Amino Acids Supplementation in Cancer: What Do We Feed, the Patient or the Tumor?
Abstract
1. Introduction
2. Cancer Cell Metabolism: An Overview
3. The Multiple Roles of Amino Acids in Cancer Cells
3.1. Glutamine
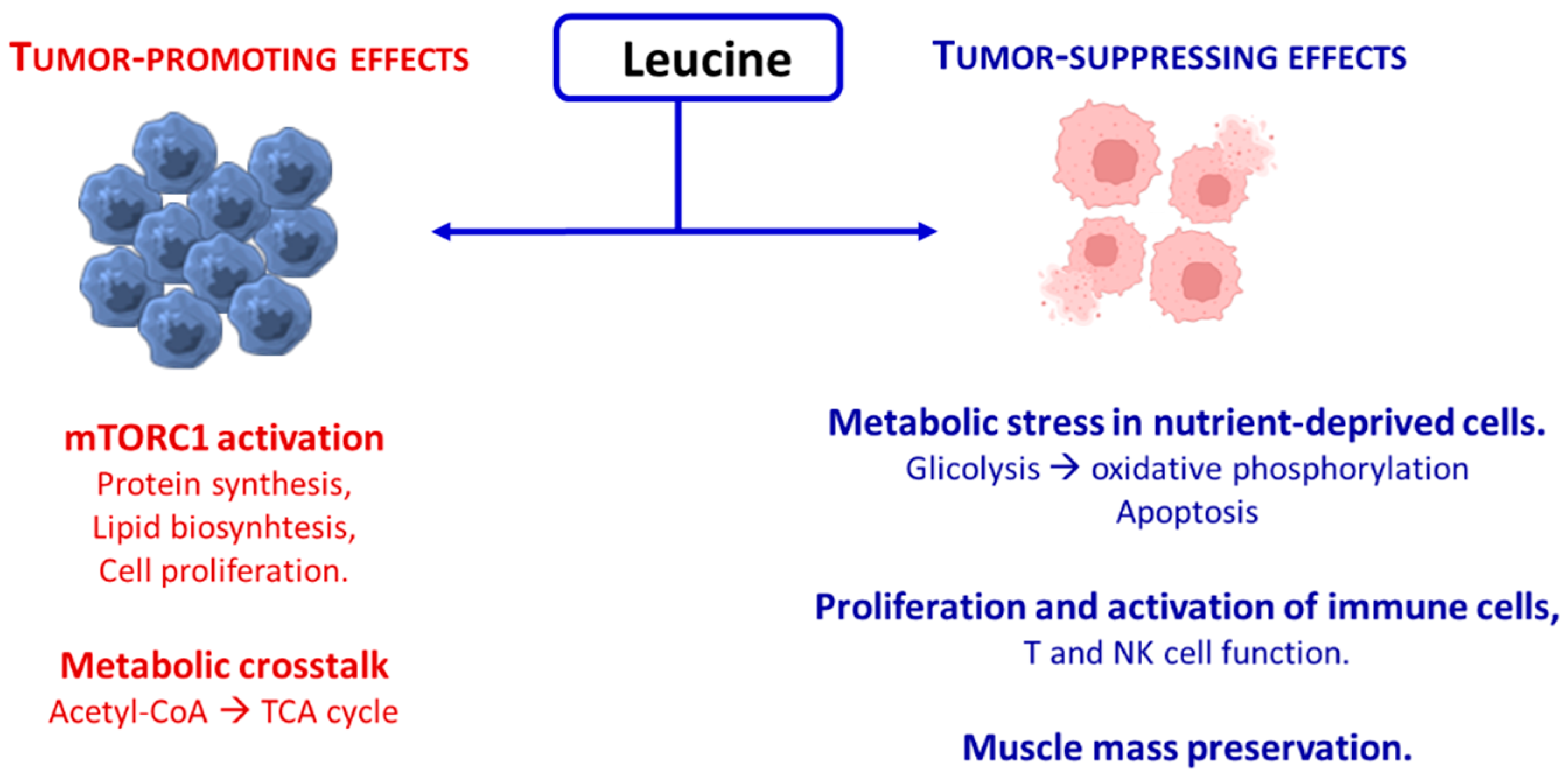
3.2. Leucine
3.3. Serine
3.4. Arginine
3.5. Asparagine
3.6. Proline and Glycine
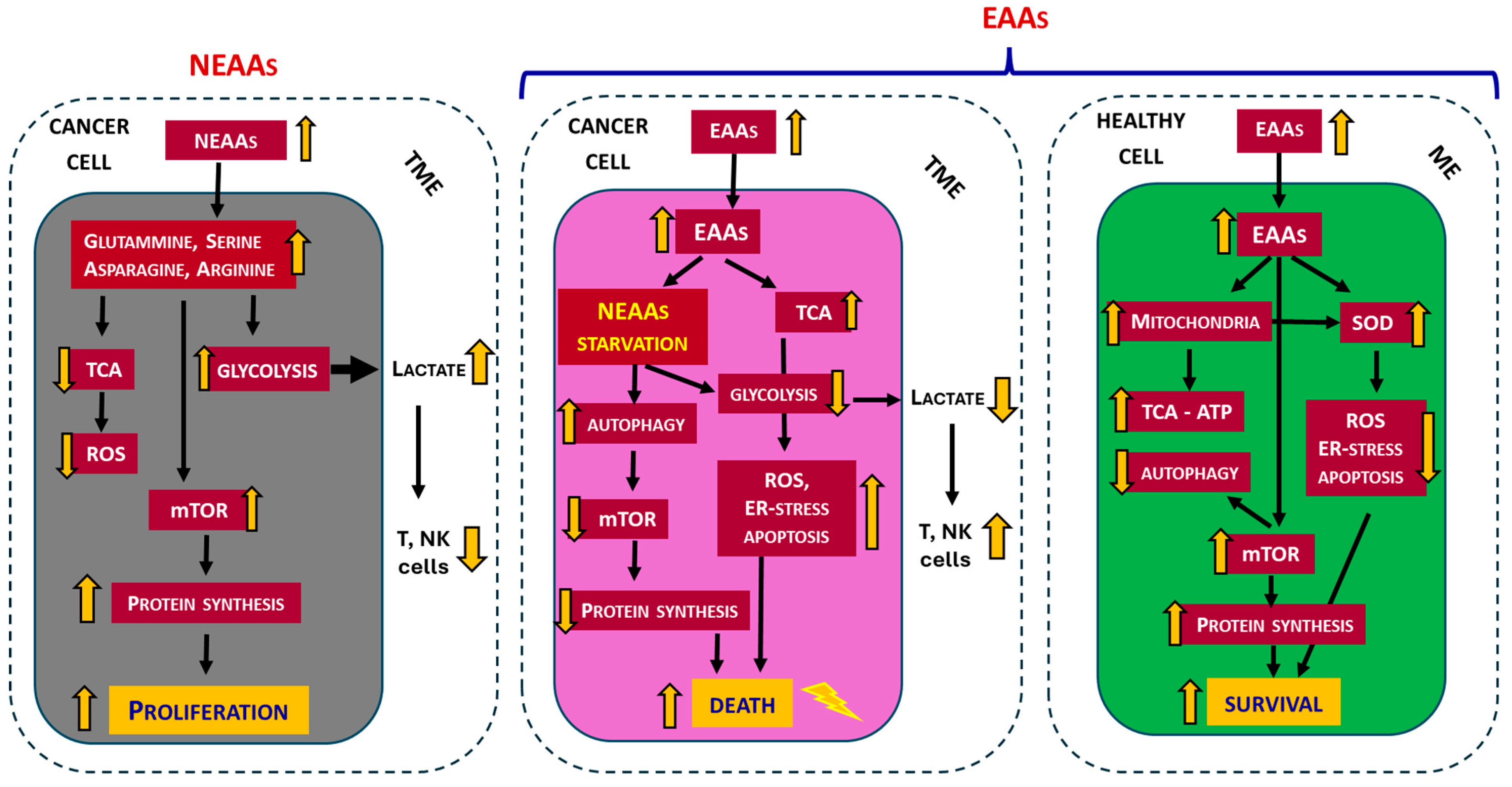
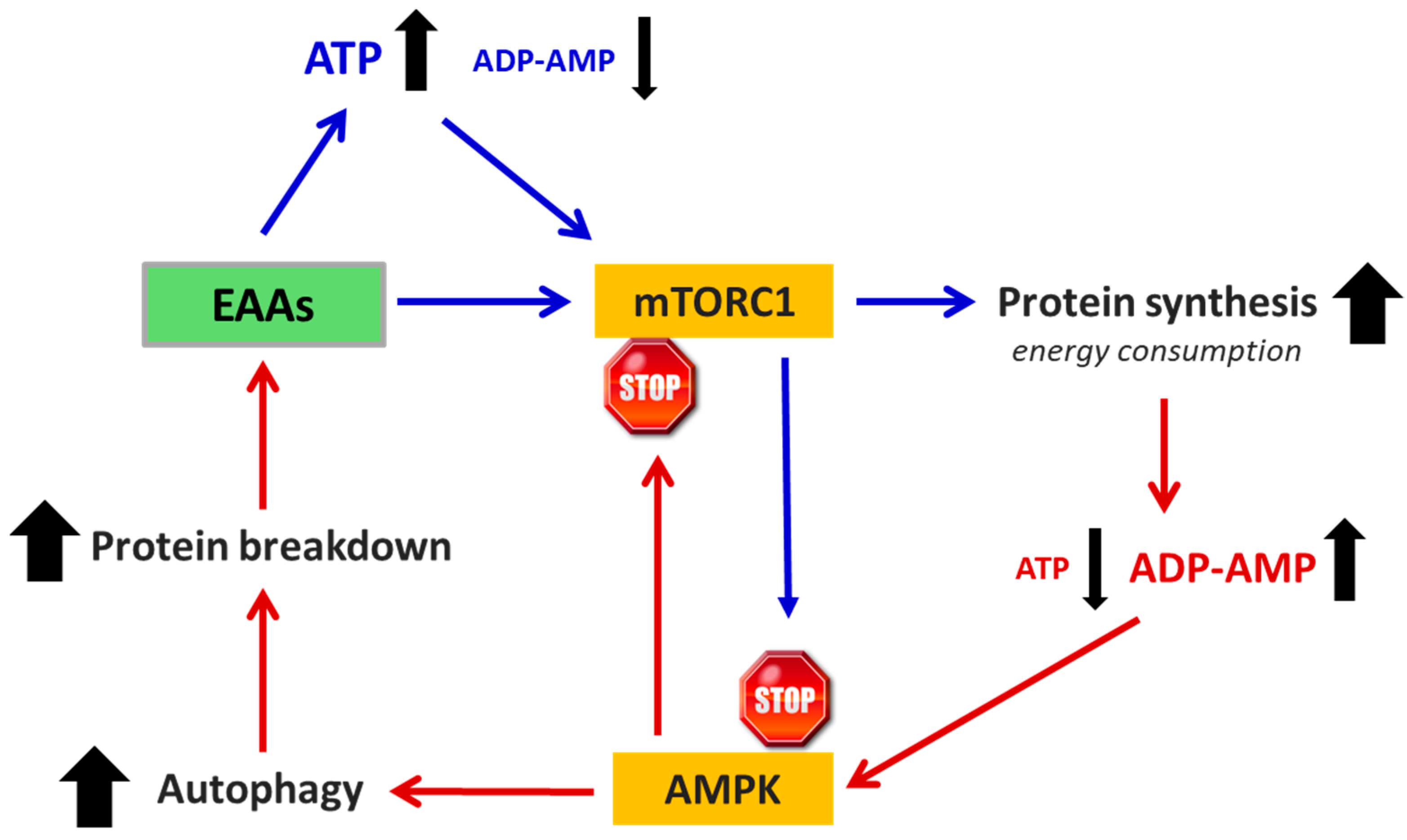
3.7. Essential Amino Acids (EAAs)
4. Amino Acids in Experimental Models of Cancer
4.1. The Traditional Approach: A Single AA as a Drug
4.2. The Innovative Approach: A Specifically Designed EAAs Mixture
| Free AAs Composition of EAAs-Mix | % |
|---|---|
| L-Leucine (BCAA) | 13.53 |
| L-Isoleucine (BCAA) | 9.65 |
| L-Valine (BCAA) | 9.65 |
| L-Lysine | 11.6 |
| L-Threonine | 8.7 |
| L-Histidine | 11.6 |
| L-Phenylalanine | 7.73 |
| L-Methionine | 4.35 |
| L-Tyrosine | 5.80 |
| L-Tryptophan | 3.38 |
| L-Cystine/Cysteine | 8.20 |
| L-Serine | 2.42 |
| Ornithine-αKG | 2.42 |
| N-acetylcysteine | 0.97 |
5. Amino Acids in Cancer Patients
Amino Acids as Prevention or Treatment of Cancer Cachexia
6. Does Food Integration with EAAs Promote Cancer?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arg-1 | Arginase 1 |
| ASS1 | Arginosuccinate synthase |
| BCAA | Branched-chain amino acids |
| CRC | Colorectal cancer |
| EAAs | Essential amino acids |
| eNOS | Endothelial nitric oxide synthase |
| IDO1 | Indoleamine 2,3 dioxygenase |
| MM | Muscle mass |
| NEAA | Non-essential amino acids |
| ROS | Reactive oxygen species |
| TME | Tumor microenvironment |
References
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116, Erratum in: Pharm. Res. 2008, 25, 2200. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Tchekmedyian, N.S. Cancer anorexia and cachexia. In Nutritional Oncology; Heber, D., Blackburn, G.L., Go, V.L., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 645–660. [Google Scholar]
- Hall, D.T.; Ma, J.F.; Marco, S.D.; Gallouzi, I.E. Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging 2011, 3, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, R.; Chapman, I.M. Undernutrition and anorexia in the older person. Gastroenterol. Clin. N. Am. 2009, 38, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Ispoglou, T.; Witard, O.C.; Duckworth, L.C.; Lees, M.J. The efficacy of essential amino acid supplementation for augmenting dietary protein intake in older adults: Implications for skeletal muscle mass, strength and function. Proc. Nutr. Soc. 2021, 80, 230–242. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef]
- Aquilani, R.; D’Antona, G.; Baiardi, P.; Gambino, A.; Iadarola, P.; Viglio, S.; Pasini, E.; Verri, M.; Barbieri, A.; Boschi, F. Essential amino acids and exercise tolerance in elderly muscle-depleted subjects with chronic diseases: A rehabilitation without rehabilitation? Biomed. Res. Int. 2014, 2014, 341603. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Antoniello, N.; Boschi, F.; Iadarola, P.; Pasini, E.; Aquilani, R.; Dioguardi, F.S. Effect of essential amino acid supplementation on quality of life, amino acid profile and strength in institutionalized elderly patients. Clin. Nutr. 2011, 30, 571–577. [Google Scholar] [CrossRef]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef]
- Choi, B.H.; Coloff, J.L. The diverse functions of non-essential amino acids in cancer. Cancers 2019, 11, 675. [Google Scholar] [CrossRef]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Shunxi, W.; Xiaoxue, Y.; Guanbin, S.; Li, Y.; Junyu, J.; Wanqian, L. Serine metabolic reprogramming in tumorigenesis, tumor immunity, and clinical treatment. Adv. Nutr. 2023, 14, 1050–1066. [Google Scholar] [CrossRef]
- Yang, J.; Shay, C.; Saba, N.F.; Teng, Y. Cancer metabolism and carcinogenesis. Exp. Hematol. Oncol. 2024, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef]
- Locasale, J.W. Serine, glycine and one-carbon units: Cancer metabolism in full circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Khan, I.R.; Khan, M.S.; Husain, F.M.; Ahmad, S.; Haris, M.; Singh, M.; Akil, A.S.A.; Macha, M.A.; et al. Glutamine Metabolism: Molecular Regulation, Biological Functions, and Diseases. MedComm 2025, 6, e70120. [Google Scholar] [CrossRef]
- Mider, G.B.; Tesluk, H.; Morton, J.J. Effects of Walker carcinoma 256 on food intake, body weight and nitrogen metabolism of growing rats. Acta Union. Int. Contra Cancrum 1948, 6, 409–420. [Google Scholar]
- Babson, A.L.; Winnick, T. Protein transfer in tumor-bearing rats. Cancer Res. 1954, 14, 606–611. [Google Scholar]
- Stehle, G.; Sinn, H.; Wunder, A.; Schrenk, H.H.; Stewart, J.C.M.; Hartung, G.; Maier-Borst, W.; Heene, D.L. Plasma protein (albumin) catabolism by the tumor itself—Implications for tumor metabolism and the genesis of cachexia. Crit. Rev. Oncol. Hematol. 1997, 26, 77–100. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Wetzel, M.; Tenner, B.; D’Andrea, L. Crosstalk between arginine, glutamine, and branched-chain amino acid metabolism in the tumor microenvironment. Trends Cancer 2023, 9, 589–604. [Google Scholar] [CrossRef]
- Wißfeld, J.; Werner, A.; Yan, X.; Ten Bosch, N.; Cui, G. Metabolic regulation of immune responses to cancer. Cancer Biol. Med. 2022, 19, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Liu, Z. Metabolic reprogramming and immune evasion in cancer: The role of glutamine metabolism. Biomark. Res. 2024, 12, 25. [Google Scholar] [CrossRef]
- Nan, D.; Yao, W.; Huang, L.; Liu, R.; Chen, X.; Xia, W.; Sheng, H.; Zhang, H.; Liang, X.; Lu, Y. Glutamine and cancer: Metabolism, immune microenvironment, and therapeutic targets. Cell Commun. Signal. 2025, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Lemos, H.; Huang, L.; Prendergast, G.C.; Mellor, A.L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat. Rev. Cancer 2019, 19, 162–175. [Google Scholar] [CrossRef]
- Li, G.W.; Burkhardt, D.; Gross, C.; Weissman, J.S. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 2014, 157, 624–635. [Google Scholar] [CrossRef]
- Vasan, K.; Werner, M.; Chandel, N.S. Mitochondrial metabolism as a target for cancer therapy. Cell Metab. 2020, 32, 341–352. [Google Scholar] [CrossRef]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Vriens, K.; Christen, S.; Parik, S.; Broekaert, D.; Yoshinaga, K.; Talebi, A.; Dehairs, J.; Escalona-Noguero, C.; Schmieder, R.; Cornfield, T.; et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer. Nature 2019, 566, 403–406. [Google Scholar] [CrossRef]
- Peck, B.; Schulze, A. Lipid desaturation—The next step in targeting lipogenesis in cancer? FEBS J. 2016, 283, 2767–2778. [Google Scholar] [CrossRef]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J. 2017, 284, 1726–1737. [Google Scholar] [CrossRef]
- Ragni, M.; Fornelli, C.; Nisoli, E.; Penna, F. Amino acids in cancer and cachexia: An integrated view. Cancers 2022, 14, 5691. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Codenotti, S.; Giugno, L.; Pasini, E.; Fanzani, A.; Scarabelli, T.; Dioguardi, F.S. Intake of special amino acids mixture leads to blunted murine colon cancer growth in vitro and in vivo. Cells 2024, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Akbay, B.; Omarova, Z.; Trofimov, A.; Sailike, B.; Karapina, O.; Molnár, F.; Tokay, T. Double-edge effects of leucine on cancer cells. Biomolecules 2024, 14, 1401. [Google Scholar] [CrossRef] [PubMed]
- Deldicque, L.; Theisen, D.; Francaux, M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur. J. Appl. Physiol. 2005, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Cuzick, J.; Thorat, M.A.; Andriole, G.; Brawley, O.W.; Brown, P.H.; Culig, Z.; Eeles, R.A.; Ford, L.G.; Hamdy, F.; Holmberg, L.; et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014, 15, e484–e492. [Google Scholar] [CrossRef]
- Howell, A.; Anderson, A.S.; Clarke, R.B.; Duffy, S.W.; Evans, D.G.; Garcia-Closas, M.; Gescher, A.J.; Key, T.J.; Saxton, J.M.; Harvie, M.N. Risk determination and prevention of breast cancer. Breast Cancer Res. 2014, 16, 446. [Google Scholar] [CrossRef]
- Courneya, K.S.; McKenzie, D.C.; Mackey, J.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Vallerand, J.R.; Adams, S.C.; Proulx, C.; et al. Subgroup effects in a randomised trial of different types and doses of exercise during breast cancer chemotherapy. Br. J. Cancer 2014, 111, 1718–1725. [Google Scholar] [CrossRef]
- Avruch, J.; Long, X.; Ortiz-Vega, S.; Rapley, J.; Papageorgiou, A.; Dai, N. Amino acid regulation of TOR complex 1. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E592–E602. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef]
- Flati, V.; Corsetti, G.; Pasini, E.; Rufo, A.; Romano, C.; Dioguardi, F.S. Nutrition, nitrogen requirements, exercise and chemotherapy-induced toxicity in cancer patients. a puzzle of contrasting truths? Anticancer. Agents Med. Chem. 2016, 16, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Sherman, C.D.; Morton, J.J.; Mider, G.B. Potential sources of tumor nitrogen. Cancer Res. 1950, 10, 374–378. [Google Scholar]
- Eagle, H. The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J. Exp. Med. 1955, 102, 37–48. [Google Scholar] [CrossRef]
- Allison, J.B.; Wannemacher, R.W.; Prosky, L., Jr.; Crossley, M.L. Nutritive value of protein and tumor-host relationship in the rat. J. Nutr. 1956, 60, 297–307. [Google Scholar] [CrossRef]
- Young, V.R.; Bier, D.M. Amino acid requirements in adult humans: How well do we know them? J. Nutr. 1987, 117, 1484–1487. [Google Scholar] [CrossRef]
- Patel, D.; Menon, D.; Bernfeld, E.; Mroz, V.; Kalan, S.; Loayza, D.; Foster, D.A. Aspartate rescuess-phase arrest caused by suppression of glutamine utilization in KR as driven cancer cells. J. Biol. Chem. 2016, 291, 9322–9939. [Google Scholar] [CrossRef]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Cluntun, A.A.; Lukey, M.J.; Cerione, R.A.; Locasale, J.W. Glutamine metabolism in cancer: Understanding the heterogeneity. Trends Cancer 2017, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, L.J.; Wice, B.M.; Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 1979, 254, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef]
- Villar, V.H.; Nguyen, T.L.; Terés, S.; Bodineau, C.; Durán, R.V. Escaping mTOR inhibition for cancer therapy: Tumor suppressor functions of mTOR. Mol. Cell Oncol. 2017, 4, e1297284. [Google Scholar] [CrossRef]
- Bodineau, C.; Tomé, M.; Courtois, S.; Costa, A.S.H.; Sciacovelli, M.; Rousseau, B.; Richard, E.; Vacher, P.; Parejo-Pérez, C.; Bessede, E.; et al. Two parallel pathways connect glutamine metabolism and mTORC1 activity to regulate glutamoptosis. Nat. Commun. 2021, 12, 4814. [Google Scholar] [CrossRef]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef]
- Neman, J.; Termini, J.; Wilczynski, S.; Vaidehi, N.; Choy, C.; Kowolik, C.M.; Li, H.; Hambrecht, A.C.; Roberts, E.; Jandial, R. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl. Acad. Sci. USA 2014, 111, 984–989. [Google Scholar] [CrossRef]
- Bodineau, C.; Tomé, M.; Murdoch, P.D.S.; Durán, R.V. Glutamine, MTOR and autophagy: A multiconnection relationship. Autophagy 2022, 18, 2749–2750. [Google Scholar] [CrossRef]
- Sener, A.; Malaisse, W.J. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature 1980, 288, 187–189. [Google Scholar] [CrossRef]
- Tennant, D.A.; Gottlieb, E. HIF prolyl hydroxylase-3 mediates alpha-ketoglutarate-induced apoptosis and tumor suppression. J. Mol. Med. 2010, 88, 839–849. [Google Scholar] [CrossRef]
- Howell, J.J.; Ricoult, S.J.H.; Ben-Sahra, I.; Manning, B.D. A growing role for mTOR in promoting anabolic metabolism. Biochem. Soc. Trans. 2013, 41, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Villar, V.H.; Merhi, F.; Djavaheri-Mergny, M.; Durán, R.V. Glutaminolysis and autophagy in cancer. Autophagy 2015, 11, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, I.; Malek, M.; Gadet, R.; Viallet, J.; Garcia, A.; Girard-Gagnepain, A.; Hesling, C.; Gillet, G.; Gonzalo, P.; Rimokh, R.; et al. Genetic and pharmacologic inhibition of mTORC1 promotes EMT by a TGF-β-independent mechanism. Cancer Res. 2013, 73, 6621–6631. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Alonso, J.J.; López-Lázaro, M. Dietary Manipulation of Amino Acids for Cancer Therapy. Nutrients 2023, 15, 2879. [Google Scholar] [CrossRef]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine Supplementation Differentially Enhances Pancreatic Cancer Growth in Lean and Overweight Mice. Cancer Metab. 2014, 2, 6. [Google Scholar] [CrossRef]
- Martin, S.B.; Reiche, W.S.; Fifelski, N.A.; Schultz, A.J.; Stanford, S.J.; Martin, A.A.; Nack, D.L.; Radlwimmer, B.; Boyer, M.P.; Ananieva, E.A. Leucine and branched-chain amino acid metabolism contribute to the growth of bone sarcomas by regulating AMPK and mTORC1 signaling. Biochem. J. 2020, 477, 1579–1599. [Google Scholar] [CrossRef]
- Hassan, Y.A.; Helmy, M.W.; Ghoneim, A.I. Combinatorial antitumor effects of amino acids and epigenetic modulations in hepatocellular carcinoma cell lines. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 2245–2257. [Google Scholar] [CrossRef]
- Hosios, A.M.; Hecht, V.C.; Danai, L.V.; Johnson, M.O.; Rathmell, J.C.; Steinhauser, M.L.; Manalis, S.R.; Vander Heiden, M.G. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 2016, 36, 540–554. [Google Scholar] [CrossRef]
- Kang, J.S. Dietary restriction of amino acids for cancer therapy. Nutr. Metab. 2020, 17, 20. [Google Scholar] [CrossRef]
- Sun, W.; Liu, R.; Gao, X.; Lin, Z.; Tang, H.; Cui, H.; Zhao, E. Targeting serine-glycine-one-carbon metabolism as a vulnerability in cancers. Biomark. Res. 2023, 11, 48. [Google Scholar] [CrossRef]
- Newman, A.C.; Maddocks, O.D.K. Serine and functional metabolites in cancer. Trends Cell Biol. 2017, 27, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Itoyama, R.; Yasuda-Yoshihara, N.; Kitamura, F.; Yasuda, T.; Bu, L.; Yonemura, A.; Uchihara, T.; Arima, K.; Hu, X.; Jun, Z.; et al. Metabolic shift to serine biosynthesis through 3-PG accumulation and PHGDH induction promotes tumor growth in pancreatic cancer. Cancer Lett. 2021, 523, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, S.L.; Heylen, E.; De Keersmaecker, K.; Kampen, K.R. The ins and outs of serine and glycine metabolism in cancer. Nat. Metab. 2021, 3, 131–141. [Google Scholar] [CrossRef]
- Li, X.; Gracilla, D.; Cai, L.; Zhang, M.; Yu, X.; Chen, X.; Zhang, J.; Long, X.; Ding, H.F.; Yan, C. ATF3 promotes the serine synthesis pathway and tumor growth under dietary serine restriction. Cell Rep. 2021, 36, 109706. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsu, S.C.; Ann, D.K.; Yen, Y.; Kung, H.J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef]
- Marigo, I.; Dolcetti, L.; Serafini, P.; Zanovello, P.; Bronte, V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 2008, 222, 162–179. [Google Scholar] [CrossRef]
- Feng, T.; Xie, F.; Lyu, Y.; Yu, P.; Chen, B.; Yu, J.; Zhang, G.; Fai To, K.; Tsang, C.M.; Kang, W. The arginine metabolism and its deprivation in cancer therapy. Cancer Lett. 2025, 620, 217680. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yin, L.; He, J.; Zeng, Q.; Liang, Y.; Shen, Y.; Zu, X. Metabolism of asparagine in the physiological state and cancer. Cell Commun. Signal. 2024, 22, 163. [Google Scholar] [CrossRef] [PubMed]
- Knott, S.R.V.; Wagenblast, E.; Khan, S.; Kim, S.Y.; Soto, M.; Wagner, M.; Turgeon, M.-O.; Fish, L.; Erard, N.; Gable, A.L.; et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 2018, 554, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Liu, W.; Xiao, X.; Gajendran, B.; Ben-David, Y. Targeting pivotal amino acids metabolism for treatment of leukemia. Heliyon 2024, 10, e40492. [Google Scholar] [CrossRef]
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C.H. L-asparaginase treatment in acute lymphoblastic leukemia: A focus on Erwinia asparaginase. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef]
- Hanada, K.; Kawada, K.; Obama, K. Targeting Asparagine Metabolism in Solid Tumors. Nutrients 2025, 17, 179. [Google Scholar] [CrossRef]
- D’Aniello, C.; Patriarca, E.J.; Phang, J.M.; Minchiotti, G. Proline metabolism in tumor growth and metastatic progression. Front. Oncol. 2020, 10, 776. [Google Scholar] [CrossRef]
- Amelio, I.; Cutruzzolá, F.; Antonov, A.; Agostini, M.; Melino, G. Serine and glycine metabolism in cancer. Trends Biochem. Sci. 2014, 39, 191–198. [Google Scholar] [CrossRef]
- Chenette, E. Glycine fuels cancer cells. Nat. Cell Biol. 2012, 14, 658. [Google Scholar] [CrossRef]
- Ericksen, R.E.; Lim, S.L.; McDonnell, E.; Shuen, W.H.; Vadiveloo, M.; White, P.J.; Ding, Z.; Kwok, R.; Lee, P.; Radda, G.K.; et al. Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 2019, 29, 1151–1165.e6. [Google Scholar] [CrossRef] [PubMed]
- Guri, Y.; Colombi, M.; Dazert, E.; Hindupur, S.K.; Roszik, J.; Moes, S.; Jenoe, P.; Heim, M.H.; Riezman, I.; Riezman, H.; et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell 2017, 32, 807–823.e12. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; van der Meer, L.T.; van Leeuwen, F.N. Amino acid depletion therapies: Starving cancer cells to death. Trends Endocrinol. Metab. 2021, 32, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.C.; Prudner, B.C.; Lange, S.E.S.; Bean, G.R.; Schultze, M.B.; Brashears, C.B.; Radyk, M.D.; Redlich, N.; Tzeng, S.C.; Kami, K.; et al. Arginine deprivation inhibits the Warburg effect and upregulates glutamine anaplerosis and serine biosynthesis in ASS1-deficient cancers. Cell Rep. 2017, 18, 991–1004. [Google Scholar] [CrossRef]
- Dioguardi, F.S. Clinical use of amino acids as dietary supplement: Pros and cons. J. Cachexia Sarcopenia Muscle 2011, 2, 75–80. [Google Scholar] [CrossRef]
- Maddocks, O.D.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine deprivation inhibits proliferation and induces apoptosis of human breast cancer cells via fatty acid synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; de Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; Brunetto, S.Q.; et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci. Rep. 2019, 9, 15529. [Google Scholar] [CrossRef]
- Li, J.T.; Yin, M.; Wang, D.; Wang, J.; Lei, M.Z.; Zhang, Y.; Liu, Y.; Zhang, L.; Zou, S.W.; Hu, L.P.; et al. BCAT2-mediated BCAA catabolism is critical for development of pancreatic ductal adenocarcinoma. Nat. Cell Biol. 2020, 22, 167–174. [Google Scholar] [CrossRef]
- Kang, Z.R.; Jiang, S.; Han, J.X.; Gao, Y.; Xie, Y.; Chen, J.; Liu, Q.; Yu, J.; Zhao, X.; Hong, J.; et al. Deficiency of BCAT2-mediated branched-chain amino acid catabolism promotes colorectal cancer development. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166941. [Google Scholar] [CrossRef]
- Jiménez-Alonso, J.J.; Guillén-Mancina, E.; Calderón-Montaño, J.M.; Jiménez-González, V.; Díaz-Ortega, P.; Burgos-Morón, E.; López-Lázaro, M. Artificial diets with altered levels of sulfur amino acids induce anticancer activity in mice with metastatic colon cancer, ovarian cancer and renal cell carcinoma. Int. J. Mol. Sci. 2023, 24, 4587. [Google Scholar] [CrossRef]
- Dioguardi, F.S.; Flati, V.; Corsetti, G.; Pasini, E.; Romano, C. Is the response of tumors dependent on the dietary input of some amino acids or ratios among essential and non-essential amino acids? All that glitters is not gold. Int. J. Mol. Sci. 2018, 19, 3631. [Google Scholar] [CrossRef]
- Maddocks, O.; Athineos, D.; Cheung, E.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Correction: Corrigendum: Modulating the therapeutic response of tumors to dietary serine and glycine starvation. Nature 2017, 548, 122. [Google Scholar] [CrossRef]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Buyukgolcigezli, I.; Tenekeci, A.K.; Sahin, I.H. Opportunities and challenges in antibody-drug conjugates for cancer therapy: A new era for cancer treatment. Cancers 2025, 17, 958. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Pasini, E.; Scarabelli, T.M.; Romano, C.; Singh, A.; Scarabelli, C.C.; Dioguardi, F.S. Importance of energy, dietary protein sources, and amino acid composition in the regulation of metabolism: An indissoluble dynamic combination for life. Nutrients 2024, 16, 2417. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Corsetti, G.; Dioguardi, F.S. Behind protein synthesis: Amino acids-metabokine regulators of both systemic and cellular metabolism. Nutrients 2023, 15, 2892. [Google Scholar] [CrossRef]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhäusl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef]
- Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of diets with varying essential/nonessential amino acid ratios on mouse lifespan. Nutrients 2019, 11, 1367. [Google Scholar] [CrossRef]
- Vlahakis, A.; Graef, M.; Nunnari, J.; Powers, T. TOR complex 2-Ypk1 signaling is an essential positive regulator of the general amino acid control response and autophagy. Proc. Natl. Acad. Sci. USA 2014, 111, 10586–10591. [Google Scholar] [CrossRef]
- Corsetti, G.; Flati, V.; Pasini, E.; Sanità, P.; Dioguardi, F.S. Protect and counterattack: Nutritional supplementation with essential amino acid ratios reduces doxorubicin–induced cardiotoxicity in vivo and promote cancer cell death in vitro. J. Cytol. Histol. 2015, 6, 5. [Google Scholar] [CrossRef]
- Corsetti, G.; Romano, C.; Pasini, E.; Scarabelli, T.; Chen-Scarabelli, C.; Dioguardi, F.S. Essential amino acids-rich diet increases cardiomyocytes protection in doxorubicin-treated mice. Nutrients 2023, 15, 2287. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cools, J. Improvements in the survival of children and adolescents with acute lymphoblastic leukemia. Haematologica 2012, 97, 635. [Google Scholar] [CrossRef]
- Aldoss, I.; Douer, D. How I treat the toxicities of peg asparaginase in adults with acute lymphoblastic leukemia. Blood 2020, 135, 987–995. [Google Scholar] [CrossRef]
- Fernandes, H.S.; Silva Teixeira, C.S.; Fernandes, P.A.; Ramos, M.J.; Cerqueira, N.M. Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert. Opin. Ther. Pat. 2017, 27, 283–297. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Kim, J.; DeBerardinis, R.J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Tajan, M.; Vousden, K.H. Dietary approaches to cancer therapy. Cancer Cell 2020, 37, 767–785. [Google Scholar] [CrossRef]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibit liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar] [CrossRef]
- Shimizu, M.; Shirakami, Y.; Hanai, T.; Imai, K.; Suetsugu, A.; Takai, K.; Shiraki, M.; Moriwaki, H. Pharmaceutical and nutraceutical approaches for preventing liver carcinogenesis: Chemoprevention of hepatocellular carcinoma using acyclic retinoid and branched-chain amino acids. Mol. Nutr. Food Res. 2014, 58, 124–135. [Google Scholar] [CrossRef]
- Rossi, M.; Mascaretti, F.; Parpinel, M.; Serraino, D.; Crispo, A.; Celentano, E.; Giacosa, A.; La Vecchia, C. Dietary intake of branched-chain amino acids and colon rectal cancer risk. Br. J. Nutr. 2021, 126, 22–27. [Google Scholar] [CrossRef]
- Mayers, J.R.; Wu, C.; Clish, C.B.; Kraft, P.; Torrence, M.E.; Fiske, B.P.; Yuan, C.; Bao, Y.; Townsend, M.K.; Tworoger, S.S.; et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 2014, 20, 1193–1198. [Google Scholar] [CrossRef]
- Katagiri, R.; Goto, A.; Nakagawa, T.; Nishiumi, S.; Kobayashi, T.; Hidaka, A.; Budhathoki, S.; Yamaji, T.; Sawada, N.; Shimazu, T.; et al. Increased levels of branched-chain amino acid associated with increased risk of pancreatic cancer in a prospective case-control study of a large cohort. Gastroenterology 2018, 155, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Sivanand, S.; Vander Heiden, M.G. Emerging roles for branched-chain amino acid metabolism in cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Nishiyama, M.; Ishizaki, S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J. Cell Physiol. 2012, 227, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S.; Chen-Scarabelli, C.; Pasini, E.; Corsetti, G.; Scarabelli, T.M. Diet, muscle protein synthesis and autophagy relationships in cancer an attempt to understand where are we going, and why. Adv. Nutr. Food Sci. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Cong, M.; Ciu, J.; Xu, H.; Chen, J.; Li, T.; Li, Z.; Liu, M.; Li, W.; Gao, Z.; et al. General rules for treating cancer-related malnutrition. Precis. Nutr. 2022, 1, e00024. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Arends, J. Malnutrition in cancer patients: Causes, consequences and treatment options. Eur. J. Surg. Oncol. 2024, 50, 107074. [Google Scholar] [CrossRef]
- Merker, M.; Felder, M.; Gueissaz, L.; Bolliger, R.; Tribolet, P.; Kägi-Braun, N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Association of baseline inflammation with effectiveness of nutritional support among patients with disease-related malnutrition: A secondary analysis of a randomized clinical trial. JAMA New Open 2020, 3, e200663. [Google Scholar] [CrossRef]
- Laird, B.J.; Kaasa, S.; McMillan, D.C.; Fallon, M.T.; Hjermstad, M.J.; Fayers, P.; Klepstad, P. Prognostic factors in patients with advanced cancer: A comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin. Cancer Res. 2013, 19, 5456–5464. [Google Scholar] [CrossRef]
- van der Meij, B.S.; Teleni, L.; Engelen, M.P.K.J.; Deutz, N.E.P. Amino acid kinetics and the response to nutrition in patients with cancer. Int. J. Radiat. Biol. 2019, 95, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.S.; Wilkinson, D.J.; Atherton, P.J. Nutrient modulation in the management of disease-induced muscle wasting: Evidence from human studies. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Beaudry, A.G.; Law, M.L. Leucine supplementation in cancer cachexia: Mechanisms and a review of the pre-clinical literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Salomão, E.M.; Toneto, A.T.; Silva, G.O.; Gomes-Marcondes, M.C.C. Physical exercise and a leucine-rich diet modulate the muscle protein metabolism in walker tumor-bearing rats. Nut. Cancer 2010, 62, 1095–1104. [Google Scholar] [CrossRef]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am. J. Cardiol. 2008, 101, 11E–15E. [Google Scholar] [CrossRef]
- Aquilani, R.; Zuccarelli Ginetto, C.; Rutili, C.; Pisano, P.; Pasini, E.; Baldissarro, E.; Verri, M.; Boschi, F. Supplemented amino acids may enhance the walking recovery of elderly subjects after hip fracture surgery. Aging Clin. Exp. Res. 2019, 31, 157–160. [Google Scholar] [CrossRef]
- Dal Negro, R.W.; Aquilani, R.; Bertacco, S.; Boschi, F.; Micheletto, C.; Tognella, S. Comprehensive effects of supplemented essential amino acids in patients with severe COPD and sarcopenia. Monaldi Arch. Chest Dis. 2010, 73, 25–33. [Google Scholar] [CrossRef]
- Buondonno, I.; Sassi, F.; Carignano, G.; Dutto, F.; Ferreri, C.; Pili, F.G.; Massaia, M.; Nisoli, E.; Ruocco, C.; Porrino, P.; et al. From mitochondria to healthy aging: The role of branched-chain amino acids treatment: MATeR a randomized study. Clin. Nutr. 2020, 39, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Flati, V.; Caliaro, F.; Speca, S.; Corsetti, G.; Cardile, A.; Nisoli, E.; Bottinelli, R.; D’Antona, G. Essential amino acids improveinsulin activation of AKT/MTOR signaling in soleus muscle of agedrats. Int. J. Immunopathol. Pharmacol. 2010, 23, 81–89. [Google Scholar] [CrossRef]
- Madeddu, C.; Macciò, A.; Astara, G.; Massa, E.; Dessì, M.; Antoni, G.; Panzone, F.; Serpe, R.; Mantovan, G. Open phase II study on efficacy and safety of an oral amino acid functional cluster supplementation in cancer cachexia. Med. J. Nutr. Metab. 2010, 3, 165–172. [Google Scholar] [CrossRef]
- Layman, D.K. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 6, 12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsetti, G.; Pasini, E.; Romano, C.; Dioguardi, F.S. Amino Acids Supplementation in Cancer: What Do We Feed, the Patient or the Tumor? Nutrients 2025, 17, 2813. https://doi.org/10.3390/nu17172813
Corsetti G, Pasini E, Romano C, Dioguardi FS. Amino Acids Supplementation in Cancer: What Do We Feed, the Patient or the Tumor? Nutrients. 2025; 17(17):2813. https://doi.org/10.3390/nu17172813
Chicago/Turabian StyleCorsetti, Giovanni, Evasio Pasini, Claudia Romano, and Francesco S. Dioguardi. 2025. "Amino Acids Supplementation in Cancer: What Do We Feed, the Patient or the Tumor?" Nutrients 17, no. 17: 2813. https://doi.org/10.3390/nu17172813
APA StyleCorsetti, G., Pasini, E., Romano, C., & Dioguardi, F. S. (2025). Amino Acids Supplementation in Cancer: What Do We Feed, the Patient or the Tumor? Nutrients, 17(17), 2813. https://doi.org/10.3390/nu17172813







