Maternal Dietary Pattern in Pregnancy and Behavioral Outcomes at 4 Years of Age in the Piccolipiù Cohort: Potential Sex-Related Differences
Abstract
1. Introduction
2. Materials and Methods
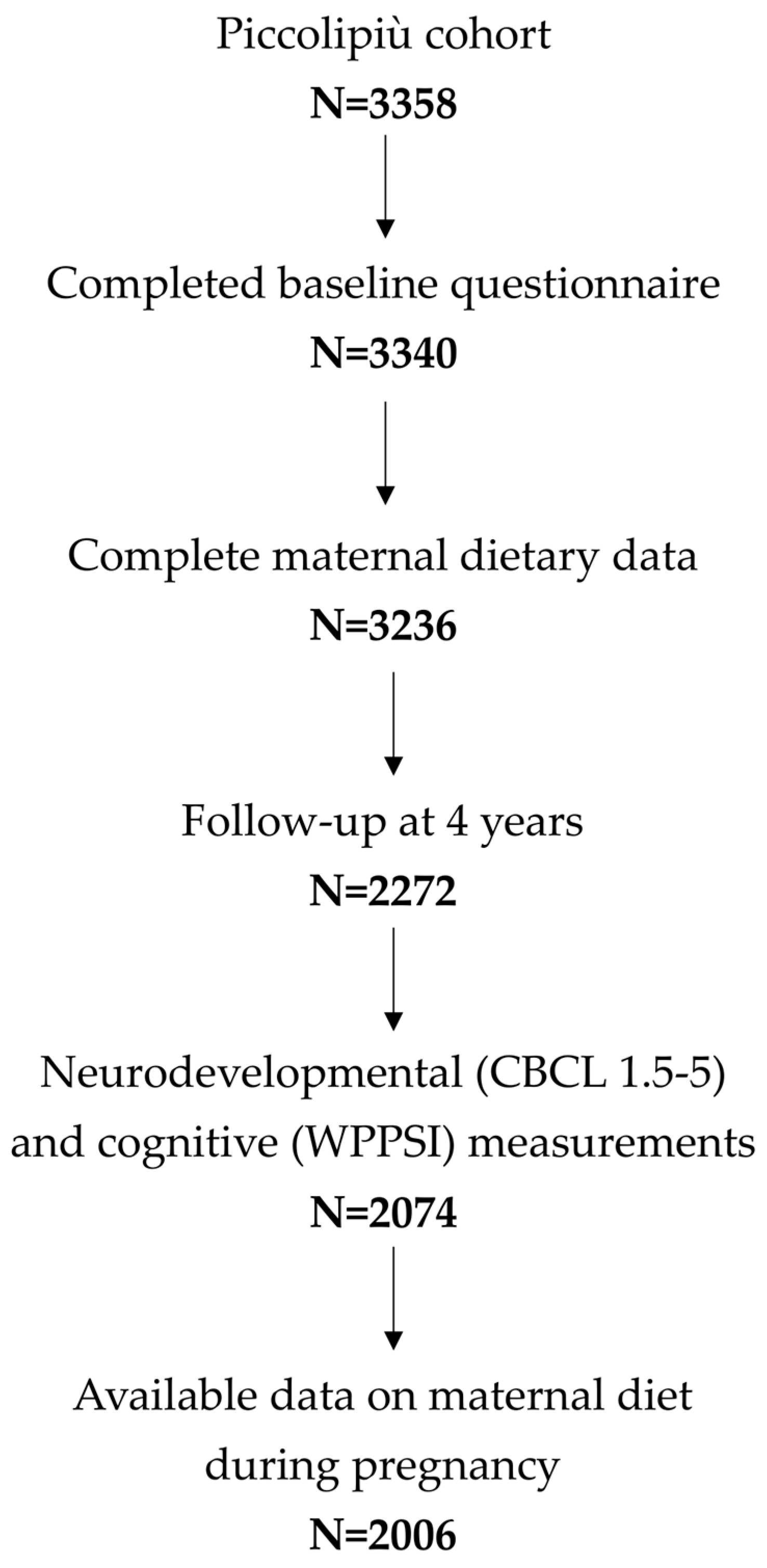
2.1. Study Population
2.2. Dietary Assessment
2.3. Neurodevelopmental and Cognitive Measurements
2.3.1. Behavioral Assessment
2.3.2. Cognitive Assessment
2.4. Covariates
2.5. Statistical Analyses
2.5.1. Descriptive Statistics
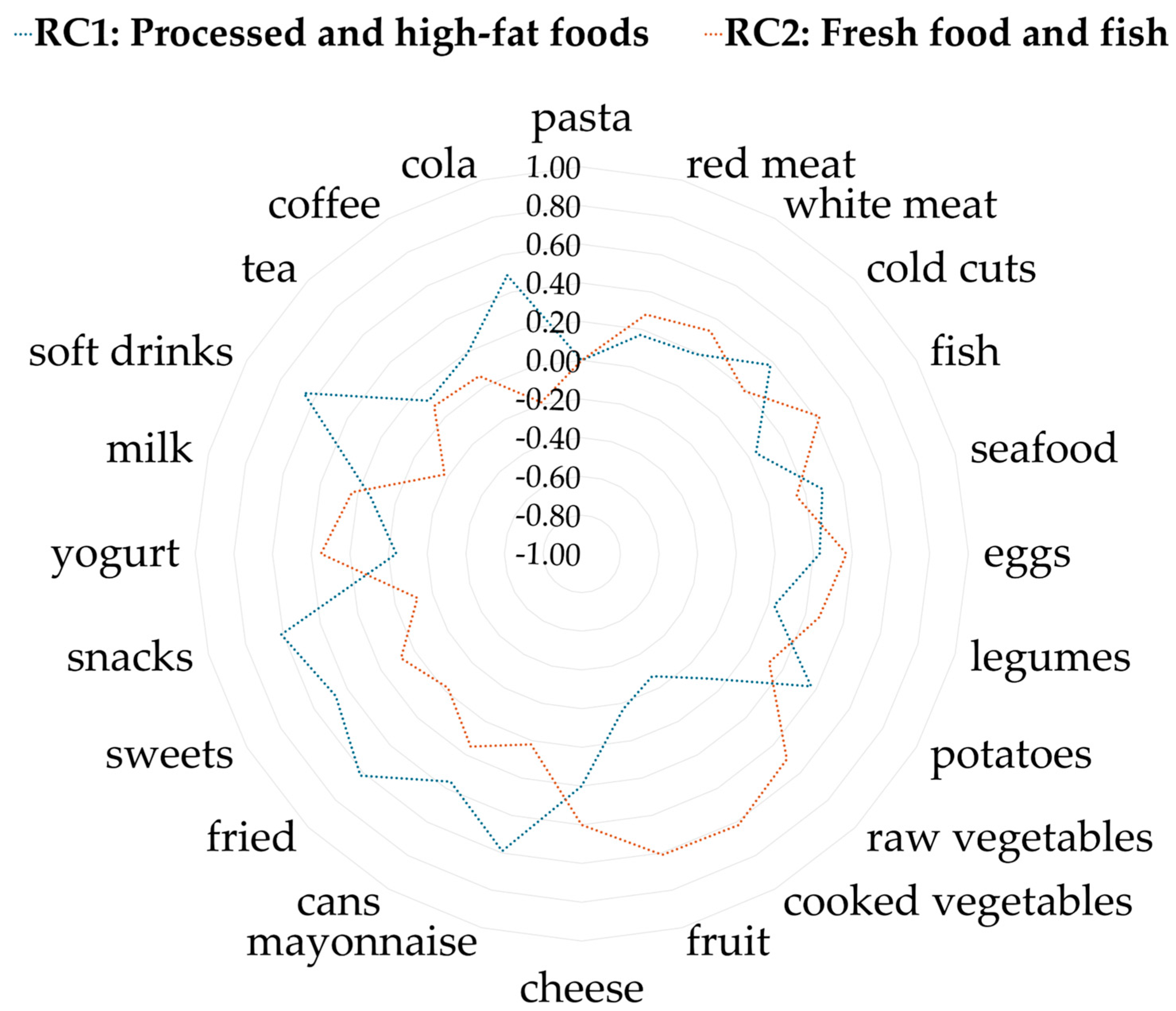
2.5.2. Principal Component Analysis (PCA) for Dietary Pattern Identification
2.5.3. Regression Analyses
3. Results
3.1. General Characteristics
3.2. Associations Between Maternal Dietary Patterns and Behavioral and Cognitive Disorders in Children at 4 Years of Age
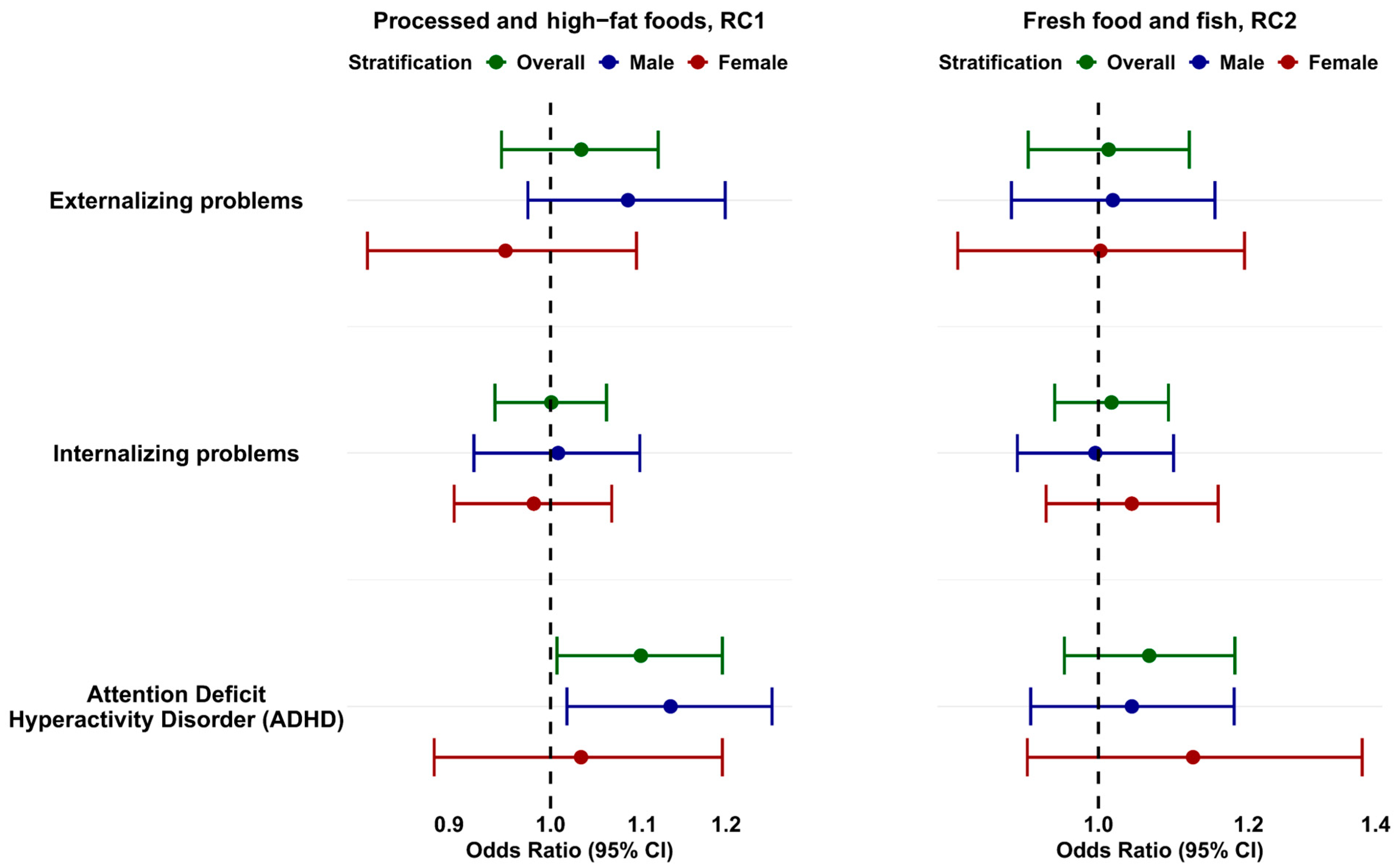
3.3. Associations Between Maternal Dietary Patterns and Clinical Risk of Behavioral Problems in Children at 4 Years of Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.P. The Developmental Origins of Adult Disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. The Developing Human: Clinically Oriented Embryology, 9th ed.; Saunders: Philadelphia, PA, USA, 2013. [Google Scholar]
- Gluckman, P.D.; Cutfield, W.; Hofman, P.; Hanson, M.A. The Fetal, Neonatal, and Infant Environments-the Long-Term Consequences for Disease Risk. In Proceedings of the Early Human Development; Elsevier Ireland Ltd.: Shannon, Ireland, 2005; Volume 81, pp. 51–59. [Google Scholar]
- Reik, W.; Dean, W.; Walter, J. Epigenetic Reprogramming in Mammalian Development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Michels, K.B. Epigenetic Epidemiology of the Developmental Origins Hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Borge, T.C.; Aase, H.; Brantsæter, A.L.; Biele, G. The Importance of Maternal Diet Quality during Pregnancy on Cognitive and Behavioural Outcomes in Children: A Systematic Review and Meta-Analysis. BMJ Open 2017, 7, e016777. [Google Scholar] [CrossRef]
- Mahmassani, H.A.; Switkowski, K.M.; Scott, T.M.; Johnson, E.J.; Rifas-Shiman, S.L.; Oken, E.; Jacques, P.F. Maternal Diet Quality during Pregnancy and Child Cognition and Behavior in a US Cohort. Am. J. Clin. Nutr. 2022, 115, 128–141. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of Maternal Obesity on the Long-Term Health of Offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary Patterns and Biomarkers of Oxidative Stress and Inflammation: A Systematic Review of Observational and Intervention Studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The Impact of Maternal High-Fat Diet on Offspring Neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Tancredi, D.J.; Ozonoff, S.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Schmidt, L.C.; Tassone, F.; Hertz-Picciotto, I. Maternal Periconceptional Folic Acid Intake and Risk of Autism Spectrum Disorders and Developmental Delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) Case-Control Study. Am. J. Clin. Nutr. 2012, 96, 80–89. [Google Scholar] [CrossRef]
- Julvez, J.; Méndez, M.; Fernandez-Barres, S.; Romaguera, D.; Vioque, J.; Llop, S.; Ibarluzea, J.; Guxens, M.; Avella-Garcia, C.; Tardón, A.; et al. Maternal Consumption of Seafood in Pregnancy and Child Neuropsychological Development: A Longitudinal Study Based on a Population with High Consumption Levels. Am. J. Epidemiol. 2016, 183, 169–182. [Google Scholar] [CrossRef]
- Ojeda-Granados, C.; Barchitta, M.; La Rosa, M.C.; La Mastra, C.; Roman, S.; Panduro, A.; Agodi, A.; Maugeri, A. Evaluating Dietary Patterns in Women from Southern Italy and Western Mexico. Nutrients 2022, 14, 1603. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.R., Jr.; Steffen, L.M. Nutrients, Foods, and Dietary Patterns as Exposures in Research: A Framework for Food Synergy. Am. J. Clin. Nutr. 2003, 78, 508–513. [Google Scholar] [CrossRef]
- Horner, D.; Jepsen, J.R.M.; Chawes, B.; Aagaard, K.; Rosenberg, J.B.; Mohammadzadeh, P.; Sevelsted, A.; Vahman, N.; Vinding, R.; Fagerlund, B.; et al. A Western Dietary Pattern during Pregnancy Is Associated with Neurodevelopmental Disorders in Childhood and Adolescence. Nat. Metab. 2025, 7, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Reinke, S.N.; Mousavi-Derazmahalleh, M.; Garssen, J.; Jenmalm, M.C.; Srinivasjois, R.; Silva, D.; Keelan, J.; Prescott, S.L.; Palmer, D.J.; et al. Maternal Prebiotic Supplementation during Pregnancy and Lactation Modifies the Microbiome and Short Chain Fatty Acid Profile of Both Mother and Infant. Clin. Nutr. 2024, 43, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, N.M.; De Meij, T.G.J.; Niemarkt, H.J. Microbiome and Its Impact on Fetal and Neonatal Brain Development: Current Opinion in Pediatrics. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 297–303. [Google Scholar] [CrossRef]
- Szczuko, M.; Szabunia, N.; Radkiewicz, J.; Jamioł-Milc, D.; Machałowski, T.; Ziętek, M. Relationship of SCFAs to Maternal and Child Anthropometric Measurements. Int. J. Mol. Sci. 2025, 26, 6424. [Google Scholar] [CrossRef]
- Cendra-Duarte, E.; Canals, J.; Becerra-Tomás, N.; Mateu-Fabregat, J.; Bulló, M.; Arija, V. Dietary Glycemic Index and Load during Pregnancy and Offspring Behavioral Outcomes: Exploring Sex Differences. Eur. J. Pediatr. 2025, 184, 178. [Google Scholar] [CrossRef]
- Edlow, A.G.; Guedj, F.; Sverdlov, D.Y.; Pennings, J.L.; Neri, C.; Daruvala, S.T.; Bianchi, D.W. 8: Males Are from Mars, Females Are from Venus: Sex-Specific Fetal Brain Gene Expression Signatures in a Mouse Model of Maternal Diet-Induced Obesity. Am. J. Obstet. Gynecol. 2016, 214, S6. [Google Scholar] [CrossRef]
- Ceasrine, A.M.; Devlin, B.A.; Bolton, J.L.; Green, L.A.; Jo, Y.C.; Huynh, C.; Patrick, B.; Washington, K.; Sanchez, C.L.; Joo, F.; et al. Maternal Diet Disrupts the Placenta–Brain Axis in a Sex-Specific Manner. Nat. Metab. 2022, 4, 1732–1745. [Google Scholar] [CrossRef]
- Musillo, C.; Ajmone-Cat, M.A.; De Simone, R.; Tassinari, R.; Maranghi, F.; Tait, S.; Samà, M.; Giona, L.; Pieroni, E.M.; Alessi, R.; et al. Maternal Stressors Disrupt Mouse Placental Proteome and Fetal Brain Development in a Sex-Specific Fashion through Inflammation and Oxidative Stress. Mol. Psychiatry 2025. [Google Scholar] [CrossRef]
- Farchi, S.; Forastiere, F.; Vecchi Brumatti, L.; Alviti, S.; Arnofi, A.; Bernardini, T.; Bin, M.; Brescianini, S.; Colelli, V.; Cotichini, R.; et al. Piccolipiù, a Multicenter Birth Cohort in Italy: Protocol of the Study. BMC Pediatr. 2014, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Toccaceli, V.; Serino, L.; Stazi, M.A. Informed Consent, and an Ethico- Legal Framework for Paediatric Observational Research and Biobanking: The Experience of an Italian Birth Cohort Study. Cell Tissue Bank. 2014, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA School-Age Forms & Profiles; ASEBA, University of Vermont: Burlington, VT, USA, 2001. [Google Scholar]
- Wechsler, D. The Wechsler Preschool and Primary Scale of Intelligence–Third Edition (WPPSI-III); The Psychological Corporation: San Antonio, TX, USA, 2002. [Google Scholar]
- Pizzi, C.; Richiardi, M.; Charles, M.A.; Heude, B.; Lanoe, J.L.; Lioret, S.; Brescianini, S.; Toccaceli, V.; Vrijheid, M.; Merletti, F.; et al. Measuring Child Socio-Economic Position in Birth Cohort Research: The Development of a Novel Standardized Household Income Indicator. Int. J. Environ. Res. Public. Health 2020, 17, 1700. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 11 July 2025).
- Revelle, W. Package “psych” Title Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, IL, USA, 2025. [Google Scholar]
- Edlow, A.G. Maternal Obesity and Neurodevelopmental and Psychiatric Disorders in Offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef]
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The Role of Nutrition in Children’s Neurocognitive Development, from Pregnancy through Childhood. In Prenatal and Childhood Nutrition: Evaluating the Neurocognitive Connections; Apple Academic Press: Palm Bay, FL, USA, 2015; pp. 35–77. ISBN 9781498714365. [Google Scholar]
- Oken, E.; Radesky, J.S.; Wright, R.O.; Bellinger, D.C.; Amarasiriwardena, C.J.; Kleinman, K.P.; Hu, H.; Gillman, M.W. Maternal Fish Intake during Pregnancy, Blood Mercury Levels, and Child Cognition at Age 3 Years in a US Cohort. Am. J. Epidemiol. 2008, 167, 1171–1181. [Google Scholar] [CrossRef]
- Getahun, D.; Jacobsen, S.J.; Fassett, M.J.; Chen, W.; Demissie, K.; Rhoads, G.G. Recent Trends in Childhood Attention-Deficit/Hyperactivity Disorder. JAMA Pediatr. 2013, 167, 282–288. [Google Scholar] [CrossRef]
- Rivera, H.M.; Christiansen, K.J.; Sullivan, E.L. The Role of Maternal Obesity in the Risk of Neuropsychiatric Disorders. Front. Neurosci. 2015, 9, 194. [Google Scholar] [CrossRef]
- Eleftheriades, A.; Koulouraki, S.; Belegrinos, A.; Eleftheriades, M.; Pervanidou, P. Maternal Obesity and Neurodevelopment of the Offspring. Nutrients 2025, 17, 891. [Google Scholar] [CrossRef]
- Ji, Y.; Riley, A.W.; Lee, L.C.; Volk, H.; Hong, X.; Wang, G.; Angomas, R.; Stivers, T.; Wahl, A.; Ji, H.; et al. A Prospective Birth Cohort Study on Maternal Cholesterol Levels and Offspring Attention Deficit Hyperactivity Disorder: New Insight on Sex Differences. Brain Sci. 2018, 8, 3. [Google Scholar] [CrossRef]
- Buss, C.; Davis, E.P.; Shahbaba, B.; Pruessner, J.C.; Head, K.; Sandman, C.A. Maternal Cortisol over the Course of Pregnancy and Subsequent Child Amygdala and Hippocampus Volumes and Affective Problems. Proc. Natl. Acad. Sci. USA 2012, 109, E1312–E1319. [Google Scholar] [CrossRef]
- Sandman, C.A.; Glynn, L.M.; Davis, E.P. Is There a Viability-Vulnerability Tradeoff? Sex Differences in Fetal Programming. J. Psychosom. Res. 2013, 75, 327–335. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shih, P.Y.; Su, C.T.; Cheng, C.F.; Lee, M.C.; Lane, H.Y. Association between Infant Feeding and ADHD Development in Childhood: A Birth Cohort Study in Taiwan. J. Child Psychol. Psychiatry 2025, 66, 881–891. [Google Scholar] [CrossRef]
- Papanastasiou, G.; Drigas, A.; Papanastasiou, P. The Association of Diet Quality and Lifestyle Factors in Children and Adults with ADHD: A Systematic Review and Meta-Analysis. Sci. Electron. Arch. 2021, 14, 39–58. [Google Scholar] [CrossRef]
| (A) Parental Characteristics | ||||
| Categorical Variables | Category | n (%) | ||
| Maternal education at childbirth | Low | 824 (41.08) | ||
| Medium | 194 (9.67) | |||
| High | 988 (49.25) | |||
| NA | - | |||
| Paternal education at childbirth | Low | 682 (34) | ||
| Medium | 382 (19.04) | |||
| High | 925 (46.11) | |||
| NA | 17 (0.85) | |||
| Equivalized Household Income Indicator (EHII) | Low/Medium | 1131 (56.38) | ||
| High | 770 (38.38) | |||
| NA | 105 (5.23) | |||
| Center | Florence | 384 (19.14) | ||
| Viareggio | 290 (14.46) | |||
| Rome | 580 (28.91) | |||
| Turin | 383 (19.09) | |||
| Trieste | 369 (18.39) | |||
| NA | - | |||
| Maternal employment (birth—48 months) | always worked | 1105 (55.08) | ||
| not continuous | 901 (44.92) | |||
| NA | - | |||
| Paternal employment (birth—48 months) | always worked | 1395 (69.54) | ||
| not continuous | 611 (30.46) | |||
| NA | - | |||
| Parity | Nulliparous | 1182 (58.92) | ||
| Uniparous or multiparous | 819 (40.83) | |||
| NA | - | |||
| Cohabiting status in the first 48 months after delivery | at least some time alone | 169 (8.42) | ||
| other cases | 1430 (71.29) | |||
| NA | - | |||
| Smoking before pregnancy | No | 1043 (51.99) | ||
| Yes | 957 (47.71) | |||
| NA | 6 (0.30) | |||
| Smoking in pregnancy | No | 1591 (79.31) | ||
| Yes | 414 (20.64) | |||
| NA | 1 (0.05) | |||
| Passive smoking in pregnancy | No | 1345 (67.05) | ||
| Yes | 510 (25.42) | |||
| NA | 151 (7.53) | |||
| Alcohol before pregnancy | No | 765 (38.14) | ||
| Yes | 1210 (60.32) | |||
| NA | 31 (1.55) | |||
| Alcohol in pregnancy | No | 1036 (51.65) | ||
| Yes | 963 (48.01) | |||
| NA | 7 (0.35) | |||
| Pregnancy complication | No | 1663 (82.90) | ||
| Yes | 328 (16.35) | |||
| NA | 15 (0.75) | |||
| Maternal stress in the first 24 months after delivery | Low | 1250 (62.31) | ||
| Medium-High | 620 (30.91) | |||
| NA | 136 (6.78) | |||
| Continuous variables | Mean ± SD | |||
| Age at delivery (years) | 33.9 ± 4.78 | |||
| Pre-pregnancy BMI (kg/m2) | 22.5 ± 3.81 | |||
| (B) Children’s Characteristics | ||||
|---|---|---|---|---|
| Categorical Variables | Category | n (%) | ||
| Exposure to smoke in the first 48 months | No | 837 (41.72) | ||
| Yes | 904 (45.06) | |||
| NA | 265 (13.21) | |||
| Gender | Female | 991 (49.40) | ||
| Male | 1015 (50.60) | |||
| NA | - | |||
| Conception season | Autumn | 399 (19.89) | ||
| Spring | 636 (31.70) | |||
| Summer | 517 (25.77) | |||
| Winter | 451 (22.48) | |||
| NA | 3 (0.15) | |||
| Breastfeeding in the first 6 months | No | 110 (5.48) | ||
| Yes | 1873 (93.37) | |||
| NA | 23 (1.15) | |||
| Daycare in the first 24 months | No | 689 (34.35) | ||
| Yes | 1135 (56.58) | |||
| NA | 182 (9.07) | |||
| Continuous Variables | Mean ± SD | |||
| Gestational age (weeks) | 40 ± 1.32 | |||
| Birth weight (grams) | 3337.6 ± 442.29 | |||
| (a) | ||||||||
| Food Item | Never | Less than Once/Week | 1–2 Times/Week | 3–5 Times/Week | 6–7 Times/Week | More than Once/Day | ||
| white meat | 47 (2.3) | 176 (8.8) | 1012 (50.4) | 669 (33.3) | 95 (4.7) | 7 (0.3) | ||
| soft drinks | 493 (24.6) | 791 (39.4) | 455 (22.7) | 170 (8.5) | 54 (2.7) | 43 (2.1) | ||
| cooked vegetables | 25 (1.2) | 121 (6) | 530 (26.4) | 794 (39.6) | 355 (17.7) | 181 (9) | ||
| raw vegetables | 221 (11) | 242 (12.1) | 453 (22.6) | 587 (29.3) | 306 (15.3) | 197 (9.8) | ||
| sweets | 41 (2) | 309 (15.4) | 471 (23.5) | 597 (29.8) | 443 (22.1) | 145 (7.2) | ||
| cheese | 73 (3.6) | 248 (12.4) | 768 (38.3) | 715 (35.6) | 174 (8.7) | 28 (1.4) | ||
| fried food | 326 (16.3) | 1338 (66.7) | 293 (14.6) | 41 (2) | 6 (0.3) | 2 (0.1) | ||
| fruit | 15 (0.7) | 55 (2.7) | 182 (9.1) | 488 (24.3) | 574 (28.6) | 692 (34.5) | ||
| seafood | 1048 (52.2) | 830 (41.4) | 114 (5.7) | 13 (0.6) | 0 (0) | 1 (0) | ||
| milk | 201 (10) | 143 (7.1) | 141 (7) | 274 (13.7) | 960 (47.9) | 287 (14.3) | ||
| legumes | 62 (3.1) | 637 (31.8) | 998 (49.8) | 256 (12.8) | 41 (2) | 12 (0.6) | ||
| mayonnaise | 949 (47.3) | 814 (40.6) | 185 (9.2) | 52 (2.6) | 5 (0.2) | 1 (0) | ||
| pasta | 1 (0) | 24 (1.2) | 166 (8.3) | 535 (26.7) | 633 (31.6) | 647 (32.3) | ||
| potatoes | 28 (1.4) | 623 (31.1) | 1062 (52.9) | 268 (13.4) | 22 (1.1) | 3 (0.1) | ||
| fish | 77 (3.8) | 510 (25.4) | 1147 (57.2) | 235 (11.7) | 27 (1.3) | 10 (0.5) | ||
| red meat | 73 (3.6) | 307 (15.3) | 996 (49.7) | 538 (26.8) | 79 (3.9) | 13 (0.6) | ||
| cold cuts | 487 (24.3) | 601 (30) | 624 (31.1) | 244 (12.2) | 44 (2.2) | 6 (0.3) | ||
| cans | 730 (36.4) | 809 (40.3) | 370 (18.4) | 87 (4.3) | 9 (0.4) | 1 (0) | ||
| snacks | 428 (21.3) | 924 (46.1) | 437 (21.8) | 165 (8.2) | 37 (1.8) | 15 (0.7) | ||
| eggs | 98 (4.9) | 845 (42.1) | 973 (48.5) | 81 (4) | 7 (0.3) | 2 (0.1) | ||
| yogurt | 196 (9.8) | 342 (17) | 529 (26.4) | 502 (25) | 357 (17.8) | 80 (4) | ||
| (b) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Beverage | Mean ± SD | Min-Max | ||||||
| tea | 1.14 ± 1.95 | 0–20 | ||||||
| coffee | 3.79 ± 4.65 | 0–70 | ||||||
| cola | 1.24 ± 2.35 | 0–60 | ||||||
| Neurodevelopmental and Cognitive Measurements | Median (IQR) |
|---|---|
| CBCL, n = 1995 | |
| Externalizing problems | 46 (19–74) |
| Internalizing problems | 45 (22–75) |
| Attention Deficit Hyperactivity Disorder (ADHD) | 39 (17–61) |
| Mean ± SD | |
| WPPSI, n = 1890 | |
| Verbal Intelligence Quotient (VIQ) | 114 ± 12 |
| Performance Intelligence Quotient (PIQ) | 112 ± 14 |
| General Language (GL) | 109 ± 11 |
| Processing Speed Quotient (PSQ) | 103 ± 14 |
| Total Intelligence Quotient (TIQ) | 114 ± 13 |
| Processed and High-Fat Foods (RC1) | Fresh Food and Fish (RC2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurodevelopmental and Cognitive Measurements | All | Females | Males | All | Females | Males | ||||||
| CBCL | β (95%CI) | p Value | β (95%CI) | p Value | β (95%CI) | p Value | β (95%CI) | p Value | β (95%CI) | p Value | β (95%CI) | p Value |
| Externalizing problems | 0.88 (0.28–1.49) | 0.004 | 0.99 (0.17–1.81) | 0.017 | 0.7 (−0.21–1.61) | 0.133 | −0.25 (−0.97–0.47) | 0.497 | −0.38 (−1.42–0.66) | 0.471 | −0.21 (−1.23–0.81) | 0.686 |
| Internalizing problems | 0.26 (−0.34–0.87) | 0.394 | 0.16 (−0.67–1.00) | 0.705 | 0.28 (−0.62–1.18) | 0.540 | −0.03 (−0.76–0.69) | 0.928 | 0.18 (−0.88–1.24) | 0.741 | −0.23 (−1.24–0.78) | 0.654 |
| Attention Deficit Hyperactivity Disorder (ADHD) | 0.37 (−0.26–1.00) | 0.254 | 0.01 (−0.85–0.87) | 0.978 | 0.77 (−0.17–1.71) | 0.110 | 0.05 (−0.70–0.81) | 0.889 | 0.43 (−0.66–1.51) | 0.442 | −0.31 (−1.37–0.75) | 0.565 |
| WPPSI | ||||||||||||
| Verbal Intelligence Quotient (VIQ) | −0.09 (−0.32–0.13) | 0.428 | −0.17 (−0.45–0.11) | 0.245 | 0.01 (−0.35–0.38) | 0.943 | 0.03 (−0.24–0.30) | 0.819 | 0.14 (−0.22–0.50) | 0.457 | −0.08 (−0.49–0.33) | 0.693 |
| Performance Intelligence Quotient (PIQ) | 0.19 (−0.07–0.45) | 0.161 | 0.20 (−0.14–0.54) | 0.247 | 0.20 (−0.21–0.61) | 0.346 | 0.14 (−0.18–0.45) | 0.396 | 0.23 (−0.20–0.66) | 0.289 | 0.02 (−0.44–0.49) | 0.916 |
| General Language (GL) | −0.16 (−0.41–0.08) | 0.189 | −0.26 (−0.56–0.03) | 0.082 | −0.04 (−0.45–0.36) | 0.843 | 0.08 (−0.20–0.37) | 0.577 | 0.19 (−0.20–0.57) | 0.337 | 0.00 (−0.44–0.43) | 0.990 |
| Processing Speed Quotient (PSQ) | 0.14 (−0.14–0.42) | 0.331 | 0.13 (−0.26–0.52) | 0.508 | 0.12 (−0.31–0.54) | 0.589 | −0.28 (−0.62–0.06) | 0.101 | −0.40 (−0.89–0.10) | 0.116 | −0.21 (−0.67–0.26) | 0.391 |
| Total Intelligence Quotient (TIQ) | 0.09 (−0.16–0.34) | 0.459 | 0.08 (−0.23–0.39) | 0.608 | 0.12 (−0.29–0.52) | 0.568 | 0.05 (−0.25–0.35) | 0.744 | 0.17 (−0.23–0.56) | 0.410 | −0.07 (−0.52–0.38) | 0.750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leccese, L.; Nisticò, L.; Culasso, M.; Pizzi, C.; Lastrucci, V.; Gagliardi, L.; Brescianini, S. Maternal Dietary Pattern in Pregnancy and Behavioral Outcomes at 4 Years of Age in the Piccolipiù Cohort: Potential Sex-Related Differences. Nutrients 2025, 17, 2814. https://doi.org/10.3390/nu17172814
Leccese L, Nisticò L, Culasso M, Pizzi C, Lastrucci V, Gagliardi L, Brescianini S. Maternal Dietary Pattern in Pregnancy and Behavioral Outcomes at 4 Years of Age in the Piccolipiù Cohort: Potential Sex-Related Differences. Nutrients. 2025; 17(17):2814. https://doi.org/10.3390/nu17172814
Chicago/Turabian StyleLeccese, Letizia, Lorenza Nisticò, Martina Culasso, Costanza Pizzi, Vieri Lastrucci, Luigi Gagliardi, and Sonia Brescianini. 2025. "Maternal Dietary Pattern in Pregnancy and Behavioral Outcomes at 4 Years of Age in the Piccolipiù Cohort: Potential Sex-Related Differences" Nutrients 17, no. 17: 2814. https://doi.org/10.3390/nu17172814
APA StyleLeccese, L., Nisticò, L., Culasso, M., Pizzi, C., Lastrucci, V., Gagliardi, L., & Brescianini, S. (2025). Maternal Dietary Pattern in Pregnancy and Behavioral Outcomes at 4 Years of Age in the Piccolipiù Cohort: Potential Sex-Related Differences. Nutrients, 17(17), 2814. https://doi.org/10.3390/nu17172814







