke-prone spontaneously hypertensive rats. BMC Complement. Altern. Med. 2016, 16, 442. [Google Scholar] [CrossRef]
- Abulnaja, K.O.; El Rabey, H.A. The Efficiency of Barley (Hordeum vulgare) Bran in Ameliorating Blood and Treating Fatty Heart and Liver of Male Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 740716. [Google Scholar] [CrossRef]
- Agyekum, A.K.; Sands, J.S.; Regassa, A.; Kiarie, E.; Weihrauch, D.; Kim, W.K.; Nyachoti, C.M. Effect of supplementing a fibrous diet with a xylanase and β-glucanase blend on growth performance, intestinal glucose uptake, and transport-associated gene expression in growing pigs. J. Anim. Sci. 2015, 93, 3483–3493. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Theil, P.K.; Purup, S.; Nørskov, N.P.; Bach Knudsen, K.E. Effects of Resistant Starch and Arabinoxylan on Parameters Related to Large Intestinal and Metabolic Health in Pigs Fed Fat-Rich Diets. J. Agric. Food Chem. 2015, 63, 10418–10430. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Piccolo, B.D.; Marco, M.L.; Kim, E.B.; Goodson, M.L.; Keenan, M.J.; Dunn, T.N.; Knudsen, K.E.; Adams, S.H.; Martin, R.J. Obese Mice Fed a Diet Supplemented with Enzyme-Treated Wheat Bran Display Marked Shifts in the Liver Metabolome Concurrent with Altered Gut Bacteria. J. Nutr. 2016, 146, 2445–2460. [Google Scholar] [CrossRef]
- Zhang, R.; Jiao, J.; Zhang, W.; Zhang, Z.; Zhang, W.; Qin, L.Q.; Han, S.F. Effects of cereal fiber on leptin resistance and sensitivity in C57BL/6J mice fed a high-fat/cholesterol diet. Food Nutr. Res. 2016, 60, 31690. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, H.; Hu, J.; Gao, H.; Fan, L.; Long, Z.; Nie, S. Arabinoxylan Attenuates Type 2 Diabetes by Improvement of Carbohydrate, Lipid, and Amino Acid Metabolism. Mol. Nutr. Food Res. 2018, 62, e1800222. [Google Scholar] [CrossRef]
- Sarma, S.M.; Singh, D.P.; Singh, P.; Khare, P.; Mangal, P.; Singh, S.; Bijalwan, V.; Kaur, J.; Mantri, S.; Boparai, R.K.; et al. Finger millet arabinoxylan protects mice from high-fat diet induced lipid derangements, inflammation, endotoxemia and gut bacterial dysbiosis. Int. J. Biol. Macromol. 2018, 106, 994–1003. [Google Scholar] [CrossRef]
- Li, L.; Pan, M.; Pan, S.; Li, W.; Zhong, Y.; Hu, J.; Nie, S. Effects of insoluble and soluble fibers isolated from barley on blood glucose, serum lipids, liver function and caecal short-chain fatty acids in type 2 diabetic and normal rats. Food Chem. Toxicol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Yang, S.C.; Huang, W.C.; Ng, X.E.; Lee, M.C.; Hsu, Y.J.; Huang, C.C.; Wu, H.H.; Yeh, C.L.; Shirakawa, H.; Budijanto, S.; et al. Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients 2019, 11, 2033. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, S.; Li, J.; Huang, X.; Cheng, J.; Jiang, X.; Qin, W.; Liu, Y.; Liu, A.; Zhang, Q.; et al. Xyloglucan compounded inulin or arabinoxylan against glycometabolism disorder via different metabolic pathways: Gut microbiota and bile acid receptor effects. J. Funct. Foods 2020, 74, 104162. [Google Scholar] [CrossRef]
- Ai, X.; Wu, C.; Yin, T.; Zhur, O.; Liu, C.; Yan, X.; Yi, C.; Liu, D.; Xiao, L.; Li, W.; et al. Antidiabetic Function of Lactobacillus fermentum MF423-Fermented Rice Bran and Its Effect on Gut Microbiota Structure in Type 2 Diabetic Mice. Front. Microbiol. 2021, 12, 682290. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Gao, H.; Li, M.; Sun, Y.; Chen, H.; Zuo, S.; Fang, Q.; Huang, X.; Yin, J.; et al. Bioactive Dietary Fibers Selectively Promote Gut Microbiota to Exert Antidiabetic Effects. J. Agric. Food Chem. 2021, 69, 7000–7015. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, N.S.; Alshammari, G.M.; El-Ansary, A.; Yagoub, A.E.A.; Amina, M.; Saleh, A.; Yahya, M.A. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients 2022, 14, 1791. [Google Scholar] [CrossRef]
- An, J.; Shi, J.; Liu, K.; Li, A.; He, B.; Wang, Y.; Duan, T.; Wang, Y.; He, J. Effects of Solid-State Fermented Wheat Bran on Growth Performance, Immune Function, Intestinal Morphology and Microflora in Lipopolysaccharide-Challenged Broiler Chickens. Animals 2022, 12, 1100. [Google Scholar] [CrossRef]
- Mio, K.; Ogawa, R.; Tadenuma, N.; Aoe, S. Arabinoxylan as well as β-glucan in barley promotes GLP-1 secretion by increasing short-chain fatty acids production. Biochem. Biophys. Rep. 2022, 32, 101343. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hamaad, F.A.; Abd Elmageed, G.M.; Ghoneum, M.H. Efficiency of Biobran/MGN-3, an Arabinoxylan Rice Bran, in Attenuating Diabetes-Induced Cognitive Impairment of the Hippocampus via Oxidative Stress and IR/Akt/NF-κB in Rats. Evid.-Based Complement. Alternat. Med. 2023, 2023, 8248576. [Google Scholar] [CrossRef]
- Fang, W.; Peng, W.; Qi, W.; Zhang, J.; Song, G.; Pang, S.; Wang, Y. Ferulic acid combined with different dietary fibers improve glucose metabolism and intestinal barrier function by regulating gut microbiota in high-fat diet-fed mice. J. Funct. Foods 2024, 112, 105919. [Google Scholar] [CrossRef]
- Agista, A.Z.; Kato, A.; Goto, T.; Koseki, T.; Oikawa, A.; Ohsaki, Y.; Yamaki, M.; Yeh, C.-L.; Yang, S.-C.; Ardiansyah; et al. Fermented Rice Bran Mitigated the Syndromes of Type 2 Diabetes in KK-Ay Mice Model. Metabolites 2024, 14, 614. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Woerle, H.J.; Neumann, C.; Zschau, S.; Tenner, S.; Irsigler, A.; Schirra, J.; Gerich, J.E.; Göke, B. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res. Clin. Pract. 2007, 77, 280–285. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Poon, T.; Harkness, L.S.; O’Shea, M.; Chu, Y. The effects of whole-grain compared with refined wheat, rice, and rye on the postprandial blood glucose response: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2018, 108, 759–774. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Xie, F.; Wang, G.; Song, Z.; Ai, L. Inhibitory mechanism of arabinoxylan and β-glucan from hull-less barley on α-amylase activity: A comparison study. Food Hydrocoll. 2024, 153, 109994. [Google Scholar] [CrossRef]
- Chenine, A.S.; Boual, Z.; Elhadj, M.D.O.; Addoun, N.; Mahfoudi, R.; Khemili, A.; Belkhalfa, H.; Bachari, K.; Fendri, I.; Modafar, C.E. Inhibitory effect of arabinoxylan oligosaccharides from Plantago ciliata Desf. seeds on α-amylase and α-d-glucosidase and the inhibition kinetics. Euro-Mediterr. J. Environ. Integr. 2023, 8, 795–805. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Lærke, H.N.; Knudsen, K.E.B. Effects of Isolated and Complex Dietary Fiber Matrices in Breads on Carbohydrate Digestibility and Physicochemical Properties of Ileal Effluent from Pigs. J. Agric. Food Chem. 2012, 60, 12469–12476. [Google Scholar] [CrossRef]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Bach Knudsen, K.E.; Hermansen, K.; Marco, M.L.; Gregersen, S.; et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef]
- Damen, B.; Cloetens, L.; Broekaert, W.F.; François, I.; Lescroart, O.; Trogh, I.; Arnaut, F.; Welling, G.W.; Wijffels, J.; Delcour, J.A.; et al. Consumption of breads containing in situ-produced arabinoxylan oligosaccharides alters gastrointestinal effects in healthy volunteers. J. Nutr. 2012, 142, 470–477. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Sharabi, K.; Tavares, C.D.; Rines, A.K.; Puigserver, P. Molecular pathophysiology of hepatic glucose production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef]
- O’Neill, H.M. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Bjursell, M.; Ahnmark, A.; Bohlooly-Y, M.; William-Olsson, L.; Rhedin, M.; Peng, X.-R.; Ploj, K.; Gerdin, A.-K.; Arnerup, G.; Elmgren, A.; et al. Opposing Effects of Adiponectin Receptors 1 and 2 on Energy Metabolism. Diabetes 2007, 56, 583–593. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Shimizu, H.; Hagio, M.; Fukiya, S.; Watanabe, M.; Tanaka, Y.; Joe, G.-H.; Iwaya, H.; Yoshitsugu, R.; Kikuchi, K.; et al. 12α-Hydroxylated bile acid induces hepatic steatosis with dysbiosis in rats. Biochim. Biophys. Acta 2020, 1865, 158811. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H. Molecular mechanisms involved in hepatic steatosis and insulin resistance. J. Diabetes Investig. 2011, 2, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Hu, J.; Chen, H.; Geng, F.; Nie, S. Arabinoxylan ameliorates type 2 diabetes by regulating the gut microbiota and metabolites. Food Chem. 2022, 371, 131106. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Park, Y.J.; Kim, Y.R.; Kim, Y.N.; Ka, S.; Lee, H.Y.; Kim, J.B. Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. FASEB J. 2015, 29, 2397–2411. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, P.; Gan, Y.; Zheng, X.; Yang, P.; Shi, A.; Liu, X.; Zhang, J.; Yu, P.; Zhang, D. Food-derived bioactive peptides as emerging therapeutic agents: Unlocking novel strategies for colorectal cancer treatment. Pharmacol. Res. 2025, 217, 107819. [Google Scholar] [CrossRef]
- Lin, J.; Liu, H.; Sun, Y.; Zou, J.; Nie, Q.; Nie, S. Arabinoxylan alleviates obesity by regulating gut microbiota and bile acid metabolism. J. Agric. Food Chem. 2024, 72, 23295–23305. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Che, Y.; He, L.; Luo, S.; Yang, C.S.; Chen, T. Interactive Effects of Arabinoxylan Oligosaccharides and Green Tea Polyphenols on Obesity Management and Gut Microbiota Modulation in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2024, 72, 16237–16249. [Google Scholar] [CrossRef]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of dietary fiber on glycemic control and insulin sensitivity in patients with type 2 diabetes: A systematic review and meta-analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- Schadow, A.M.; Revheim, I.; Spielau, U.; Dierkes, J.; Schwingshackl, L.; Frank, J.; Hodgson, J.M.; Moreira-Rosário, A.; Seal, C.J.; Buyken, A.E.; et al. The Effect of Regular Consumption of Reformulated Breads on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2023, 14, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as Functional Food Ingredients: A Review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436. [Google Scholar] [CrossRef]
- Chen, H.; Fu, Y.; Jiang, X.; Li, D.; Qin, W.; Zhang, Q.; Lin, D.; Liu, Y.; Tan, C.; Huang, Z. Arabinoxylan activates lipid catabolism and alleviates liver damage in rats induced by high-fat diet. J. Sci. Food Agric. 2018, 98, 253–260. [Google Scholar] [CrossRef]
- Tong, L.-T.; Zhong, K.; Liu, L.; Qiu, J.; Guo, L.; Zhou, X.; Cao, L.; Zhou, S. Effects of dietary wheat bran arabinoxylans on cholesterol metabolism of hypercholesterolemic hamsters. Carbohydr. Polym. 2014, 112, 1–5. [Google Scholar] [CrossRef]
- Lair, B.; Laurens, C.; Van Den Bosch, B.; Moro, C. Novel Insights and Mechanisms of Lipotoxicity-Driven Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6358. [Google Scholar] [CrossRef]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef]
- Juhász, A.E.; Greff, D.; Teutsch, B.; Gede, N.; Hegyi, P.; Horváth, E.M.; Deák, P.Á.; Nyirády, P.; Ács, N.; Juhász, R. Galactomannans are the most effective soluble dietary fibers in type 2 diabetes: A systematic review and network meta-analysis. Am. J. Clin. Nutr. 2023, 117, 266–277. [Google Scholar] [CrossRef]
- Gunness, P.; Williams, B.A.; Gerrits, W.J.J.; Bird, A.R.; Kravchuk, O.; Gidley, M.J. Circulating triglycerides and bile acids are reduced by a soluble wheat arabinoxylan via modulation of bile concentration and lipid digestion rates in a pig model. Mol. Nutr. Food Res. 2016, 60, 642–651. [Google Scholar] [CrossRef]




| Parameter | Description |
|---|---|
| Population | Animals; Human subjects (mean age ≥ 19 years). |
| Intervention | Consuming arabinoxylan or arabinoxylan-containing cereal food; Consuming higher dosages of arabinoxylan or arabinoxylan-containing cereal food. |
| Comparison | Not consuming arabinoxylan or arabinoxylan-containing cereal food, or consuming placebo; Consuming lower dosages of arabinoxylan or arabinoxylan-containing cereal food. |
| Outcomes | Primary outcome: postprandial glycemic control-related biomarkers (postprandial glucose AUC, iAUC, Peak, iPeak, and postprandial insulin AUC, iAUC, Peak, iPeak); chronic glycemic control-related biomarkers (fasting glucose, fasting insulin, HbA1c, and HOMA-IR). |
| Study Design | Animal studies; Randomized controlled trials. |
| Outcomes | SMD | 95% CI | I2 (%) | τ2 | Model | Comparisons Included (n) |
|---|---|---|---|---|---|---|
| Glucose AUC | −0.34 | [−0.58; −0.10] | 51% | 0.14 | Random | 19 |
| Glucose iAUC | −0.41 | [−0.57; −0.25] | 0% | 0 | Fixed | 18 |
| Insulin AUC | −0.42 | [−0.67; −0.17] | 0% | 0 | Fixed | 9 |
| Insulin iAUC | −0.28 | [−0.44; −0.12] | 0% | 0 | Fixed | 18 |
| Glucose Peak | −0.47 | [−0.65; −0.29] | 47% | 0.11 | Fixed | 30 |
| Glucose iPeak | −0.52 | [−0.80; −0.25] | 63% | 0.29 | Random | 23 |
| Insulin Peak | −0.29 | [−0.44; −0.15] | 0% | 0 | Fixed | 23 |
| Insulin iPeak | −0.24 | [−0.41; −0.06] | 0% | 0 | Fixed | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liang, Y.; Kim, J.E. Impact of Arabinoxylan Consumption on Glycemic Control: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Nutrients 2025, 17, 2840. https://doi.org/10.3390/nu17172840
Xu Y, Liang Y, Kim JE. Impact of Arabinoxylan Consumption on Glycemic Control: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Nutrients. 2025; 17(17):2840. https://doi.org/10.3390/nu17172840
Chicago/Turabian StyleXu, Yujing, Yuxin Liang, and Jung Eun Kim. 2025. "Impact of Arabinoxylan Consumption on Glycemic Control: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies" Nutrients 17, no. 17: 2840. https://doi.org/10.3390/nu17172840
APA StyleXu, Y., Liang, Y., & Kim, J. E. (2025). Impact of Arabinoxylan Consumption on Glycemic Control: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Nutrients, 17(17), 2840. https://doi.org/10.3390/nu17172840





